Abstract
The microenvironment of cancer cells has proven to be a critical component of tumors that strongly influences cancer development and progression into invasive and metastatic disease. Compared to normal tissue, dramatic differences in gene expression occur in multiple cell types that constitute the tumor microenvironment including cancer-associated fibroblasts (CAFs) that are important stromal components of growing tumors. In this review, we present recent advances in understanding how microRNAs are deregulated in cancer-associated fibroblasts (CAFs) and how this affects tumor biology. The microRNA signature of CAFs is discussed with respect to their functional relevance to tumor cells as well as other cell types involved in tumor homeostasis.
Keywords: Cancer-associated fibroblasts, MicroRNA, Microenvironment, Tumor, CITIM 2011
Introduction
Cells that compose the tumor microenvironment are increasingly recognized as critical components of tumor progression. Co-evolution and “reprogramming” of the stromal compartment are prerequisite for cancer to progress to advanced stages [1–3]. Cross talk and interactions between neoplastic cells and their neighboring cells in the microenvironment provide critical factors that can determine whether tumor cells remain dormant or progress into invasive and metastatic cancer [4–7].
Although the cells within the microenvironment do not undergo malignant transformation, significant changes occur in their pattern of gene expression and consequently in their function, which distinguish these cells from their normal counterparts [8–10]. The tumor microenvironment consists of various non-transformed cells including fibroblasts, myofibroblasts, inflammatory immune cells, endothelial cells and bone marrow-derived mesenchymal progenitor cells. These cells are recruited to transformed tumor cells where they undergo “reprogramming” as a result of complex cross talk with the tumor cells and other components of the microenvironment. This results in critical changes in the production of extracellular matrix proteins, cytokines, growth factors and proteases that promote the development and progression of cancer [1, 11]. This review will focus on changes that occur in microRNA expression in stromal fibroblasts that become reprogrammed to become cancer-associated fibroblasts (CAFs) as tumors develop and how these changes appear critical for the maintenance and progression of cancer. Understanding what changes occur and how they are regulated may provide new avenues for intervention to prevent or treat many cancers.
Cancer-associated fibroblasts
Cancer-associated fibroblasts comprise one of the most abundant cell types in the stroma of solid tumors [12, 13]. Several studies have demonstrated that normal fibroblasts restrict oncogenic growth of adjacent epithelial neoplastic cells [14–16]. However, at advanced stages of tumor growth, the stromal fibroblast population can induce angiogenesis, secrete a plethora of growth factors and enzymes involved in remodeling of the extracellular matrix and suppress host immune responses that normally would inhibit tumor development [8, 17–19]. The degree of activated CAF infiltration in a tumor correlates with higher grades of malignancy and poorer prognostic outcome [20, 21]. It has been shown that CAFs are a heterogeneous population of fibroblastic cells including myofibroblasts expressing alpha-smooth muscle actin (SMA) and other fibroblastic cells that do not express alpha-SMA but nevertheless promote tumor growth [22–24].
Origin of CAFs
A key question in cancer biology is how normal fibroblasts transition into tumor-promoting, but non-transformed CAFs. Studies using mouse xenograft models have demonstrated that 20–40 percent of CAFs originate from the bone marrow [25, 26]. These progenitor cells are recruited to the sites of neoplasia where they transdifferentiate into myofibroblasts and secrete chemokines, including CCL5, which promote tumor cell growth and metastasis [27]. Experimental data also suggest that the growth factor TGF-β secreted by tumor cells may “educate” resident fibroblasts to become CAFs, further triggering an autocrine loop of TGF-β synthesis by CAFs themselves [28–30]. Another recent study using a mouse model of squamous cell carcinoma demonstrated that resident normal fibroblasts are stimulated by immune cells or tumor cells that produce IL-1β to activate an inflammatory signature mediated by the NF-κB pathway [31]. This event initiates a conversion of normal fibroblasts into CAFs and promotes tumor progression by orchestrating pro-angiogenic programs during carcinogenesis.
Interestingly, although CAFs play an important role in supporting tumor growth, they do not display features of transformed cells, i.e., they do not produce tumors when injected into immunocompromised mice. When grown in culture, they senesce after 10–15 population doublings and they exhibit a normal karyotype [8, 18]. Mutations in p53 and PTEN genes have been reported in CAFs [32–35], but it is likely that these mutations accumulate as these cells adapt survival mechanisms. This is supported by the fact that most solid tumors create a hypoxic environment. p53 has been shown to play an important role in mediating apoptosis in hypoxic regions. Therefore, tumor hypoxia may act as a selective pressure for the inactivation of p53 in order for these cells to survive and flourish in this hostile, hypoxic environment [36]. In addition, more recent studies of CAFs using array CGH and SNP analysis have shown that genetic alterations are extremely rare [17, 37, 38]. This raises the possibility that the significant changes in the gene expression in CAFs may result from epigenetic modifications. Indeed, altered methylation patterns have been found not only in tumor cells but also in stromal fibroblasts [39–41].
Another important mechanism that may be involved in the activation of fibroblasts and transdifferentiation of progenitor cells to CAFs is post-transcriptional gene regulation by microRNAs. MicroRNAs are small, non-coding RNAs that can regulate hundreds of genes and have proven to play key roles in a variety of processes including cellular differentiation, development, cell motility and senescence, as well as pathological conditions such as oncogenic transformation and inflammation [42].
MicroRNA properties
MicroRNAs are small 19–25 nucleotides RNA that play a regulatory role in diverse biological processes [43, 44]. They regulate gene expression at the post-transcriptional level by hybridizing to the complementary sites in the 3′UTR of their target genes that result in translational inhibition or mRNA degradation. Most microRNAs are transcribed by RNA polymerase II as long primary RNAs that contain a 5′CAP and 3′ polyA tail. About 50 percent of all microRNAs are located within the introns of protein-coding genes and are released by splicing of the introns after parental mRNA processing. Primary miRNAs, which may be several kilobases long, are further truncated within the nucleus by RNAse III Drosha and its partner DGCR8. Pre-miRNA is then transported to the cytoplasm by Exportin 5 where it is further cleaved by another RNAse III enzyme, Dicer, into a 22-bp double-stranded RNA. Subsequently, a guiding strand of the miRNA is loaded into the RNA Induced Silencing Complex (RISC) that binds to the target protein-coding mRNAs and represses its activity by either mRNA destabilization in the case of a near-perfect complementarity between the miRNA and mRNA or translational inhibition in the case of an imperfect match between the miRNA and the target mRNA. Recent data clearly indicate that microRNAs may function as oncogenes or tumor suppressors in a variety of cancers (for recent review see [42]).
Recently, exciting studies have demonstrated that exosomes produced by the shedding of the plasma membrane from tumor and other cells contain functional microRNAs that were internalized by neighboring cells and delivered to the sites of microRNA-mediated mRNA targeting [45]. Exosome-mediated delivery of microRNAs provides a novel mechanism for cell–cell communication that had previously been considered to be mediated only through the secretion of soluble factors like hormones, chemokines and cytokines that are released by one cell type and the signal through interaction with receptors on the neighboring cells. In addition, microRNAs may also be transferred to other cells through circulating blood cells including monocytes and T cells [46]. If microRNAs can be transferred from CAFs to the other cells within the tumor stroma, this could have profound effects on the biology of the multiple immune cell types as well as endothelial cells. Therefore, we will consider the potential role of miRNAs expressed by CAFs in regulating processes not only in the CAFs but also in other cells within the tumor microenvironment.
MicroRNA signature of CAFs
We investigated the role of microRNAs in CAFs isolated from endometrial cancer or normal tissue and identified 11 microRNAs that were differentially regulated in CAFs (Fig. 1) [47]. miR-31 was the most downregulated microRNA in CAFs. Further analysis revealed that re-expression of miR-31 in CAFs impairs their ability to stimulate endometrial tumor cell migration and invasion, implying that miR-31 targets gene(s) are responsible for the secretion of soluble factor(s) implicated in tumor cell dissemination. Matched mRNA gene expression profiling of the same CAF samples revealed a reciprocal activation of the homeobox gene SATB2, a chromatin-remodeling factor capable of activating or repressing gene expression that is dependent upon the cellular context [48]. Indeed, when we overexpressed SATB2 in fibroblasts derived from normal endometrium, we identified a number of genes that were upregulated and responsible for promoting tumor expansion, metastasis and angiogenesis (Fig. 2).
Fig. 1.
MicroRNAs differentially expressed in endometrial tumor CAFs versus fibroblasts from normal endometrium. The green triangle contains microRNAs expressed in normal fibroblasts that are downregulated in CAFs. The red triangle contains microRNAs upregulated in CAFs
Fig. 2.
miR-31 targets the homeobox gene SATB2 that triggers expression of multiple protumorigenic and angiogenic factors
Interestingly, two of the microRNAs suppressed in CAFs, miR-31 and miR-148a, were found to act as metastasis suppressors in various tumor types. Screening of a large panel of breast cancer cell lines revealed an inverse correlation between miR-31 expression and metastatic potential of the cells [49]. This in vitro observation was then correlated to patient tumor samples where the abundance of miR-31 in primary breast tumors was inversely associated with the propensity of tumors to metastasize. Moreover, using mouse xenograft models, it was shown that overexpression of miR-31 impedes multiple steps of the metastatic cascade including invasion, survival in the blood stream and colonization at distant organs [49].
The cross talk between CAFs and immune cells in the tumor microenvironment has been well established. It is possible that the exchange of microRNAs between these cellular components may be another important means of cell–cell communication. It is important to keep in mind that targeting the expression of a given microRNA in a tumor may affect multiple cell types. Therefore, it is critical to understand how a particular microRNA may affect multiple cellular components within the microenvironment. For instance, recent data about the role of miR-31 in regulatory T cells (Tregs) provide additional insight into a possible function of miR-31 in Treg-mediated suppression. Tregs may be partially responsible for the absence of an adequate immune response against tumor cells. miR-31 inhibits expression of the forkhead box P3 (FoxP3) transcription factor that is necessary for active Treg function [50]. Additional experiments are needed to determine whether forced miR-31 expression will reduce Treg suppressive properties and hence augment tumor-specific immunity. Thus, miR-31 may not only be a direct tumor suppressor of tumor cells, but may also inhibit tumorigenesis by improving an immune response against tumor development.
Another microRNA that is downregulated in CAFs is miR-148a. Importantly, miR148 is uniformly silenced in all tumor types that have been studied. miR-148a is an intergenic microRNA transcribed from its own promoter, and its tumor suppressor role is further supported by the discovery that its promoter is silenced by methylation in a variety of tumors [51, 52]. Moreover, downregulation of miR-148a was reported as a metastatic marker [53, 54], and its expression negatively correlated with lymph node-positive disease [52].
There is ample evidence that miR-148a is acting as a tumor suppressor in various tumor types, however, not much is known about the role of miR-148a in other tumor stromal cells. The available data indicate that miR-148 function is also important for dendritic cells maturation. Dendritic cells, which contribute another important component of the microenvironment, play an important role in tissue remodeling. Some subpopulations of dendritic cells accumulate in solid tumors and induce immune tolerance and suppress antitumor immune responses. Through direct targeting of calcium-/calmodulin-dependent protein kinase II (CAMKII), the enzyme required for maturation and function of dendritic cells, miR-148 was shown to inhibit the function of these cells and the secretion of inflammatory cytokines, including IL-6, IL-12 and TNF-α by these cells [55].
The RIP-Tag2 mouse model of pancreatic cancer has proven to be very useful for characterizing the process of multistep tumor development. Tumors initially arise from multiple hyperplastic/dysplastic islets which may undergo an “angiogenic switch” and progress into encapsulated solid tumors, some of which form highly invasive carcinomas and acquire metastatic properties [56]. Each stage of tumor progression has been characterized by the induction or downregulation of a subset of specific microRNAs [54]. Two of the microRNAs, miR-146a and miR-424, that we found upregulated in CAFs were also induced during the angiogenic switch early in tumor development. Interestingly, treatment of mice bearing RT2 tumors with sunitinib, a potent inhibitor of angiogenesis, for 7 days reversed the expression of miR-424 to normal levels. Also, the same study indicated that miR-148a was the most downregulated microRNA in metastatic lesions compared to primary tumor cells. miR-148a together with the other miR-148 family members miR-148b and miR-152 constitutes a metastatic signature of pancreatic cancer. Remarkably, the majority of microRNAs found differentially expressed at different stages of these mouse neuroendocrine tumors were similarly affected in a number of human tumors implying the generality of these observations.
As discussed previously, the origin of CAFs remains under active investigation. It seems most likely that these heterogeneous fibroblast populations that form CAFs transdifferentiate from several progenital or resident host cells within the tumor microenvironment. A recent study using an HPV16-driven mouse model of multistep squamous cell carcinogenesis showed that the carcinoma cells “educate” resident fibroblasts to express pro-inflammatory genes through activation of NF-κB signaling [31]. This gene signature persisted in CAFs through subsequent carcinoma stages and was present in mouse models of mammary and pancreatic tumors. Inhibition of NF-κB signaling in CAFs resulted in a slower growth of tumor cells co-injected with these fibroblasts compared to control CAFs.
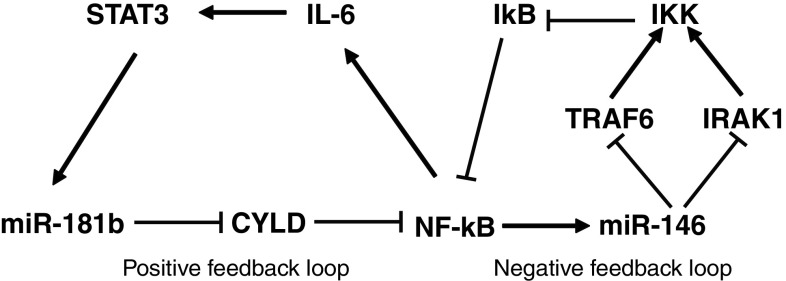
One of the possible mechanisms of NF-κB stimulation in CAFs was secretion of IL-1β by neoplastic cells. Indeed, IL-1β is a potent inducer of NF-κB and neutralizing IL-1β in conditioned media reduced the expression of known NF-κB target genes in fibroblasts [31]. Relevant to this observation, we found two upregulated microRNAs in CAFs that are involved in NF-κB signaling. miR-181b-1 was recently identified as a key player that links inflammation to cellular transformation of MCF10A human mammary epithelial cells [57]. Non-transformed MCF10A cells were generated to express an activated Src oncogene that induces morphological transformation accompanied by increased growth in soft agar, cell invasion and tumor growth in mouse xenografts. In this model, inflammatory genes were activated by STAT3-mediated induction of miR-181b-1 that targets the tumor suppressor CYLD. In turn, CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activity. The positive feedback loop is completed by induction of IL-6 by NF-κB, which phosphorylates and activates STAT3 (Fig. 3). Another microRNA upregulated in CAFS, miR-146a, is directly activated by NF-κB through multiple NF-κB-binding sites in the promoter region of miR-146 gene [58]. Importantly, miR-146 was shown to target and suppress two key adapter molecules in the NF-κB pathway, IRAK1 and TRAF6, thus limiting NF-κB activation. Therefore, activation of miR-146 by NF-κB constitutes a negative feedback loop. Taken together, it is clear that the inflammatory pathway mediated by NF-κB is under strict control of multiple regulatory factors including microRNAs (Fig. 3).
Fig. 3.
miR-181b and miR-146a induced in CAFs are involved in the regulation of the NF-κB signaling pathway
Future directions
While we have learned much about the miRNA expressions’ changes associated with the formation of CAFs, much remains to be studied about what role these miRNAs play within CAFs and whether CAFs export miRNAs to influence the biology of tumor cells and other components of the microenvironment. Interactions between tumor epithelial cells and CAFs are critical for development and progression of tumorigenesis. In order to invoke microRNAs as potential therapeutic agents, we need to understand the functions of miRNAs in the various cell types that constitute a tumor and contribute to tumor progression.
Another yet unanswered question is the commonality of microRNA signatures in CAFs and other stromal cells in different tumor types. It is quite possible that microRNA signatures in CAFs may be unique for different types of tumor. It has been shown that miR-15 and miR-16 are downregulated in CAFs isolated from prostate cancer and that reconstitution of these microRNAs partially reduces the prostate tumor burden in mouse xenograft models [59]. miR-15 and miR-16 were not found to be downregulated in CAFs from endometrial cancer [47]. Similarly, a specific pro-inflammatory gene signature was identified in dermal, mammary and pancreatic CAFs, but not in CAFs isolated from cervical tumors [31].
Future studies will analyze microRNA expression from additional cellular components of the tumor microenvironment and determine the functional consequences of altering miRNA expression. Since miRNAs in CAFs appear to play an important role in tumor biology, one can imagine interfering with miRNA expression or function to potentially inhibit critical aspects of tumorigenesis that could be translated clinically in the future.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Conflict of interest
Authors declared no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Second International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2011), held in Budapest, Hungary, 2nd–5th May 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM, Gauldie J, Green JE. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett S, Khanna C, Chambers AF, Green JE. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin H. Contact interactions between cells that suppress neoplastic development: can they also explain metastatic dormancy? Adv Cancer Res. 2008;100:159–202. doi: 10.1016/S0065-230X(08)00006-7. [DOI] [PubMed] [Google Scholar]
- 8.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117:3155–3163. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 11.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829–836. doi: 10.5858/2002-126-0829-ISOMIN. [DOI] [PubMed] [Google Scholar]
- 13.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 14.Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43:194–199. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 16.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, Naidu S, Wei G, Sharma SM, Stephens JA, Fernandez SA, Gurcan MN, Weinstein MB, Barsky SH, Yee L, Rosol TJ, Stromberg PC, Robinson ML, Pepin F, Hallett M, Park M, Ostrowski MC, Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardone A, Tolino A, Zarcone R, Borruto Caracciolo G, Tartaglia E. Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med. 1997;39:174–177. [PubMed] [Google Scholar]
- 21.Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, Kowalski LP, Coletta RD. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 23.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004;45(Suppl 2):S163–S175. doi: 10.1016/j.lungcan.2004.07.977. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 25.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 26.Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J, Emura M, Ochiya T, Ochiai A. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–240. doi: 10.1016/S0006-291X(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 27.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal E, McCrory A, Talbert M, Young G, Murphy-Ullrich J, Gladson C. Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol Carcinog. 2004;40:116–121. doi: 10.1002/mc.20024. [DOI] [PubMed] [Google Scholar]
- 29.San Francisco IF, DeWolf WC, Peehl DM, Olumi AF. Expression of transforming growth factor-beta 1 and growth in soft agar differentiate prostate carcinoma-associated fibroblasts from normal prostate fibroblasts. Int J Cancer. 2004;112:213–218. doi: 10.1002/ijc.20388. [DOI] [PubMed] [Google Scholar]
- 30.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Fukino K, Shen L, Matsumoto S, Morrison CD, Mutter GL, Eng C. Combined total genome loss of heterozygosity scan of breast cancer stroma and epithelium reveals multiplicity of stromal targets. Cancer Res. 2004;64:7231–7236. doi: 10.1158/0008-5472.CAN-04-2866. [DOI] [PubMed] [Google Scholar]
- 33.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 35.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- 36.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 37.Qiu W, Hu M, Sridhar A, Opeskin K, Fox S, Shipitsin M, Trivett M, Thompson ER, Ramakrishna M, Gorringe KL, Polyak K, Haviv I, Campbell IG. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40:650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter K, Omura N, Hong SM, Griffith M, Goggins M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008;7:882–888. doi: 10.4161/cbt.7.6.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiegl H, Millinger S, Goebel G, Muller-Holzner E, Marth C, Laird PW, Widschwendter M. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res. 2006;66:29–33. doi: 10.1158/0008-5472.CAN-05-2508. [DOI] [PubMed] [Google Scholar]
- 40.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Gonda TA, Gamble MV, Salas M, Seshan V, Tu S, Twaddell WS, Hegyi P, Lazar G, Steele I, Varro A, Wang TC, Tycko B. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68:9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci (Lond) 2011;121:141–158. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 43.Ambros V. MicroRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 44.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small RNA. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aprelikova O, Yu X, Palla J, Wei BR, John S, Yi M, Stephens R, Simpson RM, Risinger JI, Jazaeri A, Niederhuber J. The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts. Cell Cycle. 2010;9:4387–4398. doi: 10.4161/cc.9.21.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobreva G, Dambacher J, Grosschedl R. SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin mu gene expression. Genes Dev. 2003;17:3048–3061. doi: 10.1101/gad.1153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothe F, Simion A, Akl H, Mourtada M, El Rifai M, Burny A, Romero P, Martiat P, Badran B. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur J Immunol. 2009;39:1608–1618. doi: 10.1002/eji.200838509. [DOI] [PubMed] [Google Scholar]
- 51.Hanoun N, Delpu Y, Suriawinata AA, Bournet B, Bureau C, Selves J, Tsongalis GJ, Dufresne M, Buscail L, Cordelier P, Torrisani J. The silencing of microRNA 148a production by DNA hypermethylation is an early event in pancreatic carcinogenesis. Clin Chem. 2010;56:1107–1118. doi: 10.1373/clinchem.2010.144709. [DOI] [PubMed] [Google Scholar]
- 52.Lujambio A, Calin GA, Villanueva A, Ropero S, Sanchez-Cespedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso MS, Faller WJ, Gallagher WM, Eccles SA, Croce CM, Esteller M. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM, Tang ZY, Wang XW. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 54.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, Li N, Cao X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIalpha. J Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 56.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/S1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 57.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Musumeci M, Coppola V, Addario A, Patrizii M, Maugeri-Sacca M, Memeo L, Colarossi C, Francescangeli F, Biffoni M, Collura D, Giacobbe A, D’Urso L, Falchi M, Venneri MA, Muto G, De Maria R, Bonci D. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene. 2011;30:4231–4242. doi: 10.1038/onc.2011.140. [DOI] [PubMed] [Google Scholar]