Abstract
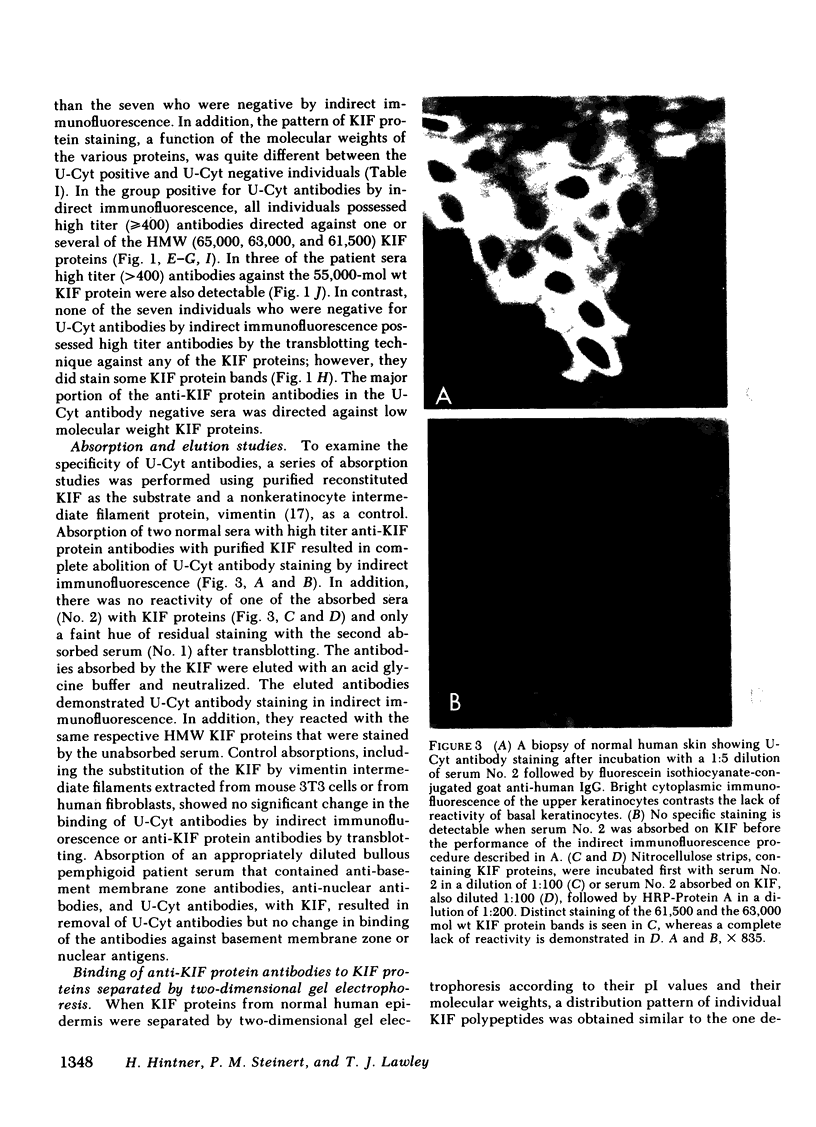
Upper cytoplasmic (U-Cyt) antibodies are directed against cytoplasmic antigens found in keratinocytes in the upper layers of the epidermis. Until now, they have been defined by indirect immunofluorescence and are known to occur in the sera of patients with cutaneous diseases such as bullous dermatoses, basal cell carcinomas, and melanomas. An increased incidence of U-Cyt antibodies has also been reported in the sera of patients with noncutaneous diseases, such as pulmonary neoplasms. They have been found in addition in the sera of some normal individuals. In this study we have identified keratin intermediate filaments (KIF) as antigens U-Cyt antibodies are directed against. KIF proteins were prepared, separated by polyacrylamide gel electrophoresis, transblotted to nitro-cellulose strips, and used as substrates for antibody binding. Sera containing U-Cyt antibodies by indirect immunofluorescence also had antibodies that were directed against high molecular weight (65,000, 63,000, 61,500) KIF proteins. When KIF proteins were separated according to their charge and their molecular weight by two-dimensional gel electrophoresis and transblotted, the anti-KIF protein antibodies bound to virtually all charge isomers of the KIF proteins at the respective molecular weight. The antibody titers measured using the transblotting technique were 10 to 160 times higher than those found by indirect immunofluorescence. To determine whether U-Cyt antibodies were directed against KIF, a series of absorption and elution experiments were performed. Absorption of test sera with purified KIF removed both U-Cyt antibodies and anti-KIF protein antibodies. Absorption with another type of intermediate filament derived from fibroblasts, vimentin, did not remove U-Cyt or anti-KIF protein antibodies. Absorbed U-Cyt and anti-KIF protein antibodies were both eluted from the same KIF preparation and shown to bind to U-Cyt antigens by indirect immunofluorescence and KIF proteins by transblotting. Absorption of a serum containing U-Cyt antibodies, anti-nuclear antibodies, and anti-basement membrane zone antibodies with purified KIF resulted in the removal of the U-Cyt antibodies but not the other types of antibody. In addition, all test sera, even those that lacked U-Cyt antibodies, were found to have low-titer antibodies against KIF proteins by the transblotting technique. These data indicate that KIF proteins bear antigens to which U-Cyt antibodies are directed and that low titer antibodies against KIF proteins may be much more common than previously appreciated.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel E. A., Bystryn J. C. Epidermal cytoplasmic antibodies: incidence and type in normal persons and patients with melanoma. J Invest Dermatol. 1976 Jan;66(1):44–48. doi: 10.1111/1523-1747.ep12478094. [DOI] [PubMed] [Google Scholar]
- Bladon P. T., Bowden P. E., Cunliffe W. J., Wood E. J. Prekeratin biosynthesis in human scalp epidermis. Biochem J. 1982 Oct 15;208(1):179–187. doi: 10.1042/bj2080179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burnham T. K. Two epidermal cytoplasmic immunofluorescent patterns detected by indirect immunofluorescence. J Invest Dermatol. 1974 Feb;62(2):100–105. doi: 10.1111/1523-1747.ep12692235. [DOI] [PubMed] [Google Scholar]
- Bystryn J. C., Nash M., Robins P. Epidermal cytoplasmic antigens: II. Concurrent presence of antigens of different specificities in normal human skin. J Invest Dermatol. 1978 Aug;71(2):110–113. doi: 10.1111/1523-1747.ep12546150. [DOI] [PubMed] [Google Scholar]
- Dellagi K., Brouet J. C., Perreau J., Paulin D. Human monoclonal IgM with autoantibody activity against intermediate filaments. Proc Natl Acad Sci U S A. 1982 Jan;79(2):446–450. doi: 10.1073/pnas.79.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M., Eisinger M., Bystryn J. C. Decreased expression of epidermal cytoplasmic antigens in cultured human keratinocytes. J Invest Dermatol. 1981 May;76(5):347–351. doi: 10.1111/1523-1747.ep12520003. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Gilmartin M. E., Culbertson V. B., Freedberg I. M. Phosphorylation of epidermal keratins. J Invest Dermatol. 1980 Sep;75(3):211–216. doi: 10.1111/1523-1747.ep12522887. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hintner H., Stingl G., Schuler G., Wolff K. In vitro complement-binding on cytoplasmic structures in normal human skin: I. Immunofluorescence studies. J Invest Dermatol. 1982 Aug;79(2):119–124. doi: 10.1111/1523-1747.ep12500038. [DOI] [PubMed] [Google Scholar]
- Janin-Mercier A., Saurat J. H., Didierjean L. Epidermal cytoplasmic antibodies as detected by hrp-labelled antisera. Arch Dermatol Res. 1978 Jul 28;262(2):235–238. doi: 10.1007/BF00455395. [DOI] [PubMed] [Google Scholar]
- Kurki P., Virtanen I., Stenman S., Linder E. Characterization of human smooth muscle autoantibodies reacting with cytoplasmic intermediate filaments. Clin Immunol Immunopathol. 1978 Dec;11(4):379–387. doi: 10.1016/0090-1229(78)90165-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder E., Hormia M., Lehto V. P., Törnroth T. Identification of cytoskeletal intermediate filaments of vascular endothelial cells as targets for autoantibodies in patient sera. Clin Immunol Immunopathol. 1981 Nov;21(2):217–227. doi: 10.1016/0090-1229(81)90210-5. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluch E. P., Bloch K. J. Antibodies to human epidermal cytoplasmic antigens: incidence, patterns, and titers. J Invest Dermatol. 1982 Aug;79(2):115–118. doi: 10.1111/1523-1747.ep12500037. [DOI] [PubMed] [Google Scholar]
- Pedersen J. S., Toh B. H., Locarnini S. A., Gust I. D., Shyamala G. N. Autoantibody to intermediate filaments in viral hepatitis. Clin Immunol Immunopathol. 1981 Nov;21(2):154–161. doi: 10.1016/0090-1229(81)90204-x. [DOI] [PubMed] [Google Scholar]
- Scaletta L. J., MacCallum D. K. A fine structural study of divalent cation-mediated epithelial union with connective tissue in human oral mucosa. Am J Anat. 1972 Apr;133(4):431–453. doi: 10.1002/aja.1001330406. [DOI] [PubMed] [Google Scholar]
- Senecal J. L., Rothfield N. F., Oliver J. M. Immunoglobulin M autoantibody to vimentin intermediate filaments. J Clin Invest. 1982 Mar;69(3):716–721. doi: 10.1172/JCI110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo J., Gibbs C. J., Jr, Gajdusek D. C. Autoantibodies against axonal neurofilaments in patients with Kuru and Creutzfeldt-Jakob disease. Science. 1980 Oct 10;210(4466):190–193. doi: 10.1126/science.6997994. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Cabral F., Gottesman M. M., Goldman R. D. In vitro assembly of homopolymer and copolymer filaments from intermediate filament subunits of muscle and fibroblastic cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3692–3696. doi: 10.1073/pnas.78.6.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Peck G. L., Idler W. W. Structural changes of human epidermal alpha-keratin in disorders of keratinization. Curr Probl Dermatol. 1980;10:391–406. doi: 10.1159/000396303. [DOI] [PubMed] [Google Scholar]
- Steinert P., Zackroff R., Aynardi-Whitman M., Goldman R. D. Isolation and characterization of intermediate filaments. Methods Cell Biol. 1982;24:399–419. doi: 10.1016/s0091-679x(08)60667-6. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Yildiz A., Sotelo J., Osung O., Holborow E. J., Kanakoudi F., Small J. V. Viral infections and IgM autoantibodies to cytoplasmic intermediate filaments. Clin Exp Immunol. 1979 Jul;37(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]