Abstract
Background
Accurate pathologic re-staging of N2 stations following neoadjuvant therapy in IIIA(N2) NSCLC is needed.
Methods
A prospective multi-institutional trial was designed to judge feasibility of VATS restaging ipsilateral nodes in mediastinoscopy proven IIIA(N2) NSCLC following 2 cycles platinum-based chemotherapy and/or > 40 Gy radiotherapy. Goals included biopsy 3 negative N2 node stations, or identify 1 positive N2 node or pleural carcinomatosis.
Results
Ten institutions accrued 68 subjects. Forty-six (68%) underwent radiation and 66 (97%) had chemotherapy. VATS successfully met pre-study feasibility in 27 pts (40%): three negative stations and confirmed at thoracotomy (7); persistent N2 disease (16) and pleural carcinomatosis (4). Twenty (29 %) found no N2 disease, but did not biopsy 3 stations due to unanticipated nodal obliteration. Thus, 47 VATS (69%, 95% CI: 57 - 80) restaged the mediastinum. VATS was unsuccessful in 21 (31 %) patients: abort procedure (11), or false negative stations (10). Of 21 failures, 15 were right-sided, and 10 had a positive 4R node. Sensitivity of VATS was 67% (95% CI: 47 – 83), and negative predictive value (NPV) was 73% (95% CI: 56 – 86) if including patients with obliterated nodal tissue. Sensitivity was 83% (95% CI: 63 – 95), NPV was 64% (95% CI: 31 – 89) if excluding those patients. Specificity was 100%. There was 1death after thoracotomy
Conclusions
VATS restaging was within 90% CI of “feasible” and provided pathology of ipsilateral nodes. VATS restaging is limited by radiation and the 4R nodal station.
Keywords: Staging; IIIA, Non-small cell lung cancer; VATS, Mediastinal nodes, Thoracoscopic
Background
Cancer & Leukemia Group B (CALGB) 8935 helped to establish the role of standardized pretreatment histopathologic staging by mediastinoscopy before neoadjuvant therapy for Stage IIIA (N2) non-small cell lung cancer (NSCLC) [1]. Mature follow-up data from both CALGB 8935 and Southwest Oncology Group (SWOG) 8805, two independent prospective multi-institutional studies, indicated that patients with residual N2 disease at the time of surgical resection after induction therapy did not enjoy the same survival as those with effective nodal downstaging [2,3]. Specifically, in SWOG 8805 there was a significant difference in median survival between those with no N2 nodal disease at thoracotomy (30 months) and those with persistent N2 disease (10 months, p = 0.005) [2]. Likewise, in CALGB 8935 the median failure free survival after landmark was 47.8 months if downstaged to no disease in the N2 nodes at thoracotomy compared to 8.2 months if persitent disease (p = 0.01) [3]. Such patients rarely benefited from surgical resections that sometimes caused perioperative morbidity, mortality, and temporary loss of independence.
Accordingly, we need a safe, accurate, minimally invasive method to restage the mediastinal nodes following neoadjuvant therapy to allow the separation of patients expected to benefit from surgical resection from those with persistent nodal disease. Pathologic restaging the mediastinal nodes may prevent futile thoracotomy for those not responding to induction therapy. A.repeat mediastinoscopy has been associated with increased risk of complications and reduced accuracy because paratracheal spaces, the subcarinal space and aortopulmonary window, are fused after previous dissection, radiation or cytotoxic therapy. [4,5]
Thoracoscopy is an attractive alternative to repeat mediastinoscopy because of its enhanced visibility and access to the posterior mediastinal, inferior mediastinal, retrohilar and aortopulmonary window nodes. While thoracoscopy can also discover clinically occult pleural carcinomatosis,it limits the surgeon to an ipsilateral assessment of the mediastinum.
Our hypothesis was that thoracoscopy could be used to provide pathologic re-staging of ispislateral nodes following neoadjuvnat therapy in those patients proven to have pathologic involvement of those nodes prior to treatment. Our primary endpoint was to determine the safety and feasibility of accessing multiple nodal stations thoracoscopically, and validating apparent down-staging with thoracotomy. If the technique of thoracoscopic restaging can be validated in this feasibility study, the concept could become an important piece of the future strategy of CALGB activity, as multimodality therapy for stage III NSCLC is refined.
Methods
A prospective, non-randomized phase II trial was designed to evaluate the feasibility of videothoracoscopy (VATS) to assess resectability in patients with stage IIIA (N2) NSCLC following prior mediastinoscopy and neoadjuvant therapy by the Cancer and Leukemia Group B (CALGB), an NCI-sponsored multi-institutional cooperative group. Unresectability was defined as the presence of a persistently positive mediastinal (N2) lymph node or pleural carcinomatosis. Secondary objectives included (1) documentation of safety, and (2) assessment of the accuracy (false-negative rate) of pre-resectional thoracoscopic mediastinal node restaging after prior mediastinoscopy and induction therapy.
Eligibility required histologic documentation of stage IIIA (N2) by mediastinoscopy performed prior to induction therapy. At least 2 cycles of chemotherapy with or without radiotherapy (> 40Gy) or radiotherapy alone (> 40 G) had to be completed within 60 days of registration. Patients with responding or stable local disease were eligible.
Patients were ineligible for this trial if they had a comorbid illness that made them unsuitable for subsequent resection, if they were unwilling to undergo subsequent anatomic resection(i.e. lobecotmy or pneumonectomy), had post-induction spirometry which suggested they could not undergo surgery, or had a currently active second malignancy other than a non-melanoma skin cancer. Previously treated relapsed lung cancer patients were not eligible. Those with local disease progression defined as a 25% increase in local tumor size or the appearance of new areas of malignant disease were ineligible. The patient could not have a history of intrapleural surgery on the ipsilateral side. An Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 was required.
Informed consent and individual institutional review board approval was obtained at each participating institution. Prior to registration, patients had to be evaluated by a CALGB credentialed thoracic surgeon and be determined suitable for surgical resection of their cancer. Surgeons were considered credentialed if they had accrued patients to previous CALGB VATS protocols. New surgeons were credentialed by the Principal Investigator after submitting the operative notes of ten minimally-invasive procedures that reflected a skill level necessary to participate in this protocol.
Before making the thoracotomy incision to resect the lung after neoadjuvant therapy, patients underwent ipsilateral video-thoracoscopy. The patient was positioned in a lateral decubitus position after selective lung ventilation was established. Three to four thoracoscopic ports were recommended, although the placement of ports was left to surgeon discretion. In general, the camera port was placed in the 6th or 7th intercostal space and the pleural space was evaluated for adhesions. Additional operative ports were recommended to be in line with the planned thoracotomy incision, including one anterior to the scapular tip and one at the posterior extent. Fourth ports, if needed, were generally placed in the axillae.
The thoracoscopic restaging was considered positive if there was histologic proof of pleural carcinomatosis, malignant effusion, or any positive mediastinal node. The diseased nodal station identified by pre-treatment mediastinoscopy was a specific target for re-staging, unless pleural carcinomatosis or another positive mediastinal nodal station was identified prior to sampling that particular station. The procedure was considered complete and negative if there were no evidences of unresectability, and at least 3 nodal stations were sampled and no evidence of malignant disease was found.
The protocol guided the surgeon to re-stage specific nodal stations as a function of the location of the primary tumor. Tumors in the right upper lobe were to have stations 2R, 4R, and 7 sampled. Tumors of the right middle and right lower lobes were to have biopsies of stations 4R, 7, and 9R, and samples of station 2R were encouraged but optional. Right hilar tumors were to have samples from stations 2R, 4R, 7 and 9R. Left upper lobe tumors were to have biopsies of stations 5, 6, and 7 and encouraged to have samples of station 4L “if feasible” (wording of the protocol). Left lower lobe tumors were to be re-staged by sampling stations 5, 7, 9L, and biopsies of station 4L were optional. Likewise, left hilar tumors were to have biopsies of stations 5, 6, 7 or 9L with optional sampling of station 4. At the time of thoracotomy, these same nodal stations were to be sampled. In particular, the original positive nodal station identified at pre-treatment cervical mediastinoscopy was a specific target at thoracotomy.
Most patients with a negative restaging procedure underwent thoracotomy and anatomic resection (lobectomy, bilobectomy or pneumonectomy) with mediastinal lymphadenectomy under the same anesthetic. Seven patients had VATS restaging and thoracotomy as two separate procedures. The days apart ranged from 6 to 29 days. Patients with a positive restaging procedure could still undergo resection at the discretion of the surgeon.
Statistical Considerations
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by CALGB Statistical Center staff and by the study chair. Statistical analyses were performed by CALGB statisticians on SAS© 9.1 (SAS Institute Inc., Cary, NC, USA). Feasibility was defined as 1) the ability to sample at least 3 negative mediastinal (N2) lymph node stations, or 2) to identify one positive mediastinal lymph node or pleural carcinomatosis. This feasibility protocol ended at the completion of surgery with either finding of persistent malignancy or successful surgical resection. Long-term survival and recurrerence patterns were not endpoints of this study.
At the outset of the study, it was agreed that further development of the surgical technique is of interest if greater than 80% of patients had a successful procedure; further investigation of the technique would not be warranted if less than 65% had a successful procedure unless there were significant modifications. With planned accrual of 75 patients, there would be 90% power to differentiate a true feasibility rate of 65% versus 80% at a one-sided significance level of 0.05.
The feasibility rate of VATS restaging as well as its 90% confidence interval are provided. The sensitivity and specificity of VATS restaging were calculated with the result of thoracotomy as gold standard. Negative prediction value (NPV) and positive prediction value (PPV) of VATS restaging were also provided. All reported p-values are two sided. Grade 3 and higher complications were tabulated. The potential variables influencing VATS restaging success were evaluated using logistic regression.
Results
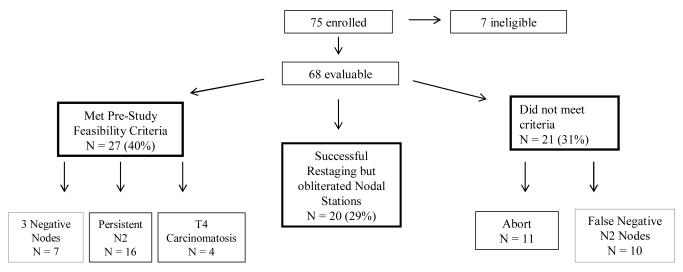
From activation on 9/15/1998 to closure on 9/30/2003, the accrual target was met by 75 participants from 10 institutions. Seven patients were unevaluable. Three subjects had PET scan staging of IIIA(N2) disease only without pre-treatment mediastinoscopy. The other four reasons (one each) were a change in the post-resection pathologic identifaction of hemangioendothelioma instead of lung cancer, a positive mediastinoscopy node that proved to be a contralateral N3 disease instead of an ipsilateral N2 mediastinal node (thus providing no pre-treatment target to restage), PET staging without mediastinoscopy prior to neoadjuvant treatment (thus no pathologically confirmed target for restaging), and bulky hilar disease with direct invasion into the mediastinum that precluded subsequent surgical rescetion. The subsequent analysis was based on 68 patients (Fig 1).
Figure 1.
Flow sheet of patient outcomes
Demographic data are summarized in Table 1. Most participants were Caucasian and 49% were male. The median age of this cohort was 61 years with a range from 40 to 77 years. ECOG performance status (PS) was either 0 (60%) or 1 (40%). All patients had either neoadjuvant chemotherapy (97% received at least 1 cycle of chemotherapy, while 49% received 3 cycles), radiotherapy (68%), or both before enrollment. About half (49%) of the participants were diagnosed with adenocarcinoma. Fifty-three (78%) had just one positive mediastinal nodal station on initial mediastinoscopy, while 14 (21%) had two positive N2 nodal stations and 1 (1%) had 3 positive N2 nodal stations. Thus, 15 of the 68 (22%) evaluable participants had multi-station N2 disease; a population which many would not consider for resection outside of an investigational protocol.
Table 1.
Patients Demographic and Baseline Clinical Characteristics (N=68)
| # (%) | ||
|---|---|---|
|
|
||
| Gender | Male | 33 (49) |
| Female | 35 (51) | |
| Age | Median (min,max) | 61 (40, 77) |
| Race | White | 66 (97) |
| Black | 1 ( 1) | |
| Asian | 1 ( 1) | |
| Performance | 0 | 41 (60) |
| Status | 1 | 27 (40) |
| Prior Treatments | ||
| Thoracic Radiotherapy | 46 (68) | |
| Induction Chemotherapy | 66 (97) | |
| Initial Diagnosis | Adeno | 33 (49) |
| Squamous | 16 (24) | |
| Undiff large | 2 ( 3) | |
| Undiff non-small | 17 (25) | |
| # positive nodes | ||
| on | 1 | 53(78%) |
| mediastinoscopy | 2 | 14 (21%) |
| 3 and 4 | 1 (1%) | |
Thoracoscopic restaging was performed on the right side in 75% of participants. The median number of thoracoscopic incisions was 3 (range from 1 to 4). The median time of the VATS restaging procedure was 63 minutes ranging from 15 to 313 minutesThoracotomy was performed on 61 patients. None of the four patients found to have pleural caricinomatosis and 3 of those found to have persistent nodal disease did not have a subsequent thoracotomy.
Postoperative morbidity data was available for 66 patients and is summarized in Table 2. There was one operative death (only Grade 5 toxicity) in this study due to Acute Respiratory Distress Syndrome (ARDS) following subsequent thoracotomy for lung resection. A total of nine individual Grade 3 toxicities were noted in 7 patients following a combination of thoracoscopic restaging followed by thoracotomy and attempted anatomic resection. There was one patient who suffered a biopsy forceps injury to the left main bronchus (Grade 3 toxicity) during restaging of the station 7 subcarinal nodes. The restaging procedure was abandoned in this patient and a thoracotomy was performed to repair the recognized injury. A Grade 4 cardiovascular arrhythmia and a Grade 4 thrombosis occurred in the same patient who died of ARDS (Grade 5).
Table 2.
Thoracic Surgical Complications (N=66) Only grade 3 and above surgery related complications shown
| Grade of Toxicity |
|||
|---|---|---|---|
| 3-Severe | 4- Life Threatening |
5-Lethal | |
| n (%) | n (%) | n (%) | |
| Cardiovascular (Arrhythmia) | |||
| Arrhythmia- Other | 2 (3%) | 1 (2%) | 0 |
| Supraventricular Arrhythmias | 3 (5%) | 0 | 0 |
| Cardiovascular (General) | |||
| Thrombosis/Embolism | 0 | 1 (2%) | 0 |
| Operative Injury of Bronchus | 1 (2%) | 0 | 0 |
| Dermatology/Skin | |||
| Wound-Infectious | 1 (2%) | 0 | 0 |
| Pulmonary | |||
| Adult Respiratory Distress Syn | 0 | 0 | 1 (2%) |
| Pneumothorax | 1 (2%) | 0 | 0 |
| Pulmonary-Other | 1 (2%) | 0 | 0 |
|
| |||
| Total Number of Patients with Toxicity: | 7 (11%) | 1 (2%) | 1 (2%) |
The median number of days with a chest tube was 4 (range 1 to 72). Prolonged chest tube durations were attributed to air leaks from the anatomic resections in 88% of evaluable IIIA patients (all of whom had undergone neoadjuvant therapy) rather than from the restaging procedure.
Feasibility Calculation
VATS restaging met pre-study criteria of feasibility in 27 of 68 eligible cases (40%). This group included 7 (10%) restaging procedures that successfully biopsied three negative mediastinal nodal stations confirmed by absent residual nodal disease at thoracotomy (including the originally diseased nodal stations discovered at mediastinoscopy), 16 (24%) participants who had one or more persistently positive mediastinal node sampled prior to thoracotomy and 4 patients (6%) with clinically occult pleural carcinomatosis (T4 disease).
Of the 68 subjects, 21 (31%) did not have a successful VATS restaging procedure for the following reasons: A) aborting the procedure due to pleural space adhesions or tumor bulk (n = 9), injury to the airway (n = 1) or inability to achieve adequate atelectasis (n = 1), B) 3 lymph node stations biopsied thoracoscopically without evidence of tumor, but residual nodal disease was found at thoracotomy (n = 4), or C) less than 3 lymph node stations biopsied thoracoscopically with obliterated nodal stations and no nodal disease found by VATS restaging, but N2 disease found at thoracotomy (n = 6). These were considered VATS restaging failures. We could detect no statistical relationship between the primary tumor locations (upper vs. lower lobe) or surgeon’s restaging experience and the likelihood of having a failed or inadequately sampled outcome.
Twenty-six patients (38%) failed the pre-study success endpoint because fewer than three nodal stations were biopsied during restaging. Despite dissection of these mediastinal N2 stations by experienced sugeons during thoracotomy and lung resection, no nodal tissue was identified in at least one N2 station for each of these patients. The neoadjuvant chemotherapy and/or radiation therapy appeared to have obliterated all nodal tissue within the anatomic station, and only scar tissue remained. Of these 26 cases, 6 have already been mentioned in the paragraph above as being a false negative sampling of persistent nodal disease found at thoracotomy, and are included in the VATS restaging failure group.
The remaining 20 subjects (29% of evaluable group) had a positive mediastinal node at mediastinoscopy, received neoadjuvant therapy, then had at least one nodal station with no remaining nodal tissue, but no nodal disease was found at thoracotomy with nodal dissection. Of these 20 patients, a VATS biopsy of the originally positive nodal stations was obtained in 14 (70%) patients, but a third nodal station had no nodal tissue. None of these 20 subjects had nodal disease found at the original site at thoracotomy, confirming downstaging of disease. Because neoadjuvant therapy obliterated at least one required nodal station, the pre-study definition of success turned out to be flawed because it was impossible to attain in some cases. Accordingly, it is reasonable to reclassify these 20 cases as part of a separate analysis.
Using the data above, if the subgroup of participants with inadequate sampling is considered a VATS failure, then the feasibility rate is 27/68 or 40% (95% CI: 28-52%) and the false negative rate (VATS did not detect the persistent nodal disease discovered at thoracotomy) is 38.5% (95% CI: 20-59.4%). If the subgroup of patients with less than 3 nodal stations sampled but in which there was no persistent nodal disease at thoracotomy are considered VATS successes, then the feasibility rate is (20+27)/68 or 69 % (95% CI:57-80%) with a false negative rate of 33% (95% CI: 17-53%). Since the estimated feasibility rate of 69% is greater than the pre-specified lower limit of continued interest (i.e. 65%), the feasibility of VATS restaging remains a question of interest for future investigation. However, as the lower limit of continued interest lies within the 95% confidence interval of 57% to 80%, our data failed to unequivocally establish the feasibility of the VATS restaging.
Effects of Radiation and Chemotherapy
Logistic regressions were conducted to examine the effect of radiotherapy (received versus not received) on VATS efficacy (success versus failure, and success versus empty nodal stations with negative thoracotomy.) The analysis indicated that VATS failure by pre-study definitions was associated with radiation (Tables 3A, 3B, 4A, 4B), although this was not dose related. There was also no statistically significant difference in efficacy between the groups of participants receiving 0-2 cycles of chemotherapy as compared to those participants receiving greater than two cycles. In addition to the radiation effect, patients with adenocarcinoma had significantly lower chance of VATS success than patients with other histologies.
Table 3A.
Number of patients received radiotherapy (N=64)*
| Radiation Doses (cGy) |
||||
|---|---|---|---|---|
| VATS outcome | none | <5000 | 5000 - < 6000 |
>= 60000 |
| success | 11 | 5 | 5 | 2 |
| failure | 5 | 6 | 6 | 4 |
| inadequate sampling |
5 | 9 | 3 | 3 |
4 Patients with pleural carcinomatosis not included
Table 3B.
Number of patients received chemotherapy (N=64)*
| Chemotherapy cycles |
||||
|---|---|---|---|---|
| VATS outcome | none | 1 - 2 | 3 - 5 | 6 - 8 |
| success | 1 | 8 | 10 | 4 |
| failure | 0 | 8 | 7 | 6 |
| inadequate sampling | 0 | 16 | 4 | 0 |
4 Patients with pleural carcinomatosis not included
Table 4A.
Statistics of logistic regression of radiotherapy on VATS successfulness
| success vs. failure | success vs. inadequate sampling |
|||
|---|---|---|---|---|
|
|
||||
| p- value |
OR (95% CI) | p- value |
OR (95% CI) | |
| radiotherapy (none vs. yes) | 0.0525 | 4.9 (1.0, 24.6) | 0.0404 | 5.4 (1.1, 26.6) |
| age | 0.8226 | 1.0 (0.9, 1.1) | 0.4116 | 0.9 (0.8, 1.0) |
| gender (male vs. female) | 0.1731 | 0.4 (0.1, 1.5) | 0.2178 | 0.4 (0.1, 1.7) |
| histology (adenocarcinomar vs. others) |
0.0267 | 5.6 (1.2, 25.6) | 0.0349 | 4.9 (1.1, 21.1) |
Table 4B.
Statistics of logistic regression of chemotherapy on VATS successfulness
| success vs. failure | success vs. inadequate sampling |
|||
|---|---|---|---|---|
|
|
||||
| p- value |
OR (95% CI) | p- value |
OR (95% CI) | |
| chemo therapy (<=2 vs. >2 cycles) |
0.7544 | 0.8 (0.2, 3.4) | 0.0059 | 0.1 (0, 0.5) |
| age | 0.7428 | 1.0 (0.9, 1.1) | 0.6509 | 1.0 (0.9, 1.1) |
| gender (male vs. female) | 0.1068 | 0.3 (0.1, 1.3) | 0.3872 | 0.5 (0.1, 2.3) |
| histology (adenocarcinomar vs. others) |
0.0598 | 3.8 (0.9, 15.6) | 0.0273 | 7.2 (1.2, 41.4) |
“Success” is equal to meeting protocol endpoints (i.e. disease found in pleura, disease in a mediastinal node, or 3 nodal stations sampled without disease). “Failure” is equal to not meeting protocol endpoints (i.e. biopsies were not obtained from 3 negative nodal stations or procedure aborted). “Inadequate sampling” means inability to sample 3 nodal stations secondary to the obliteration of nodal tissue.
Anatomic Correlation with Restaging Failure
An analysis of the location of persistently positive mediastinal nodes found at thoracotomy when the VATS restaging procedure failed to detect positive nodes is presented in Table 5. Nearly half of all the false negative nodes were in the station 4R position.
Table 5.
Nodal stations when VATS negative and thoracotomy positive
| side | station | N |
|---|---|---|
| right | 2 Upper paratracheal |
2 |
| 4 Lower paratracheal |
10 | |
| 7 Subcarinal | 3 | |
|
| ||
| left | 5 Aortopulmonary | 4 |
| 6 Anterior mediastinal |
1 | |
| 9 Pulmonary ligament |
1 | |
Discussion
This study demonstrated that VATS may be a useful tool to detect or exclude persistent nodal disease, and VATS restaging may have a superior negative predictive value in patients who do not receive radiation therapy as part of their induction therapy. It proved particularly useful in discovering occult pleural carcinomatosis (6% in this prospective trial) following neoadjuvant therapy for IIIA(N2) disease. Restaging thoracoscopy did have trouble reliably restaging the station 4R nodes.
Neoadjuvant therapy prior to surgical resection of locally advanced stage IIIA (N2) NSCLC improves survival for those patients who have had sterilization of their mediastinal nodes [2-3]. More specifically, patients with persistent N2 disease at surgical resection have similar long-term survival to those treated non-operatively [6]. Mature analyses of both SWOG 8805 [2] and CALGB 8935 trials [3] showed that nodal downstaging is the most important predictor of long-term survival.
The mediastinal nodal downstaging rate of 21-50% seen in the two American Cooperative Group trials, and the clinical significance of persistent disease, emphasize the need to accurately restage the mediastinum after neoadjuvant therapy. Imaging alone has been unable to provide sufficient accuracy. Although PET scans have shown value in predicting persistent viable tumor in the primary tumor bed, they are unreliable in assessing the mediastinal nodes. The specificity for persistent mediastinal nodal disease in restaging PET scans is generally 85 – 90%, but the sensitivity is disappointingly low at 50 – 60% [7-12]. This may be due to the inability to detect a small focus of persistent disease within fibrosis, or altered perfusion to the nodes that affect PET tracer.
The inability to accurately predict persistent nodal disease by non-invasive imaging has led to the use of surgical restaging of the mediastinum. Although some authors have reported an accuracy of repeat mediastinoscopy approaching that of an initial mediastinoscopy [13, 14], other large series have reported lower sensitivities and accuracies. A series of 104 repeat mediastinoscopies from 2 European academic centers in Spain and Belgium reported a sensitivity of 71%, specificity of 100%, and an accuracy of 84%. That series also reported a 2% rate of massive hemorrhage (one aortic arch and one superior vena cava) and a 1% mortality [5]. A second combined German series of 104 repeat mediastinoscopies reported a sensitivity of 61%, and accuracy of 88% [15]. A prospective trial comparing the accuracy of repeat mediastinoscopy to PET-CT fusion scans in 30 patients reported that the repeat surgical procedure was technically feasible but inaccurate due to severe adhesions and fibrosis [7]. The sensitivity to detect residual mediastinal disease in this study was only 29% with an accuracy of 60%. Fibrosis not only affects the accuracy of a repeat procedure, but negatively affects the completeness of the mediastinal evaluation [4].
It was unanticipated at the time of this trial’s design that high dose neoadjuvant chemotherapy and radiotherapy would become widespread. The recognition that complete obliteration of all nodal tissue within a station of the lymph node map can occur after aggressive neoadjuvant therapy is one of the contributions of this study. Twenty patients (nearly a third of the evaluable group) had histologically proven N2 disease at initial mediastinoscopy, with subsequent obliteration of all nodal tissue within that station at both VATS restaging and thoracotomy. As a result, we felt compelled to perform a dual statistical analysis for the 26 (39%) participants who fell into this category. If we accept the original protocol criteria that this subgroup is defined as a VATS failure for inadequate node sampling, then the feasibility rate is only 39.7%. If we are willing to consider that this subgroup is a VATS success because no nodal disease was found at thoracotomy, then the feasibility rate is 69.1%, which is marginally feasible. Subset analysis revealed that radiotherapy was linked to lack of nodal tissue found within the fibrosis of these stations, although not dose related. Further subset analysis also reveals a disproportionate rate of VATS failure in the station 4R position. There may be a several causes for this outcome, including the bulk of tumor in the right upper lobe preventing exposure of the 4R position, the relationship of the azygous-caval junction and the pulmonary artery to the nodal bed, the scar from the previous mediastinoscopy, and the radiation field effect on that location.
The strengths of this prospective multi-institutional surgical study are considerable. All participants underwent pre-treatment cervical mediastinoscopy with histologically confirmed N2 (not N3) disease by a CALGB thoracic surgeon. Neoadjuvant treatments were then set by each individual institution with minimum criteria set by the protocol. All participating surgeons underwent a quality assurance credentialing process.
This trial suffers from weaknesses as well. The original trial design was created prior to our recognition that high dose neoadjuvant therapy can completely obliterate all nodal tissue at certain stations. This placed us in a position of doing a post-hoc analysis of the procedure in light of the impossibility of sampling a node that no longer exists. As a result, the true feasibility likely lies somewhere between the two extremes of data analysis that we presented: analysis by original trial design (missing nodal tissue is a failure) and analysis by assumption that missing nodal tissue be considered equivalent to a sterilized node biopsy. The possibility of bias exists. If a surgeon failed to find nodal tissue during the thoracoscopic dissection of an N2 nodal station, it might have influenced the degree of surgical resection of the same nodal station at thoracotomy and lung resection. Considering the level of thoracic surgical expertise at the 10 participating centers, these seems unlikely. It is important to remember that 68 participants limits power on sub-analysis. Also, this particular study was dominated by right-sided procedures (76%). That limits observations of the technique on the left, but did allow us to further characterize the problem with sampling the station 4R position. Finally, the accrual of patients ended in 2003, and staging and restaging technology has improved since that time. However, we believe this prospective trial still provides valuable data to thoracic surgeons who seek to individualize treatment for patients with stage IIIA(N2) non-small cell lung cancer.
Technology has continued to advance since the time of this prospective study. Many centers have now gained experience with minimally invasive endoscopic techniques such as TransBronchial Needle Aspiration (TBNA), Endo-esophageal UltraSound Fine-Needle Aspiration (EUS-FNA), and EndoBronchial UltraSound Fine-Needle Aspiration (EBUS-FNA). In one trial, the overall sensitivity and accuracy of 194 TBNA samples without neoadjuvant therapy was 71% and 73% respectively [16]. Interestingly, the accuracy was highest in the station 4R position, and least in the left paratracheal position. Although there are no large prospective studies using endobronchial ultrasound specifically for restaging the mediastinum following neoadjuvant therapy for stage IIIA(N2) NSCLC, there are data for endo-esophageal ultrasound. EUS-FNA has been used to restage 19 patients treated with 3 cycles of platinum-based neoadjuvant chemotherapy without radiation [17]. The positive predictive value, negative predictive value, sensitivity, specificity and diagnostic accuracy were 100%, 67%, 75%, 100%, and 83%, respectively. Another 128 patient EBUS restaging series, however, showed a negative predictive value of only 20% [18]. Another large series using EUS to stage untreated mediastinal nodal disease reported a sensitivity of only 72.6% [19] and a meta-analysis of this same disease group reports a pooled sensitivity of 83%, with lower sensitivities of 39 – 75% for normal sized nodes [20]. Needle samples from fibrotic nodes following high dose chemoradiation would likely have even lower sensitivities. Alternatively, a potential strategy can involve using a non invasive technique such as EBUS/EUS at the time of diagnosis, and then saving the mediastinoscopy for the restaging after induction therapy. This may also allow the surgeon to assess the higher and contralateral nodes in a “virgin” mediastinum.
We conclude that thoracoscopic restaging of the mediastinum following neoadjuvant chemotherapy or radiotherapy is feasible in a prospective multi-institutional setting, although feasibility decreases with radiation or attempts to sample the 4R nodal station. This suggests that thoracoscopy could likely be combined with other new surgical staging techniques. EBUS and EUS could likely be used as an initial re-staging modality guided by results of a repeat PET-CT scan. Patients found to have histologic confirmation of mediastinal nodes by these endoscopic procedures may go on to additional non-surgical therapy. Those patients with negative mediastinal node biopsies would then have further histologic confirmation of mediastinal nodal sterilization and lack of pleural carcinomatosis by ipsilateral thoracoscopic restaging. Again, positive nodal disease might be treated non-surgically. Thoracotomy and surgical resection might be offered for those patients with negative needle restaging followed by negative thoracoscopic restaging. Although intuitive, this restaging plan would need confirmation in an additional prospective randomized trial.
ACKNOWLEDGEMENTS
The research for CALGB 39803 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, M.D., Chair) and to the CALGB Statistical Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Cedars-Sinai Medical Center, Los Angeles, CA-Alan T. Lefor
Dana-Farber Cancer Institute, Boston, MA-Harold J Burstein, M.D., Ph.D., supported by CA32291
Duke University Medical Center, Durham, NC-Jeffrey Crawford, M.D., supported by CA47577
Massachusetts General Hospital, Boston, MA-Jeffrey W. Clark, M.D., supported by CA32291
Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC-James N. Atkins, M.D., supported by CA45808
State University of New York Upstate Medical University, Syracuse, NY-Stephen L. Graziano, M.D., supported by CA21060
University of Iowa, Iowa City, IA-Daniel A. Vaena, M.D., supported by CA47642
University of Maryland Greenebaum Cancer Center, Baltimore, MD-Martin Edelman, M.D., supported by CA31983
University of Minnesota, Minneapolis, MN-Bruce A Peterson, M.D., supported by CA16450
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO-Michael C Perry, M.D., supported by CA12046
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Sugarbaker DJ, Herndon J, Kohman LJ, KrasnaMJ GreenMR, and the, Cancer and Leukemia Group B Thoracic Surgery Group Results of cancer and leukemia group B protocol 8935. A multiinstitutional phase II trimodality trial for stage IIIA (N2) non-small cell lung cancer. J Thorac Cardiovasc Surg. 1995;109:473–83. doi: 10.1016/s0022-5223(95)70278-4. [DOI] [PubMed] [Google Scholar]
- 2.Albain KS, Rusch VW, Crowley JJ, Rice TW, TUrrisi AT, 3rd, Weick JK, Lonchyna VA, Presant CA, McKenna RJ, Gandara DR, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–92. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 3.Jaklitsch MT, Herndon JE, III, DeCamp MM, Jr, Richards WG, Kumar P, Krasna MJ, Green MR, Sugarbaker DJ. Nodal Downstaging predicts survival following induction chemotherapy for stage IIIA (N2) non-small cell lung cancer in CALGB protocol #8935. J Surg Oncol. 2006;94:599–606. doi: 10.1002/jso.20644. [DOI] [PubMed] [Google Scholar]
- 4.Meersschaut D, Vernassen F, Brutel de la Riviere A, Knaepen PJ, Van den Bosch JM, Vandershueren Repeat mediastinoscopy in the assessment of new and recurrent lung neoplasm. Ann Thorac Surg. 1992;53:120–22. doi: 10.1016/0003-4975(92)90769-z. [DOI] [PubMed] [Google Scholar]
- 5.De Waele M, Serra-Mitjans M, Hendriks J, Lauwers P, Belda-Sanchis J, Van Schil P, Rami-Porta R. Accuracy and survival of repeat mediastinoscopy after induction therapy for non-small cell lung cancer in a combined series of 104 patients. Eur J Cardiothorac Surg. 2008;33:824–8. doi: 10.1016/j.ejcts.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Bueno R, Richards WG, Swanson SJ, Jaklitsch MT, Lukanich JM, Mentzer SJ, Sugarbaker DJ. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg. 2000;70:1826–31. doi: 10.1016/s0003-4975(00)01585-x. [DOI] [PubMed] [Google Scholar]
- 7.De Leyn P, Stoobants S, Dewever W, Lerut T, Coosemans W, Decker G, Nafteux P, Van Raemdonck D, Martelmans L, Nackaerts K, Vansteenkiste J. Prospective comparative study of integrated PET-CT with remediastinoscopy in the assessment of residual mediastinal disease after induction chemotherapy for mediastinoscopy proven stage IIIA-N2 non-small cell lung cancer. J Clin Oncol. 2006;24:3333–9. doi: 10.1200/JCO.2006.05.6341. [DOI] [PubMed] [Google Scholar]
- 8.Akhurst T, Downey RJ, Ginsberg MS, Gonen M, Bains M, Korst R, Ginsberg RJ, Rush VW, Larson SM. An initial experience with FDG-PET in the imagines of residual disease after induction therapy for lung cancer. Ann Thorac Surg. 2002;73:259–66. doi: 10.1016/s0003-4975(01)03257-x. [DOI] [PubMed] [Google Scholar]
- 9.Ryu JS, Choi NC, Fischman AJ, Lynch TJ, Mathisen DJ. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002;35:179–87. doi: 10.1016/s0169-5002(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 10.Cerfolio RJ, Ojha B, Mukherjee S, Pask AH, Bass CS, Katholi CR. Positron emission tomography scanning with 2-fluoro-2-deoxy-d-glucose as a predictor of response of neoadjuvant treatment for non-small cell carcinoma. J Thorac Cardiovasc Surg. 2003;125:938–44. doi: 10.1067/mtc.2003.381. [DOI] [PubMed] [Google Scholar]
- 11.Port JL, Kent MS, Korst RJ, Keresztes R, Levin MA, Altorki NK. Positron emission tomography poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg. 2004;77:254–9. doi: 10.1016/s0003-4975(03)01457-7. [DOI] [PubMed] [Google Scholar]
- 12.Hellwig D, Graeter TP, Ukena D, Georg T, Kirsch CM, Schafers HJ. Value of F-18-fluorodesoxyglucose positron emission tomography after induction therapy of locally advanced bronchogenic carcinoma. J Thorac Cardiovasc Surg. 2004;128:802–9. doi: 10.1016/j.jtcvs.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Pauwels M, Van Schil P, De Backer W, Van den Brande F, Eyskens E. Repeat mediastinoscopy in the staging of lung cancer. Eur J Cardiothorac Surg. 1998;14:271–3. doi: 10.1016/s1010-7940(98)00196-1. [DOI] [PubMed] [Google Scholar]
- 14.Granone P, Van Schil P. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: A closer look at redo mediastinoscopy. Letter to Editor. J Thor Cardiovasc Surg. 2007;133(1):275–6. doi: 10.1016/j.jtcvs.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 15.Marra A, Hillejan L, Fechner S, Stamatis G. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg. 2008;135:843–9. doi: 10.1016/j.jtcvs.2007.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Patelli M, Lazzari A, Poletti V, et al. Role of fiberscopic transbronchial needle aspiration in the staging of N2 disease due to non-small cell lung cancer. Ann Thorac Surg. 2002;73:407–11. doi: 10.1016/s0003-4975(01)03447-6. [DOI] [PubMed] [Google Scholar]
- 17.Annema J, Veselic M, Versteegh MIM, Willems LNA, Rabe KF. Mediastinal restaging: EUS-FNA offers a new perspective. Lung Cancer. 2003;42:311–8. doi: 10.1016/s0169-5002(03)00364-7. [DOI] [PubMed] [Google Scholar]
- 18.Herth FJF, Annema JT, Eberhardt R, Yasufuku K, Ernst A, Krasnik M, Rintoul RC. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol. 2008;26(20):3346–3350. doi: 10.1200/JCO.2007.14.9229. [DOI] [PubMed] [Google Scholar]
- 19.Witte B, Neumeister W, Huertgen M. Does endoesophageal ultrasound-guided fine-needle aspiration replace mediastinoscopy in mediastinal staging of thoracic malignancies? Eur J Cardiothorac Surg. 2008;33:1124–8. doi: 10.1016/j.ejcts.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Micames CG, McCrory DC, Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound-guided fine needle aspiration for non-small cell lung cancer staging. A systematic review and metaanalysis. Chest. 2007;131:539–48. doi: 10.1378/chest.06-1437. [DOI] [PubMed] [Google Scholar]