Abstract
Background
Few studies have evaluated the relationship between high-sensitivity C-reactive protein (hs-CRP) and vascular events in the elderly with chronic kidney disease (CKD).
Methods
The relationship of hs-CRP with vascular events was examined according to CKD status in 3166 participants of the Intervention Project on Cerebrovascular Diseases and Dementia in the Community of Ebersberg, Bavaria (INVADE study). CKD was defined as a creatinine clearance <60mL/min estimated by the Cockcroft-Gault formula. hs-CRP was used as a binary variable > or < 2.1mg/l (median value). Vascular events were defined as a composite of myocardial infarction, stroke and vascular death.
Results
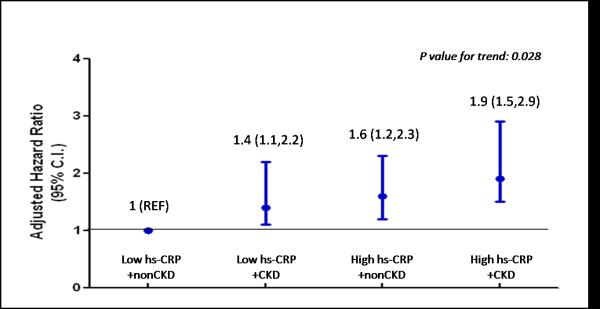
After 4 years of follow-up, 204 (6.4%) participants experienced a major cardiovascular event. High hs-CRP levels and CKD at baseline were associated with a greater risk of vascular events. Compared to patients with low hs-CRP/non-CKD, the adjusted HR (95% CI) for vascular events was 1.42 (1.11; 2.21) for low hs-CRP/CKD,1.57 (1.21; 2.34) for high hs-CRP/non-CKD, and 1.93 (1.45; 2.89) for high hs-CRP/CKD.
Conclusions
These results suggest that high CRP provides prognostic information in patients with CKD.
Keywords: C-reactive protein, inflammation, CKD, cardiovascular disease
Introduction
Multiple studies have shown CKD to be an independent risk factor for cardiovascular events and mortality (1-5). This increased risk of cardiovascular disease is evident even with mild decrements of kidney function (1, 6), and appears to increase incrementally as CKD advances (2, 7). Despite the higher prevalence of traditional (Framingham) risk factors for cardiovascular disease in patients with CKD (8-9), the increased risk of cardiovascular disease in this patient population is only partially explained by the traditional risk factors of the general population (10). Several non-traditional risk factors have been proposed to play a role in the development and progression of accelerated atherosclerosis noted in individuals with CKD such as chronic inflammation (11).
The association between inflammation and cardiovascular disease is best described in patients with ESRD (12), where inflammatory markers such as high-sensitivity C-reactive protein (hs-CRP) and Interleukin-6 (IL-6) are powerful predictors of cardiovascular events and mortality (13-16). Recent evidence suggests that even patients with early kidney disease suffer from inflammation (17-19). Yet, results of studies in patients with pre-ESRD CKD have been conflicted on whether CRP is an independent risk factor for cardiovascular events and mortality in patients with CKD (20-24). Furthermore, a paucity of studies have addressed the association of inflammation with cardiovascular disease in elderly patients with CKD (22, 25).
Using data from the Intervention Project for Cardiovascular Diseases and Dementia in Ebersberg, Germany Study (INVADE), we tested the hypothesis that elevated serum hs-CRP levels are associated with increased risk of cardiovascular events in elderly patients with CKD and kidney disease progression.
Subjects and Methods
Study Population
All the residents of Ebersberg, Germany who were born prior to 1946 and were members of the health insurance company AOK (Allgemeine Ortskrankenkasse) were eligible for enrollment in the study (26). A total of 10325 individuals (40% of Ebersberg residents) were identified in the AOK database and invited to participate. 3905 subjects responded to the invitation during the recruitment phase (2001-2003) and 3166 subjects were included in the current analysis. The remaining participants (n=739) were excluded because of incomplete data or labs. The baseline assessment was conducted by the primary care physicians of the community (n=65) and included a standardized questionnaire, medical history, physical examination, evaluation of several risk factors, a 12-lead electrocardiogram, in addition to overnight fasting venous blood sample for analysis in a central laboratory. Once enrolled, the subjects were evaluated every 3 months for a total of 4 years. The local institutional review board approved this study, and all participants provided informed consent prior to enrolling in the study.
Predictor & Outcomes Definitions
As previously reported (26), hs-CRP was measured by a high-sensitivity assay (hs-CRP; N High Sensitivity CRP, DADE Behring, Germany). The assay has a lower detection level of 0.175 mg/L and a coefficient of variation of 7.6%; the intra-assay precision ranges from 3.1% for a hs-CRP content of 0.5 mg/L to 4.0% for a CRP content of 15 mg/L; and the interassay precision was 2.5% and 2.6% respectively. hs-CRP was entered into the model as a binary variable with values either less than or greater than the median value (2.1mg/L).
The primary study outcome was a major cardiovascular event defined as a composite of myocardial infarction, stroke, and vascular death. Information about the designated outcomes was obtained from the general practitioners visits (every 3 months as above), AOK database, or the municipality. Additional information was obtained from the hospital records, autopsy records, and death certificates. All fatal and non-fatal events were coded independently by 2 physicians.
CKD defined as creatinine clearance (Ccr) < 60 mL/min (25, 27). Serum creatinine was measured using a kinetic alkaline picrate (Jaffe) method (28). Glomerular filtration rate (GFR) was calculated as Ccr (clearance of creatinine) by the Cockroft-Gault (CG) equation: Creatinine clearance (mL/min) = [(140-Age) × (weight) × (0.85 if female)] / [(72) × (serum creatinine)]. The CG prediction equation has been used in other analyses of the INVADE study (29-30) and has been validated in older European adults (31-32). In addition, in previous publications, the four-variable abbreviated Modification of Diet in Renal Disease (33) had provided similar results and hence is not described further.
Baseline Covariates
The following were obtained from a computerized questionnaire: age, sex, pack years smoking, and prior history of hypertension, diabetes, ischemic heart disease, peripheral artery disease, stroke, and use of aspirin or a statin. History of hypertension was defined as treatment with antihypertensive medications, systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg measured according to JNC VII guidelines. A subject was considered diabetic if they were treated with antidiabetic drugs, if they had an overnight fasting serum glucose ≥ 7.0 mmol/L, or if they had HbA1C >6.0%. History of ischemic heart disease was documented by previous myocardial infarction or angina pectoris, previous bypass surgery, or > 50% angiographic stenosis of ≥ 1 major coronary artery. Stroke was defined as neurological deficit for > 24 hours as evaluated by a neurologist [30].
Serum samples were analyzed in a central laboratory after an overnight fast, and included: low density lipoprotein (LDL)- and high density lipoprotein (HDL)-cholesterol and triglycerides, glycosylated hemoglobin (HbAIc), and homocysteine levels [30].
Statistical Analyses
Baseline characteristics are presented according to CG-GFR quartiles. All values are given as mean (SD), or as median and interquartile range (IQR), or as counts and percentages. χ2 tests, independent t-tests, Mann–Whitney U-tests, and the Spearman rank correlation were applied for univariate analysis, as appropriate. Least square means (95% C.I.) were calculated for hs-CRP and are shown in table 2 by CG-GFR. In order to examine the relationship between hs-CRP with cardiovascular events across kidney function status we defined four (4) groups on the basis of serum hs-CRP levels and CKD status as the interaction between CKD and hs-CRP was significant (P = 0.012): low hs-CRP/non-CKD, high hs-CRP/non-CKD, low hs-CRP/CKD, and high hs-CRP/CKD. hs-CRP levels below the median of 2.1 mg/L were considered low, and > 2.1 mg/L were considered high. CKD was defined as CG-GFR <60 mL/min. Kaplan-Meier survival analysis was used to compute the cumulative probability of reaching a primary endpoint and curves were compared with the log-rank test. The hazard of reaching the study outcome (composite of myocardial infarction, stroke, and vascular death) in study groups compared to group A (reference) was estimated with the Cox proportional hazard model. To evaluate whether measuring hs-CRP conveys additional prognostic value to the traditional cardiovascular risk factors, we calculated the Framingham risk score (FRS), first for all participants then stratifying by CKD status. We calculated the area under the curve (AUC) with FRS alone then adding in hs-CRP. All calculations were performed using the JMP 7.0 software (SAS, Cary, NC, USA). P-values <0.05 were considered to be statistically significant.
Table 2. Least square means (95% C.I.) for hs-CRP levels according to CG-estimated GFR category at the Initiation of the Study.
| Model | CG- estimated GFR in mL/min | P-value for trend |
|||
|---|---|---|---|---|---|
| < 64 (n=749) |
64-75 (n=837) |
75-89 (n=788) |
> 89 (n=792) |
||
|
| |||||
| Unadjusted hs-CRP | 0.45 (0.39,0.52) |
0.38 (0.32,0.45) |
0.35 (0.28,0.41) |
0.34 (0.27,0.40) |
0.018 |
|
| |||||
| Age- adjusted hs- CRP |
0.47 (0.41,0.53) |
0.38 (0.31,0.45) |
0.34 (0.27,0.43) |
0.35 (0.28,0.42) |
0.019 |
|
| |||||
| Fully-adjusted hs- CRP |
0.49 (0.41,0.61) |
0.47 (0.36,0.57) |
0.42 (0.33,0.52) |
0.41 (0.33,0.52) |
0.032 |
Values are expressed as least square means (mg/L) and 95% confidence intervals. Fully adjusted results were adjusted for: age, sex, pack years smoking, history of hypertension, diabetes, ischemic heart disease, and stroke, BMI, systolic and diastolic blood pressure, aspirin and statin use, LDL- and HDL- cholesterol and triglycerides, HbA1c, baseline CG- estimated GFR, and homocysteine levels
Results
Baseline characteristics
Mean hs-CRP and CG-GFR for the participants were 0.40 (SD 0.88) and 77 (SD 21) respectively. Baseline characteristics for the entire population are shown in Table 1. Baseline levels of hs-CRP according to CG-GFR quartile are shown in Table 2. After adjusting for age, sex, pack years smoking, history of hypertension, diabetes, ischemic heart disease, and stroke, BMI, systolic and diastolic blood pressure, aspirin and statin use, LDL- and HDL-cholesterol and triglycerides, HbAIc, baseline CG-GFR, and homocysteine levels, CRP levels increased from 0.41 mg/L (0.33, 0.52) in the highest CG-GFR quartile to 0.49 mg/L (0.41, 0.61) in the lowest CG-GFR quartile (P value for trend 0.032).
Table 1. Baseline Characteristics of Participants According to GFR Quartiles.
| Characteristic | CG- estimated GFR in mL/min | P-value | |||
|---|---|---|---|---|---|
| <64 | 64-75 | 75-89 | > 89 | ||
| (n=749) | (n=837) | (n=788) | (n=792) | ||
|
| |||||
| Age (years) | 76 ± 8 | 70 ± 7 | 67 ± 6 | 65 ± 5 | < 0.0001 |
|
| |||||
| Gender (%) | 0.002 | ||||
| Male | 35 | 41 | 44 | 43 | |
| Female | 65 | 59 | 56 | 57 | |
|
| |||||
| Smoking pack year | 5.3[4.4-6.3] | 7.5[6.4-8.6] | 8.4[7.2-9.6] | 9.4[8.0-10.7] | <0.0001 |
|
| |||||
| Smoking (%) | |||||
| Current | 6 | 9 | 11 | 13 | <0.01 |
| Former | 21 | 26 | 24 | 24 | 0.02 |
| Never | 73 | 65 | 64 | 63 | 0.03 |
|
| |||||
| Diabetes (%) | 25 | 16 | 15 | 16 | 0.01 |
|
| |||||
| Hypertension (%) | 69 | 54 | 53 | 52 | < 0.0001 |
|
| |||||
| History of Cardiovascular Disease (%) | |||||
| Myocardial infarction | 7 | 4 | 4 | 2 | < 0.0001 |
| Ischemic heart disease | 21 | 10 | 10 | 8 | < 0.0001 |
| Stroke | 5 | 2.5 | 3 | 1 | < 0.0001 |
|
| |||||
| Aspirin Use (%) | 34 | 25 | 20 | 19 | < 0.0001 |
|
| |||||
| Statin Use (%) | 17 | 16 | 18 | 14 | 0.2 |
|
| |||||
| Systolic Blood Pressure | 140 ± 2 | 140 ± 1 | 139 ± 2 | 139 ± 1 | 0.5 |
|
| |||||
| Diastolic Blood Pressure | 81 ± 1 | 82 ± 0.7 | 82 ± 0.5 | 83 ± 1 | 0.01 |
|
| |||||
| BMI (kg/m2) | 26.3 ± 0.3 | 27 ± 0.3 | 27.6 ± 0.3 | 30 ± 0.2 | < 0.0001 |
|
| |||||
| Baseline Serum Creatinine (mg/dL) | 1.11 ± 0.03 | 0.9 ± 0.02 | 0.81 ± 0.01 | 0.70 ± 0.02 | < 0.0001 |
|
| |||||
| LDL-C (mg/dL) | 129 ± 3 | 132 ± 2 | 132 ± 3 | 128 ± 2 | 0.14 |
|
| |||||
| HDL- C (mg/dL) | 59 ± 1 | 60 ± 2 | 59 ± 2 | 55 ± 1 | < 0.0001 |
|
| |||||
| Triglycerides (mg/dL) | 140 ± 6 | 136 ± 5 | 142 ± 5 | 157 ± 6 | < 0.0001 |
|
| |||||
| HbA1C (%) | 5.9 ± 0.5 | 5.8 ± 0.1 | 5.8 ± 0.1 | 5.6 ± 0.1 | < 0.0001 |
|
| |||||
| Homocysteine (mg/dL) | 8.5 ± 0.3 | 7.0 ± 0.2 | 6.4 ± 0.2 | 6.3 ± 0.3 | < 0.0001 |
Values are expressed as means ± standard deviation or %=percent of patients; BMI=body mass index; GFR=glomerular filtration rate; hs- CRP= high sensitivity C reactive protein; LDL-C=low density lipoprotein cholesterol; HDL-C= high density lipoprotein cholesterol
High hs-CRP predicts increased risk of vascular events
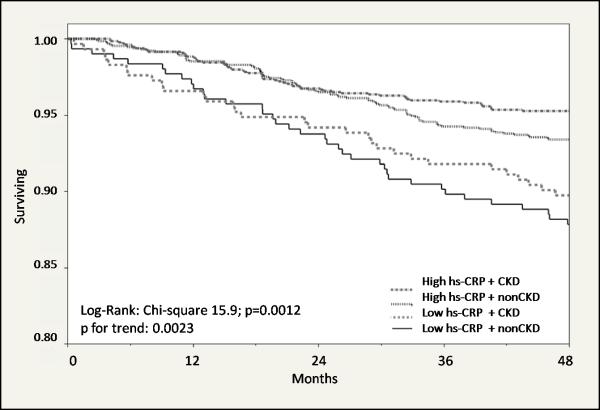
Of the 3166 participants, 724 individuals (23%) had CKD at baseline. The mean CG-GFR in the CKD and non-CKD groups at the initiation of the study was 50± 9 and 83±18 mL/min respectively. After 4 years follow up, 204 (6.4%) individuals experienced a major cardiovascular event. Kaplan-Meier survival curves are presented in Figure 1 and illustrate that the majority of vascular events occurred in subjects with high hs-CRP/CKD. Results of multivariable Cox proportional hazard analysis are presented in Figure 2. After adjusting for important traditional and non-traditional cardiovascular risk factors, the group with high hs-CRP/CKD had the highest risk of a major cardiovascular event; the adjusted hazard ratio (95% CI) for a major cardiovascular event was 1.93 (1.45, 2.89) for the high hs-CRP/CKD group as compared to the reference group (low hs-CRP/no CKD).
Figure 1.
Figure 2.
Using only FRS, the AUC was 0.60 (95% C.I. 0.56, 0.63) for the entire population. After adding CRP, the AUC increased to 0.68 (95% C.I. 0.67, 0.72).The findings in non CKD participants were simiilar: using only FRS the AUC was 0.62 (95% C.I. 0.58, 0.66) and after adding CRP, the AUC increased to 0.67 (95% C.I. 0.63, 0.72).For the CKD participants: the AUC was 0.58 (95% C.I. 0.53, 0.64) using FRS alone, and increased to 0.70 (95% C.I. 0.64, 0.76) after hs-CRP was added.
Discussion
We report that individuals with increased hs-CRP and CKD at baseline are at the highest risk of cardiovascular events after 4 years of follow up in an elderly population in Germany. This association is independent of demographics and other risk factors for cardiovascular disease, such as smoking, hypertension, diabetes, obesity, and dyslipidemia, in addition to prior cardiovascular disease, and use of aspirin or statins.
Evidence to the direct involvement of CRP in the atherosclerotic process primarily stems from in vitro experiments that suggest CRP induces leukocyte activation (34), endothelial cell dysfunction (35-36), and oxidative stress (37). Our observation that hs-CRP levels are increased in individuals with lower GFR is consistent with previous reports in the literature (17, 38). The predictive value of CRP in patients with underlying CKD has been explored in a few cohorts. Consistent with our results high serum levels of CRP were found to be an independent risk factor for cardiovascular mortality in participants of the MDRD study (24). In this analysis, patients with prior history of cardiovascular events were excluded. However, a study of individuals with CKD in Cardiovascular Health Study (CHS) (22) found that traditional risk factors conveyed the majority of the risk of cardiovascular mortality (AUC of 0.73, 95% C.I. 0.70,0.77). Similarly, an examination of Irbesartan Diabetic Nephropathy Trial (IDNT) (21) found CRP was not predictive of cardiovascular events in patients with overt type II diabetic nephropathy. In IDNT, adjusting for prior cardiovascular events alone was sufficient to offset the univariate association between CRP and cardiovascular events. The higher risk factor profile of the participants in these other studies may explain the conflicted findings. In our current analysis, the FRS alone yielded an AUC of 0.58 that increased to 0.7 when hs-CRP was added in the model suggesting that measuring hs-CRP may be of value in certain groups.
Our study has several limitations. Our analysis was conducted on a population of Ebersberg Germany, and although our results are consistent with other studies in the US, our results may not be generalizeable to other populations. Furthermore, the study cohort did not include young or black patients with CKD, hence we are unable to comment on the predictive value of CRP in individuals with either of these demographics. In addition, although prospective, this is an observational study, and the evaluation of inflammation as a therapeutic target for patients with CKD requires interventional studies with novel inflammation-modulating agents.
Notwithstanding these limitations, our results suggest that high hs-CRP levels provide prognostic information in elderly patients with CKD. The role of inflammation in the morbidity and mortality of patients with CKD remains incompletely understood, and prospective studies are needed to evaluate the impact of modulating inflammation on cardiovascular events and mortality in patients with CKD.
Acknowledgements
The work in this study was supported by funds from the following grants: AOK (Allgemeine Ortskrankenkasse), Bavaria, Germany, 1 R01 DK081473-01A1 +1R01DK078112-01A2, and by the Denver Veteran’s Administration Medical Center.
Footnotes
Disclosures: The authors have nothing to disclose
References
- 1.Brantsma AH, Bakker SJ, Hillege HL, et al. Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008;23:3851–8. doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Jurkovitz CT, Pergola PE, et al. Independent components of chronic kidney disease as a cardiovascular risk state: results from the Kidney Early Evaluation Program (KEEP) Arch Intern Med. 2007;167:1122–9. doi: 10.1001/archinte.167.11.1122. [DOI] [PubMed] [Google Scholar]
- 4.McCullough PA, Li S, Jurkovitz CT, et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008;156:277–83. doi: 10.1016/j.ahj.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Weiner DE, Tighiouart H, Stark PC, et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44:198–206. doi: 10.1053/j.ajkd.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–8. [PubMed] [Google Scholar]
- 7.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Jama. 2001;286:421–6. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 8.Whaley-Connell AT, Sowers JR, Stevens LA, et al. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51:S13–20. doi: 10.1053/j.ajkd.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Rao MV, Qiu Y, Wang C, et al. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999-2004. Am J Kidney Dis. 2008;51:S30–7. doi: 10.1053/j.ajkd.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Sarnak MJ, Coronado BE, Greene T, et al. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57:327–35. doi: 10.5414/cnp57327. [DOI] [PubMed] [Google Scholar]
- 11.Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–38. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 12.Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2008;4:672–81. doi: 10.1038/ncpneph0954. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–58. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeun JY, Levine RA, Mantadilok V, et al. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–76. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 15.Iseki K, Tozawa M, Yoshi S, et al. Serum C-reactive protein (CRP) and risk of death in chronic dialysis patients. Nephrol Dial Transplant. 1999;14:1956–60. doi: 10.1093/ndt/14.8.1956. [DOI] [PubMed] [Google Scholar]
- 16.Pecoits-Filho R, Barany P, Lindholm B, et al. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–8. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 18.Landray MJ, Wheeler DC, Lip GY, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43:244–53. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–16. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 20.Knight EL, Rimm EB, Pai JK, et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol. 2004;15:1897–903. doi: 10.1097/01.asn.0000128966.55133.69. [DOI] [PubMed] [Google Scholar]
- 21.Friedman AN, Hunsicker LG, Selhub J, et al. C-reactive protein as a predictor of total arteriosclerotic outcomes in type 2 diabetic nephropathy. Kidney Int. 2005;68:773–8. doi: 10.1111/j.1523-1755.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 22.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. Jama. 2005;293:1737–45. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 23.Weiner DE, Tighiouart H, Elsayed EF, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51:212–23. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–72. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–91. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 26.Sander D, Schulze-Horn C, Bickel H, et al. Combined effects of hemoglobin A1c and C-reactive protein on the progression of subclinical carotid atherosclerosis: the INVADE study. Stroke. 2006;37:351–7. doi: 10.1161/01.STR.0000199034.26345.bc. [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Klein BE, Moss SE, et al. The 10-year incidence of renal insufficiency in people with type 1 diabetes. Diabetes Care. 1999;22:743–51. doi: 10.2337/diacare.22.5.743. [DOI] [PubMed] [Google Scholar]
- 28.Walser M. Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis. 1998;32:23–31. doi: 10.1053/ajkd.1998.v32.pm9669420. [DOI] [PubMed] [Google Scholar]
- 29.Desbien AM, Chonchol M, Gnahn H, et al. Kidney function and progression of carotid intima-media thickness in a community study. Am J Kidney Dis. 2008;51:584–93. doi: 10.1053/j.ajkd.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Chonchol M, Gnahn H, Sander D. Impact of subclinical carotid atherosclerosis on incident chronic kidney disease in the elderly. Nephrol Dial Transplant. 2008;23:2593–8. doi: 10.1093/ndt/gfn021. [DOI] [PubMed] [Google Scholar]
- 31.Pizzarelli F, Lauretani F, Bandinelli S, et al. Predictivity of survival according to different equations for estimating renal function in community-dwelling elderly subjects. Nephrol Dial Transplant. 2009;24:1197–205. doi: 10.1093/ndt/gfn594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannelli SV, Patel KV, Windham BG, et al. Magnitude of underascertainment of impaired kidney function in older adults with normal serum creatinine. J Am Geriatr Soc. 2007;55:816–23. doi: 10.1111/j.1532-5415.2007.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 34.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 35.Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 36.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 37.Qamirani E, Ren Y, Kuo L, et al. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- 38.Muntner P, Hamm LL, Kusek JW, et al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]