Abstract
The presence of human immunodeficiency virus type 1 (HIV-1) in the brain is believed to be responsible for mediating the pathogenesis of neurological abnormalities through the viral toxins gp120 and Tat. Numerous studies indicate neurotoxic effects of the HIV-1-protein Tat, with demonstrated neurobehavioral and cognitive alterations. However, less clear is the neurotoxic effect of gp120 on neurobehavior. This study was designed to characterize the potential deficits in sensory-motor and preattentive functions, following intrahippocampal administration of gp120. Using a randomized-block design, male and female pups of eight Sprague–Dawley litters were injected bilaterally with either vehicle (VEH) (1 μl volume) or one of the three gp120 doses (1.29, 12.9, or 129 ng/μl) at postnatal day (P)1. Sensory-motor functions were assessed at P3, as measured by the righting reflex and at P8, as measured by negative geotaxis. At P24 animals were tested on preattentive processes, as indexed by sensorimotor gating. Sensorimotor gating was measured by prepulse inhibition (PPI) of the auditory startle response (ASR) (ISIs of 0, 8, 40, 80, 120, and 4000 ms, six trial blocks, Latin-square design). Results indicated gp120-induced neurotoxicity on the righting reflex but not negative geotaxis. For sensorimotor gating, the PPI test demonstrated a reduced inhibition response on peak ASR latency as the dose of gp120 increased. No effect was noted for response inhibition on peak ASR amplitude. These data suggest that intrahippocampal injection of gp120 (0, 1.29, 12.9, or 129 ng/μl) had transient neurotoxic effects on sensory-motor function and limited effects on preattentive processes early in development.
Keywords: Gp120, HIV-1, Hippocampus, Sensory-motor system, Preattentive processes, Prepulse inhibition
1. Introduction
Infection by human immunodeficiency virus type 1 (HIV-1) is often complicated by a variety of neurological abnormalities. In infants and children, the HIV-1 virus enters the organism in an immature stage of a developing immune and nervous system. The adverse effects of pediatric HIV-1 infection in a developing central nervous system (CNS) often result in a more rapid onset of clinical symptoms and progression to death (Belman, 1997). HIV-1-associated encephalopathy is a term used to describe HIV-1 infection in children with CNS dysfunction and is analogous to the HIV-1 associated dementia complex (HAD) seen in adults. Encephalopathy can be associated with impaired brain growth, loss of developmental milestones, progressive motor dysfunction, as well as attentional and cognitive deficits (Belman, 1997; Epstein et al., 1986; Fauci, 1988).
Numerous studies suggest that brain dysfunction and neuronal alteration is caused by HIV-1 being carried into the CNS through infected macrophages (Catani et al., 2003; Nath et al., 2000). Because neurons in the CNS are not productively infected by the virus itself, it is suggested that HIV-1-induced neurodegeneration is mediated by HIV-1 products. The HIV-1 proteins Tat and gp120 are released from HIV-1-infected cells and are present extracellularly in the infected brain. It has been well established that Tat and gp120 are neurotoxic and elevated in brain tissue of patients with HAD (Jones et al., 2000; Valle et al., 2000).
In vitro and in vivo studies give evidence that both HIV-1 proteins cause histological changes, consistent with those seen in patients with neuropsychological deficits (Hill et al., 1993; Maragos et al., 2003). Neurotoxicity induced by the nonstructural transactivating HIV-1 protein Tat has been reported to be caused via oxidative stress (Aksenov et al., 2001, 2003; Aksenova et al., 2005). For the envelope glycoprotein gp120, in vitro studies report detrimental effects of gp120 on neurons with the induced neurotoxicity being primarily mediated by N-methyl-d-aspartate (NMDA) receptor mechanisms (Barks et al., 1997; Lipton and Gendelman, 1995).
Studies examining effects of the HIV-1 proteins on neurobehavior and development indicate adverse effects of Tat on the developing CNS of perinatal animals (Barks et al., 1997; Fitting et al., 2006b). A developmental study on prepulse inhibition of the auditory startle response (ASR) demonstrated that Tat-induced neurotoxicity impaired the cognitive processes involved in sensorimotor gating that were already evident very early in development (Fitting et al., 2006b). Neurobehavioral studies for the envelope glycoprotein gp120, however, are not as compelling. Exposure of gp120 in utero and subcutaneous injection of gp120 during the neonatal period failed to cause any measurable neurotoxicity on behavior and histology (Bussiere et al., 1999). Another study examining immature rats reported gp120-induced neurotoxicity in complex motor skills but failed to alter developmental milestones, including measures of physical and sensory development (Hill et al., 1993). It is important to note that the authors used categorical behavioral measures, such as the day of onset, e.g., examined sensory development on the day of the first auditory startle as defined when a ‘clapping sound causes immediate startle response’ (Hill et al., 1993). Another more sophisticated way of assessing sensory development is the assessment of sensorimotor gating with examining prepulse inhibition (PPI), as measured by the reduction in the startle reflex that occurs when the startling stimulus is preceded by a weak prepulse (Hoffman and Ison, 1980).
The present study was undertaken to determine whether gp120 causes neurobehavioral effects in pre and postweanling rats following intrahippocampal gp120-injection on postnatal day (P)1. For the neurobehavioral assessment the righting reflex at P3, negative geotaxis at P8, and PPI at P24 were tested to examine the immediate impact of gp120-induced neurotoxicity on specific sensory-motor and preattentive processes early in development.
2. Methods
2.1. Animals
Sprague-Dawley pregnant dams (n = 8) were ordered from Harlan Laboratories Inc. (Indianapolis, IN) and delivered to the vivarium before embryonic day 7. Dams were housed singly with food (Pro-Lab Rat, Mouse Hamster Chow #3000, NIH diet #31) and water available ad libitum. The day pups were found in the cage was designated as P0. On P1, litters were culled to 10 offspring of equal sexes, if possible. No more than one female and one male per litter were assigned to a single condition. At 21 days of age animals were weighted, weaned and pair housed throughout the experiment. The animal facility was maintained at 21 ± 2°C, 50 ± 10% relative humidity and had a 12-h light:12-h dark cycle with lights on at 07:00 h (EST). The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina, Columbia.
2.2. Dose-response gp120 study
Purified gp120 LAV (T-tropic) envelope protein was purchased from Protein Sciences Corp. (Meriden, CT) with a concentration of 100 μg at 1.0 ml. Gp120 was stored at −20°C until used for testing. Solutions of gp120 were prepared in order to get the following three doses: 1.29, 12.9, or 129 ng/μl. These three doses were sub-threshold doses and used in the present study to determine the gp120-dose response function with no gross behavioral impairments being anticipated. Previous research demonstrated that doses of 250 ng gp120 or higher were necessary to cause significant loss of striatal-tissue and significantly increased the number of glial fibrillary acidic protein reactive cells (Bansal et al., 2000).
2.3. Surgery
Standard stereotaxic surgery techniques modified for neonates, were used for treatment injection. Individual pups were removed from the dam and cryogenically anesthetized (AVMA, 2001) before being placed in a modified stereotaxic holder for surgery of neonates (Kopf Inc.), which included a chilled base to maintain cryogenic anesthesia. Rubber head bars held the skull in place while bilateral microinjections of vehicle (VEH; physiological saline) or gp120 were made directly into hippocampus using stereotaxic coordinates for injections and a microsyringe (Hamilton Co., Nevada, USA (Microliter #701 RN, 10 μl). The set of coordinates used for the hippocampus were: right hemisphere −0.3 mm anterior to the bregma, 0.7 mm lateral to bregma, −3.0 mm dorsal from dura; left hemisphere −0.3 mm anterior to the bregma, −0.7 mm medial to bregma, −3.0 mm dorsal from dura. The VEH and gp120-treated animals were injected with the same volume (1 μl) and gp120 was dissolved in saline. The 1 μl injection volume was released over 1 min after a 1-min resting period that allowed the tissue to return to its original conformation. The injection needle was withdrawn over 2 min to prevent reflux. After the bilateral injections, the piercings in the skin of the head were closed with surgical glue and the pups warmed under a heat lamp (35°C) before being returned to the dam, where they were closely observed for indications of rejection. No pups were rejected or abused by the dam. Gp120 had no significant effect on body weight or growth. Analyses of Nissl-stained sections through the hippocampus confirmed placement of injection sites into the hippocampal dentate hilar region.
2.4. Experimental design
A randomized block design was employed, with litter as the blocking factor, in which all experimental gp120-dose treatments were represented. Thus, animals were randomly assigned to one of four treatment groups that received bilateral hippocampal injections at P1 of either 1 μl VEH (0 ng gp120, n = 8), 1.29 ng gp120 as the low-dose group (n = 8), 12.9 ng gp120 as the medium-dose group (n = 7), and 129 ng gp120 as the high-dose group (n = 8). In each of the groups, male and female rats were included. Weighing and behavioral assessment started at P3. All rats were tested for alterations in sensory-motor processes by assessing the righting reflex at P3 and negative geotaxis at P8. Preattentive processes were assessed by PPI of the ASR at P24.
2.5. Behavioral motor tasks
The righting reflex was examined at P3 in order to assess preweanling alterations in the sensory-motor system. The goal was to assess the development of the sensory-motor system at a time point when other developmental milestones were still undeveloped and thus, not confounded by secondary variables, such as eye opening and ear opening. The righting reflex is defined as the ability to assume an upright position when there has been a departure from it (Walton et al., 2005). A pup was placed on its back and time in seconds was recorded to return to the upright position by turning over onto its belly. In this task two of the animals were defined as outliers with more than 20 S.D. away from the mean and thus, not included in the data analysis.
Another test to determine sensory-motor function was the assessment of time in seconds to perform negative geotaxis at P8. Negative geotaxis is defined as an orienting response and movement expressed in opposition to cues of a gravitational vector (Motz and Alberts, 2005). The test was conducted by placing the pup’s head down on a 25° tilted plane. The dependent variable was latency to turn 180° and climb up toward the high end of the plane.
2.6. ASR and PPI assessment
2.6.1. Apparatus
The startle chamber (SR-Lab Startle Reflex System, San Diego Instruments Inc.) was enclosed in a 10 cm thick double-walled, 81 cm × 81 cm × 116 cm isolation cabinet (external dimensions) (Industrial Acoustic Company Inc., Bronx, NY). Each animal was tested individually in the dark with a high-frequency loudspeaker that produced a background white noise (70 dB(A)) and was mounted inside the chamber 31 cm above the Plexiglas cylinder. The startle chamber consisted of a Plexiglas cylinder 8.75 cm in internal diameter resting on a 12.5 cm × 20 cm Plexiglas stand. The startle stimulus was 100 dB(A) and the prepulse stimulus was 85 dB(A) in intensity. Both stimuli had a duration of 20 ms. The animal’s response to the startle stimulus produced deflection of the Plexiglas cylinder, which was converted into an analog signal by a piezoelectric accelerometer. Acoustic stimulus intensities and response sensitivities were calibrated using a SR-LAB Startle Calibration System. Sound levels were measured and calibrated with a sound level meter (Extech Instruments, Waltham, MA) with the microphone placed inside the Plexiglas cylinder. The signals were then digitized (12 bit A–D) and saved to a hard disk on a Pentium class computer.
2.6.2. Testing procedures
All rats were tested for approximately 20 min. Animals were first exposed to a 5-min acclimation period of 70 dB(A) background of white noise, followed by six single white noise stimuli of 100 dB(A), and 36 PPI trials with 0, 8, 40, 80, 120, and 4000 ms interstimulus intervals (ISIs), assigned by a Latin-square design. The stimulus duration was 20 ms. The six single stimuli assessed the baseline acoustic startle response (ASR) without a prior prepulse stimulus and the PPI trials 0 and 4000 ms ISIs were defined as control trials in order to provide the baseline ASR within the PPI test. The ISI represents the time from the offset of the prepulse stimulus to the onset of the startle stimulus. For PPI the dependent measures analyzed were peak ASR amplitude, peak ASR latency (from startle stimulus onset to the peak response), and percent PPI. Percent PPI is typically expressed as percent of inhibition in startle amplitude (Geyer and Swerdlow, 2004), most commonly used in applied settings at a prepulse of 100 ms ISI relative to pulse only trials (0 ms ISI) (e.g. Caine et al., 2001; Davis et al., 1990). PPI for ISI 100 ms was calculated using the average of PPI trials 80 and 120 ms ISIs. Percent PPI was computed according to the following formula: %PPI = [(0 ms ISI trials − 100 ms ISI trials)/0 ms ISI trials] × 100.
2.7. Data analysis
Data are expressed as mean (±S.E.M.). All data were analyzed using analysis of variance (ANOVA) techniques (SYSTAT 11.0 for Windows, SYSTAT Inc.). Planned comparisons (comparing VEH-treated animals with each of the three gp120 dose-treated animals) and orthogonal component analyses were performed to determine specific treatment effects. Separate orthogonal component analyses for each treatment on PPI trials were employed to describe the shape of the function by determining its significance (e.g., linear, quadratic, etc. equations) (Winer, 1971). In addition, the ISI at which the maximal response inhibition occurred was recorded across all PPI trials (8–120 ms ISIs) and categorized into two categories. Category one included ISIs 8 and 40 ms, and category two included ISIs 80 and 120 ms. The ISI data is categorical in nature with some frequencies less than 5, thus, the Fisher’s exact test was applied. An alpha level of p ≤ 0.05 was considered significant for all statistical tests used.
3. Results
3.1. Body weight
A 3 (age: 3-, 8-, and 21-day old) × 4 (treatment: 0.0, 1.29, 12.9, or 129 ng/μl gp120 dose) mixed-model ANOVA conducted on body weight revealed a significant age effect [F(2,54) = 12413.92, p ≤ 0.0001] with a prominent linear increase of growth with age [F(1,27) = 17168.2, p ≤ 0.0001]. Treatment did not interact with age, indicating no gp120-induced effects on pup’s growth. Table 1 presents mean (±S.E.M.) body weight at P3, P8, and P21 separate for each of the four treatment groups.
Table 1.
Mean (±S.E.M.) of body weight across 3 days of age for each of the four treatment groups
| P3 | P8 | P21 | |
|---|---|---|---|
| 0.0 ng | 12.42 (0.21) | 19.90 (0.24) | 58.67 (0.81) |
| 1.29 ng | 12.48 (0.24) | 19.88 (0.69) | 59.00 (0.93) |
| 12.9 ng | 12.64 (0.13) | 20.50 (0.58) | 60.50 (0.38) |
| 129 ng | 12.30 (0.15) | 20.00 (0.43) | 58.35 (0.58) |
3.2. Behavioral motor tasks
A one-way ANOVA on righting reflex at P3 revealed a significant treatment effect [F(3,25) = 3.0, p ≤ 0.05] with a linear component [F(1,25) = 7.0, p ≤ 0.01], indicating a linear dose-dependent gp120-induced alteration in the development of the early sensory-motor reflex. Contrast analyses comparing the VEH-treated animals, as the control group, to the gp120-dose-treated animals, revealed a significant difference of the 129 ng gp120-dose group compared to the VEH-treated animals (p ≤ 0.01). A one-way ANOVA on negative geotaxis at P8 revealed no treatment effect, indicating no adverse alterations in sensory-motor processes at P8 by any of the gp120 doses. Fig. 1 illustrates for each of the four treatment groups (A) latency for the righting reflex and (B) latency for the negative geotaxis.
Fig. 1.

Mean (±S.E.M.) for each of the four treatment groups illustrating (A) the significant linear effect of treatment for latency on the righting reflex at P3, and (B) latency for the negative geotaxis test at P8.
3.3. ASR and PPI test
3.3.1. Baseline trials
A one-way ANOVA on peak ASR amplitude and peak ASR latency revealed no treatment effects, indicating similar baseline ASR for all treatment conditions. Fig. 2 illustrates for each of the four treatment groups (A) peak ASR amplitude and (B) peak ASR latency.
Fig. 2.
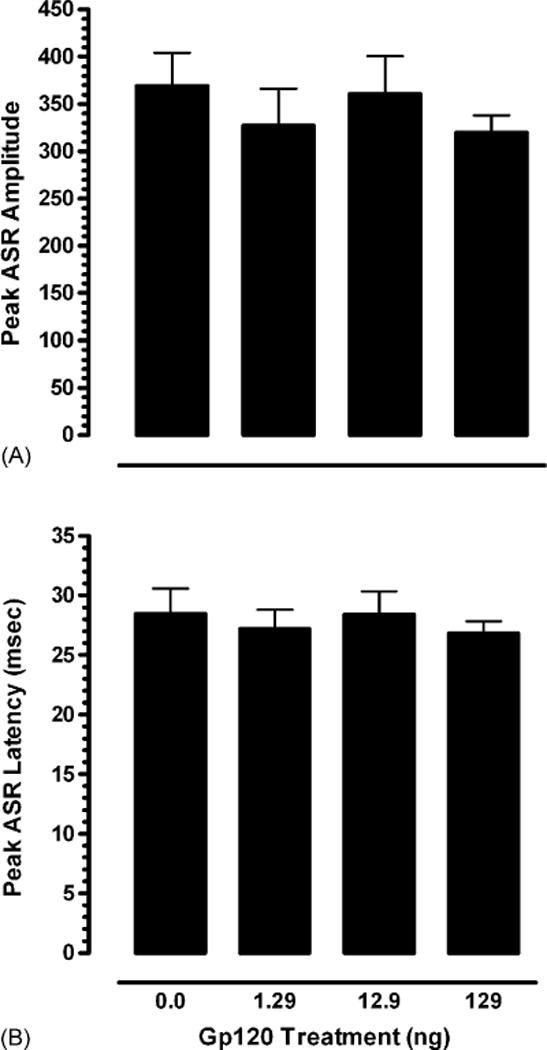
Mean (±S.E.M.) on baseline trials for each of the four treatment groups illustrating (A) peak ASR amplitude, and (B) peak ASR latency.
3.3.2. Control trials (0, 4000 ms ISI combined)
A one-way ANOVA on peak ASR amplitude revealed no treatment effect, indicating similar ASR for all treatment conditions on the control trials. On peak ASR latency a significant treatment effect was noted [F(3,27) = 3.5, p ≤ 0.03] with a significant cubic component [F(1,27) = 5.8, p ≤ 0.02]. Post hoc comparisons supported the cubic component with a significant difference between low and medium gp120-dose-treated animals (p ≤ 0.05), indicating gp120-dose-dependent alterations on the startle response. Fig. 3 illustrates for each of the four treatment groups (A) peak ASR amplitude, and (B) the significant treatment effect on peak ASR latency.
Fig. 3.

Mean (±S.E.M.) on control trials (0 and 4000 ms) within PPI for each of the four treatment groups illustrating (A) peak ASR amplitude, and (B) the significant cubic treatment effect on peak ASR latency.
3.3.3. PPI trial (100 ms ISI)
A one-way ANOVA conducted on percent PPI of the startle amplitude for ISI 100 ms revealed no treatment effect, indicating no significant alteration in percent inhibition by any of the given gp120-dose treatments (data not shown).
3.3.4. PPI trials (8–120 ms ISIs)
A mixed-model ANOVA conducted on peak ASR amplitude revealed a significant PPI trial effect with a prominent linear trend [F(1,27) = 57.3, p ≤ 0.001], but no effect for treatment or treatment × PPI interaction, suggesting the lack of a gp120-induced effect on the shape of the peak ASR amplitude of the inhibition response across PPI trials (08–120 ms ISIs). In addition, a Chi-square test on the ISI in which the maximal inhibition occurred revealed no significant effects, indicating that the ISI in which the peak response occurred, was not altered by any of the given gp120-doses relative to the VEH-treated animals. A mixed-model ANOVA conducted on peak ASR latency revealed a significant effect for PPI trials with a prominent linear trend, [F(1,27) = 193.4, p ≤ .001], indicating a linear slowing in the inhibition response with an increase in ISIs. Treatment did not interact with PPI trials, but revealed a significant main effect [F(3,27) = 3.0, p ≤ 0.05], suggesting that gp120 to some extend altered cognitive processes, as indexed by PPI. Data suggest a linear flattening of the peak ASR latency collapsed across PPI (08–120 ms ISIs) [F(1,27) = 4.2, p ≤ 0.05] as the dose of gp120 increased. Fig. 4 illustrates for each of the four treatment groups (A) peak ASR amplitude and (B) peak ASR latency across PPI trials (8–120 ms ISIs).
Fig. 4.
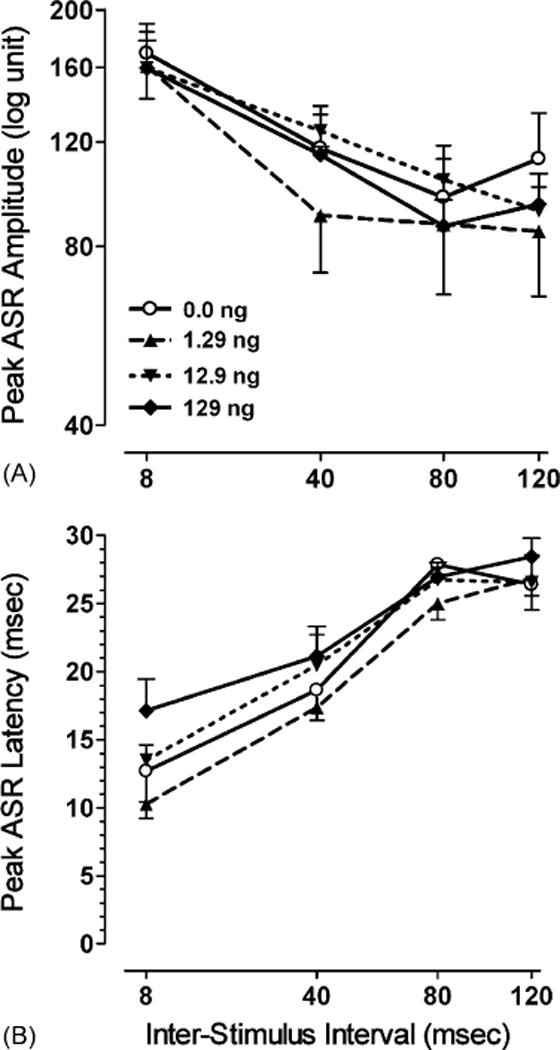
Mean (±S.E.M.) for each of the four treatment groups across PPI trials (8–120 ms ISIs) illustrating (A) peak ASR amplitude, and (B) peak ASR latency.
4. Discussion
In the present study the HIV-1 protein gp120 was bilaterally injected into the hippocampus at P1 with testing its neurobehavioral effects early in development. Results indicate that the injected gp120 doses (varying from 1.29 to 129 ng) induced a linear dose-dependent effect on sensory-motor function with a delayed onset of the early sensory-motor reflex development, and only limited effects on preattentive processes.
For sensory-motor function, the righting reflex was assessed very early in development at P3. A linear gp120-dose dependent slowing of the latency response with increased gp120 treatment was noted, suggesting a gp120-induced alteration on early sensory-motor reflex development. However, negative geotaxis assessed at P8 did not reveal any significant treatment effect. These results suggest that the onset of the development of the sensory-motor system was delayed for gp120-treated rats, albeit transiently, as indicated by negative geotaxis assessed at P8.
In the sensorimotor startle system limited alterations were noted. Whereas, no alterations in the baseline ASR trials were found, dose-dependent gp120-induced alterations in ASR on the control trials occurred. A significant cubic gp120-effect on peak ASR latency to the startle stimuli was noted, although the non-monotonicity of the dose effect is difficult to interpret. The nonmonotonicity of the dose effect could be an indicator that the early treatment with a low dose of gp120 may require a longer duration for the cognitive effects to become present. Further, no differences were noted between VEH-treated animals and any of the gp120-induced doses, thus, limiting the interpretation of a gp120-induced effect on the ASR.
Assessment of preattentive processes during the postweanling period suggested that gp120 had at least some effects on the process of sensorimotor gating, as indexed by PPI. No effects on peak ASR amplitude, and percent PPI were noted. In contrast, peak ASR latency indicated a significant linear gp120-dose effect on PPI with a slowing in response when gp120 dose increased. An attenuation of peak ASR latency across the inhibition ISIs of 8–120 ms, with a flattening of the latency curves, was noted, that was most evident for the high dose gp120-treated animals. In contrast to previous studies that used only a categorical measure to assess sensory development (Bussiere et al., 1999; Hill et al., 1993) the present study used a more fine-grain measure to assess sensorimotor gating, indexed by PPI. Sensorimotor gating reflects a fundamental principle of the neural control of behavior within the CNS (Hoffman and Ison, 1980). Recent studies that used Tat as a viral HIV-1 protein and focused on PPI of the ASR demonstrated that Tat-induced neurotoxicity impaired the cognitive processes involved in sensorimotor gating (Fitting et al., 2006a,b).
Several additional points should be considered when interpreting the results of the current study. In the present study the highest gp120-induced dose was 129 ng/μl. Literature indicates that gp120 (200 ng) but not gp120 (50 ng) elicited minimal focal pyramidal cell loss immediately adjacent to the injection track (Barks et al., 1997), and co-injection of 50 ng gp120 with 5 nmol NMDA increased the severity of hippocampal injury (Barks et al., 1997). Interestingly, a previous in vivo study assessing prepulse inhibition in adulthood demonstrated long-term gp120-induced neurotoxic effects after perinatal gp120 exposure at P1 (1.29–129 ng gp120) (Fitting et al., 2006c). Also synergistic effects of combined gp120 and Tat on histology were noted (Bansal et al., 2000), revealing the question for future research about combined gp120 and Tat synergistic effects on neurobehavior and development.
In summary, gp120-doses between 1.29–129 ng injected bilaterally into the hippocampus on P1, delayed but did not preclude the onset of the sensory-motor reflex development, as measured by the righting reflex at P3, and negative geotaxis at P8, and had only limited effects on preattentive processes, as indexed by PPI on P24.
Acknowledgments
The authors thank Dr. Heidi M. Carman and Dr. Guanghan Wu for technical assistance. This work was supported by grants from the National Institute on Drug Abuse (DA013137, DA014401) and the National Institute of Child Health and Human Development (HD043680).
References
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, et al. Oxidative damage induced by the injection of HIV-1 Tat in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, et al. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Curr Neurovasc Res. 2005;2:73–89. doi: 10.2174/1567202052773463. [DOI] [PubMed] [Google Scholar]
- AVMA. Report of the AVMA panel of euthanasia. J Am Vet Med Ass. 2001;218:671–96. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–9. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Barks JDE, Liu X-H, Sun R, Silverstein FS. Gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience. 1997;76:397–409. doi: 10.1016/s0306-4522(96)00373-9. [DOI] [PubMed] [Google Scholar]
- Belman AL. Infants, children, and adolescents. In: Berger JR, Levy RM, editors. AIDS and the nervous system. Lippincott-Raven Publishers; 1997. pp. 223–53. [Google Scholar]
- Bussiere JL, Hardy LM, Peterson M, Foss JA, Garman RH, Hoberman AM, et al. Lack of developmental neurotoxicity of MN rgp 120/HIV-1 administered subcutaneously to neonatal rats. Toxicol Sci. 1999;48:90–9. doi: 10.1093/toxsci/48.1.90. [DOI] [PubMed] [Google Scholar]
- Caine SB, Humby T, Robbins TW, Everitt BJ. Behavioral effects of psycho-motor stimulants in rats with dorsal or ventral subiculum lesions: locomotion, cocaine self-administration, and prepulse inhibition of startle. Behav Neurosci. 2001;115:880–94. doi: 10.1037//0735-7044.115.4.880. [DOI] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Ranalli M, Amantea D, Litovchick A, Lapidot A, et al. The Tat antagonist neomycin B hexa-arginine conjugate inhibits gp-120-induced death of human neuroblastoma cells. J Neurochem. 2003;84:1237–45. doi: 10.1046/j.1471-4159.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology. 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Oleske JM, Connor EM, Goudsmit J, Bagdon L, et al. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986;78:678–87. [PubMed] [Google Scholar]
- Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–22. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Intrahippocampal injections of Tat: effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol Biochem Behav. 2006a;84:189–96. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition of the auditory startle response. Int J Dev Neurosci. 2006b;24:275–83. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal gp120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006c;318:1352–8. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of the startle response, prepulse inhibition, and habituation. In: Crawley JN, et al., editors. Current protocols in neuroscience. New York: Wiley; 2004. p. S61S61. [unit 8.7]. [DOI] [PubMed] [Google Scholar]
- Hill JM, Mervis RF, Avidor R, Moody TW, Brenneman DE. HIV envelope protein-induced neuronal damage and retardation of behavioral development in rat neonates. Brain Res. 1993;603:222–33. doi: 10.1016/0006-8993(93)91241-j. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–89. [PubMed] [Google Scholar]
- Jones MV, Bell JE, Nath A. Immunolocalization of HIVenvelope gp120 in HIV encephalitis with dementia. AIDS. 2000;14:2709–13. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–40. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, et al. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Motz BA, Alberts JR. The validity and utility of geotaxis in young rodents. Neurotoxicol Teratol. 2005;27:529–33. doi: 10.1016/j.ntt.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodefiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–94. [PubMed] [Google Scholar]
- Valle LD, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–8. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Walton KD, Harding S, Anschel D, Harris YT, Llinás R. The effects of microgravity on the development of surface righting in rats. J Physiol. 2005;565:593–608. doi: 10.1113/jphysiol.2004.074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. New York: McGraw-Hill; 1971. [Google Scholar]


