Abstract
Purpose
To test the hypothesis that interleukin (IL)-6 prevents photoreceptor cell death during periods of retinal separation from the retinal pigment epithelium (RPE).
Methods
Retinal-RPE separation was created in wild-type C57BL mice, IL-6−/− mice, and Brown Norway rats by subretinal injection of 1% hyaluronic acid. In some animals, anti-IL-6 neutralizing antibody (NAB) or exogenous IL-6 was administered into the subretinal space at the time of separation or at specified times afterward. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was performed 3 days after separation to detect apoptotic photoreceptors. Photoreceptor cell counts were performed after 1 and 2 months.
Results
Loss of IL-6 function through genetic ablation (IL-6−/− mice) or injection of anti–IL-6 NAB resulted in significantly increased levels of TUNEL-positive staining 3 days after retinal–RPE separation. One month after separation, outer nuclear layer (ONL) cell counts were significantly lower in IL-6−/− mice or in animals injected with anti–IL-6 NAB than in controls. Gain of IL-6 function through the addition of exogenous IL-6 resulted in significantly increased ONL counts at 1 month but not at 2 months. Reinjection of IL-6 at 1 month led to continued preservation of ONL counts compared with controls. A window of opportunity for treatment was detected because delaying injection of exogenous IL-6 to 2 weeks after retinal–RPE separation still resulted in significantly greater ONL cell counts compared with controls.
Conclusions
IL-6 may serve as a photoreceptor neuroprotectant in the setting of retinal–RPE separation.
Retinal detachment (RD), defined as the separation of the neurosensory retina from subjacent retinal pigment epithelium (RPE), results in the apoptotic death of photoreceptor cells.1-4 Rodent and feline models of RD have demonstrated the activation of proapoptotic pathways nearly immediately after the retina becomes separated from the RPE.1-4 Histologic markers of apoptosis, such as terminal deoxynucleotidyl transferase nick-end label (TUNEL) staining, reach a peak at approximately 3 days after RD, with apoptotic activity and progressive cell death persisting for the duration of the detachment period. Clinical experience in the repair of retinal detachments, however, has demonstrated that there is a window of opportunity for repair with preservation of good visual acuity. Retrospective case series have demonstrated that significant numbers of patients with macula-off RDs repaired within 5 to 10 days after onset of detachment can retain relatively good visual function but that visual acuity drops significantly as the time between detachment and repair extends.5-7 The delayed time between the activation of proapoptosis pathways and the clinical onset of visual loss suggests that intrinsic neuroprotective factors may become activated within the neural retina and may serve to counterbalance the effects of the proapoptotic pathways activated by retinal–RPE separation.
Previous work in our laboratory using gene microarray analysis of experimental detachment in rats revealed the increased expression of genes involved in stress–response pathways.8 Of particular interest was the increased transcription and translation of interleukin (IL)-6 and downstream components of its associated signal transduction pathway. IL-6 is a pleiotropic cytokine with a role in inflammation, hematopoiesis, angiogenesis, cell differentiation, and neuronal survival.9-11 In the central nervous system, IL-6 is synthesized by microglia, astrocytes, and neurons.12,13 In the retina, IL-6 is synthesized by Müller cells and the RPE.14,15 A neuroprotective role for IL-6 has been previously suggested in different models of ocular injury. In rat models of retinal ischemia–reperfusion injury, IL-6 protein levels are upregulated within 8 hours after injury.16 Furthermore, intravitreal injection of exogenous IL-6 immediately after ischemia–reperfusion injury or before N-methyl-d-aspartate (NMDA)-induced toxicity increases survival of retinal ganglion cells.16,17 In vitro studies have shown that IL-6 increases the duration of rat retinal ganglion cell survival in primary tissue culture.18,19 Collectively, these data suggest that IL-6 upregulation after injury may serve to function as a neuronal survival factor.
The goal of this study is to test the hypothesis that IL-6 functions as an inhibitor of photoreceptor apoptosis after RD. Understanding the effect of IL-6 on photoreceptor survival may provide insight into potential therapeutic strategies for protecting photoreceptors during periods of photoreceptor–RPE separation.
Methods
Experimental Model of Retinal Detachment
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines established by the University Committee on Use and Care of Animals of the University of Michigan. Detachments were created in adult male Brown-Norway rats (300–400 g; Charles River Laboratories, Wilmington, MA), wild-type C57BL mice (age 3–6 weeks; Jackson Laboratory, Bar Harbor, ME), and IL-6−/− mice on a C57BL background (age 3–6 weeks; Jackson Laboratory), as previously described.20 Briefly, rodents were anesthetized with a 50:50 mix of ketamine (100 mg/mL) and xylazine (20 mg/mL), and pupils were dilated with topical phenylephrine (2.5%) and tropicamide (1%). A 20-gauge microvitreoretinal blade (Walcott Scientific, Marmora, NJ) was used to create a sclerotomy 2 mm posterior to the limbus, carefully avoiding lens damage. A Glaser subretinal injector (32-gauge tip; BD Ophthalmic Systems, Sarasota, FL) was introduced through the sclerotomy into the vitreous cavity and then through a peripheral retinotomy into the subretinal space. Sodium hyaluronate (10 mg/mL; Pharmacia and Upjohn Co., Kalamazoo, MI) was slowly injected to detach the neurosensory retina from the underlying RPE. In all experiments, approximately one third to one half of the neurosensory retina was detached. Detachments were created in the left eye, leaving the right eye as the control. In some eyes, either 0.15 μg anti–human IL-6 neutralizing antibody (NAB; R&D Systems, Minneapolis, MN) or 15 ng exogenous human IL-6 (R&D Systems) was injected into the subretinal space of the detachment in a 10-μL volume at the time of creation of the detachment or at a subsequent time point. Doses were based on manufacturers’ recommendations based on in vitro activity assays.
Western Blot Analysis
Retinas from experimental eyes with detachments and control eyes without detachments were dissected from the RPE–choroid at 3 days after retinal detachment, homogenized, and lysed in buffer containing 10 mM HEPES (pH 7.6), 0.5% IgEPal, 42 mM KCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol (DTT), 5 mM MgCl2, and 1 tablet of protease inhibitors per 10 mL buffer (Complete Mini; Roche Diagnostics GmbH, Mannheim, Germany). The homogenates were incubated on ice and centrifuged at 22,000g at 4°C for 60 minutes. The protein concentration of the supernatant was then determined (DC Protein Assay kit; Bio-Rad Laboratories, Hercules CA). Protein samples were loaded and run on SDS-polyacrylamide gels (Tris-HCl Ready Gels; Bio-Rad Laboratories). After electrophoretic separation, the proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P; Amersham Pharmacia Biotech, Piscataway, NJ). Protein bands were visualized with Ponceau S staining, and the lanes were assessed for equal loading by densitometry of a nonspecific band present across all lanes. Membranes were then immunoblotted for phospho-STAT1 (Tyr701) or phospho-STAT3 (Tyr705) using an immunoblotting kit (PhosphoPlus Stat1 [Tyr701] Antibody Kit 9170 or PhosphoPlus Stat3 [Tyr705] Antibody Kit 9130, respectively; Cell Signaling Technology, Danvers, MA) according to the manufacturer’s instructions using a 1:1000 dilution of the primary antibody.
TUNEL Staining and Histology
At varying intervals after creation of the detachment, the animals were humanely killed, and the eyes were enucleated. For TUNEL staining, whole eyes were fixed overnight at 4°C in phosphate-buffered saline with 4% paraformaldehyde (pH 7.4). The specimens were embedded in paraffin and sectioned at a thickness of 5 to 6 μm. TUNEL staining was performed on the sections with a fluorescein in situ apoptosis detection kit (ApopTag; Millipore, Billerica, MA) according to the manufacturer’s instructions. For light microscopic analysis, paraffin sections were stained with 0.5% toluidine blue in 0.1% borate buffer.
Data Analysis
Photoreceptor cell apoptosis was quantified as the percentage of total cells in the outer nuclear layer (ONL) that was TUNEL positive. Three nonoverlapping high-power fields (40×) at the maximal height of the RD were selected per section and were averaged unless there were fewer than three nonoverlapping high-power fields, in which case fewer fields were used. One representative section was used per eye. The total number of cells in the ONL was measured in a similar fashion. The total thickness of the retina (measured from the outer edge of the ONL to the inner limiting membrane) was measured in three places in each of three nonoverlapping high-power fields (40×) at the maximal height of the RD per section and was averaged for each eye. Photoreceptor inner and outer segments were not included in the total retinal thickness measurement given the variable retraction of these elements after detachment of the neurosensory retina, which does not necessarily correlate with post-reattachment viability of the photoreceptors.21,22 For toluidine blue–stained specimens, normalization of ONL cell count to the total retinal thickness of each section (i.e., ONL cell count divided by total retinal thickness) was performed to account for possible differences in angles of sectioning and to allow for intersample comparison. ONL cell counts and total retinal thicknesses in each group of the rat experiments were also normalized to corresponding values of attached retinas in that group to allow intersample comparison. For each experimental group, measurements were performed on three sections from four to 11 eyes, each eye from a separate animal.
Statistical analysis comparing percentages of TUNEL-positive cells in the ONL between groups and comparing the ONL cell count/total retinal thickness ratio between groups was performed using two-tailed Student’s t-tests without assuming equal variance. Differences were considered significant at P ≤ 0.05.
RESULTS
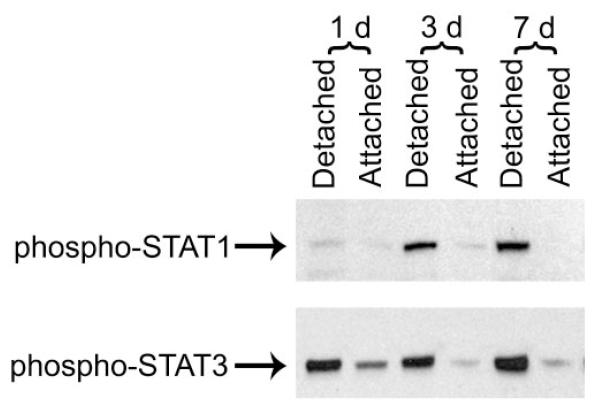
The immediately downstream transducers of IL-6 receptor signaling are signal transducers and activators of transcription (STATs).10,23 Our previous work has shown that in the context of retinal–RPE separation, STAT1 and STAT3 transcript and protein levels are increased.8 Figure 1 demonstrates increased phosphorylation (i.e., activation) of STAT1 and STAT3 in detached retinas compared with attached retinas. Injection of the IL-6–neutralizing antibody into the subretinal space at the time of detachment resulted in approximately a 50% reduction in the level of phosphorylated STAT3. There was not, however, any reduction in the level of phosphorylated STAT1. These data suggest that after retinal–RPE separation, the IL-6 effect is mediated predominantly through STAT3 but not STAT1.
Figure 1.
Western blot analysis of levels of activated forms of STAT1 and STAT3 in attached and detached retinas. Leftmost 2 lanes: one day after detachment. Middle 2 lanes: three days after detachment. Right-most 2 lanes: seven days after detachment. Retina–RPE separation was created in the left eye. Attached retina was obtained from the contralateral eye of the same animal. Equal loading was verified across all lanes.
To further define the role of IL-6 in controlling photoreceptor apoptosis after RD, retinal–RPE separation was created in wild-type C57BL and IL-6−/− mice. Three days after detachment, the eyes were harvested, and apoptosis within the retina was evaluated with TUNEL staining. TUNEL-positive cells were confined to the ONL of photoreceptors, consistent with earlier studies of experimental RD.3 The percentage of TUNEL-positive cells in the ONLs of detached retinas was significantly greater in the IL-6−/− mice than in the wild-type mice (P = 0.002; Fig. 2).
Figure 2.
TUNEL staining in wild-type versus IL6−/− mouse retinas harvested 3 days after detachment. (A, B) Wild-type mice. (C, D) IL-6−/− mice. (A, C) Fluorescein isothiocyanate (FITC) fluorescence of TUNEL-positive cells. (B, D) Propidium iodide (PI) fluorescence of all nuclei. (E) Graph summarizing TUNEL staining of wild-type and IL-6−/− mouse retinas 3 days after detachment. Results are mean ± SEM. *P = 0.002; IL-6−/− mice compared with wild-type mice.
To investigate the effect of genetic ablation of IL-6 on longer term changes in retinal morphology after RD, detachments in wild-type and IL-6−/− mice were created and maintained for 1 and 2 months. Eyes were then harvested for toluidine blue staining. The ONL cell count/total retinal thickness ratio was similar between attached retinas of wild-type mice and attached retinas of IL-6−/− mice (Fig. 3). There was a decline in the normalized ONL cell count at the 1-month time point for wild-type and IL-6−/− mice compared with attached retinas at time 0, but the rate of photoreceptor cell death was significantly higher in the IL-6−/− mice (P = 0.02; Fig. 3). Two months after detachment, there was further decline in the ONL cell count/total retinal thickness ratio. The IL-6−/− group had lower final ONL cell counts than wild-type animals, but the difference did not reach significance at this time (P = 0.10; Fig. 3).
Figure 3.
Outer nuclear layer cell counts in wild-type versus IL6−/− mice after retinal detachment. (A–C) Wild-type mice. (D–F) IL-6−/− mice. (A, D) Attached retinas. (B, E) Retinas harvested 1 month after creation of the detachment. (C, F) Retinas harvested 2 months after creation of the detachment. (G) Graph summarizing ONL cell counts/total retinal thickness in wild-type and IL-6−/− mice 1 and 2 months after retinal detachment. Results are mean ± SEM. *P = 0.02; IL-6−/− mice compared with wild-type mice.
To further support the data from IL-6−/− mice that abrogating IL-6 activity increases photoreceptor loss after RD, retinal–RPE separation was created in Brown Norway rats, and either vehicle only or vehicle plus 0.15 μg anti–human IL-6 NAB was injected subretinally at the time of detachment. TUNEL staining of rat eyes 3 days after detachment revealed significantly higher percentages of TUNEL-positive cells in the ONLs of retinas treated with anti–IL-6 NAB compared with those treated with vehicle only (P = 0.01; Fig. 4A–D, G). To determine whether administering exogenous IL-6 would reduce the peak of TUNEL-positive staining seen 3 days after RD, 15 ng recombinant IL-6 was injected subretinally at the time of creation of the detachment. The percentage of TUNEL-positive cells in the group of rats treated with subretinal exogenous IL-6 was no different from that of rats treated with vehicle alone, but it was still significantly less than that of rats treated with subretinal anti–IL-6 NAB (P = 0.01; Fig. 4).
Figure 4.
TUNEL staining of detached rat retinas treated with IL-6–neutralizing antibody or exogenous IL-6. (A, B) Subretinal injection of vehicle only at the time of creation of the detachment. (C, D) Subretinal injection of 0.15 μg anti–IL-6 NAB at the time of creation of the detachment. (E, F) Subretinal injection of 15 ng exogenous IL-6 at the time of creation of the detachment. (A, C, E) FITC fluorescence of TUNEL-positive nuclei. (B, D, F) Propidium iodide (PI) fluorescence of all nuclei. (G) Graph summarizing effects of subretinal anti–IL-6 NAB and exogenous IL-6 on TUNEL staining of rat retinas 3 days after detachment. Results are mean ± SEM. *P = 0.01; anti–IL-6 NAB versus control and anti–IL-6 NAB versus exogenous IL-6.
When the period of retinal–RPE separation was extended to 4 and 8 weeks, subretinal injection of anti–IL-6 NAB at the time of detachment resulted in significantly lower normalized ONL cell counts 4 weeks after detachment compared to subretinal injection of vehicle only (P = 0.001; Figs. 5B, 5D, 5H). In contrast, subretinal administration of exogenous IL-6 at the time of detachment resulted in significantly higher ONL cell count/total retinal thickness ratios 4 weeks after detachment compared to controls injected with vehicle only (P = 0.05; Figs. 5B, 5F, 5H). Subretinal administration of exogenous IL-6 appeared to slow the rate of photoreceptor cell loss during the first month after detachment, but the rate accelerated during the second month after detachment such that the protective benefit of exogenous IL-6 was lost. ONL cell count/total retinal thickness ratios were similar between groups treated with vehicle only, exogenous IL-6, and anti–IL-6 NAB 8 weeks after detachment (Figs. 5C, 5E, 5G, 5H).
Figure 5.
Effects of IL-6–neutralizing antibody compared with effects of exogenous IL-6 on rat retina ONL cell counts. (A) Attached retina. (B, C) Retina harvested 1 and 2 months after subretinal injection of vehicle only at the time of creation of the detachment, respectively. (D, E) Retina harvested 1 and 2 months after subretinal injection of 0.15 μg anti–IL-6 NAB at the time of creation of the detachment, respectively. (F, G) Retina harvested 1 and 2 months after subretinal injection of 15 ng exogenous IL-6 at the time of creation of the detachment, respectively. (H) Graph summarizing effects of subretinal anti–IL-6 NAB and exogenous IL-6 on ONL cell count of rat retinas 1 and 2 months after retinal detachment. Results are mean ± SEM. *P = 0.001; control versus anti–IL-6 NAB. †P = 0.05; control versus exogenous IL-6. ‡P = 0.02; control versus delayed exogenous IL-6. §P = 0.02; control versus exogenous IL-6 reinjection at 1 month. The apparent increase in cell counts between 4 and 8 weeks for animals treated with exogenous IL-6 is within the accepted variability and did not reach statistical significance.
It was hypothesized that the protective effect of exogenous IL-6 seen at 4 weeks could be extended by a second subretinal injection of exogenous IL-6 at that time. To test this hypothesis, rats were injected with exogenous IL-6 at the time of creation of the detachment followed by a second injection of the same dose of exogenous IL-6 at 4 weeks after detachment. At 8 weeks after creation of the RD, the ONL cell count/total retinal thickness was still significantly higher in animals with repeat IL-6 injection at 4 weeks than in control animals (P = 0.02; Fig. 5H).
Clinically, there is often a delay from the occurrence of the detachment to the onset of symptoms to the diagnosis and treatment by the ophthalmologist. We sought to determine whether delaying subretinal administration of IL-6 by 2 weeks after the creation of the detachment could still prevent or delay photoreceptor apoptosis. This was tested by creating the retinal–RPE separation according to the usual protocol, followed, 2 weeks after creation of the RD, by the injection of exogenous IL-6. Eyes were harvested 4, 6, and 8 weeks after detachment (i.e., 2, 4, and 6 weeks after subretinal IL-6 injection, respectively) and stained with toluidine blue. The ONL cell count/total retinal thickness was minimally lower in the group, in which IL-6 injection was delayed 2 weeks compared with the group in which IL-6 injection was administered at the time of creation of the detachment (P = 0.70), and it was significantly higher than in control animals (P = 0.02; Fig. 5H). As with the animals treated with IL-6 at the time of retinal–RPE separation, the effect of delayed IL-6 injection seemed to diminish after the 4-week postdetachment time point, with the number of ONL cells reaching the control values by 8 weeks after detachment.
DISCUSSION
Our results strongly support a role for IL-6 as a controller of photoreceptor apoptosis after separation of the neurosensory retina from the underlying RPE. Inhibition of IL-6 with genetic ablation or through administration of an IL-6–neutralizing antibody significantly increased the rate of photoreceptor apoptosis. One month after detachment, both the IL-6−/− mice and the rats receiving subretinal IL-6 NAB had significantly decreased ONL cell counts compared with their respective controls. These data support our hypothesis that IL-6 is necessary for the survival of photoreceptors after detachment from the RPE.
Experiments looking at the effect of increasing IL-6 levels had mixed effects, depending on the time point examined and the assay measured. When compared with injection of vehicle only, subretinal injection of exogenous IL-6 did not significantly affect the percentage of TUNEL-positive cells 3 days after RD but did significantly increase the number of photoreceptors that survived longer term detachments. A possible explanation for this difference is that the dose of exogenous IL-6 might have been insufficient to prevent apoptosis 3 days after creation of the detachment, but RD may cause surrounding cells, such as Müller cells and RPE cells, to express IL-6 in a delayed fashion, which, in addition to the exogenous IL-6, is enough to inhibit apoptosis in the longer term. It has been shown that subretinal IL-6 levels in patients with RD are higher than in the vitreous of controls,24 and the amount of subretinal IL-6 in patients with RD is highest 5 to 8 weeks after RD.25 Another possible explanation is that a cofactor may need time to be upregulated to maximally transduce IL-6 signaling. IL-6 signals through a combination of its ligand-binding subunit (gp80, also known as IL-6R) and a common signal-transducing subunit (gp130).26 Although we administered very high doses of exogenous IL-6, perhaps there was not enough gp80 or gp130 to transduce the signal, and there might have been a delay in upregulation such that maximal IL-6 signal transduction might not have been seen 3 days after RD but was manifest 1 month after RD. Some studies have shown that the administration of exogenous IL-6 alone does not have as much antiapoptotic activity as the administration of IL-6 in conjunction with a soluble form of IL6-R.17,27 The time to upregulation of gp80 and gp130 after retinal detachment is unknown.
Despite the activation of endogenous IL-6, there was still a relatively linear rate of cell loss. Our results demonstrate that the rate of this death can be significantly decreased by the addition of exogenous IL-6. Correlating this to clinical outcomes of retinal detachment repair described in the literature, in which a significant portion of patients with prolonged detachment do not achieve visual acuity greater than 20/40,5-7 our data suggest that adding exogenous IL-6 might serve to increase that percentage. We do not know whether treatment with exogenous IL-6 is sufficient to preserve visual acuity, but certainly the presence of an increased number of photoreceptor cells appears to be a requisite starting point for improved outcomes.
Although there was significantly greater preservation of photoreceptors in the group of rats treated with exogenous IL-6 at the time of detachment than in the control group 1 month after RD, the difference was lost 2 months after RD because of accelerated photoreceptor loss in the exogenous IL-6 group in the second month. Reinjection of exogenous IL-6 at 1 month suggested that the duration of the effect of IL-6 could be extended, perhaps allowing for a greater therapeutic “window of opportunity” to achieve retinal–RPE reattachment. The half-life of IL-6 in the subretinal space was uncertain in our experiments. Further work is necessary to clarify the optimal dosing amount and frequency to achieve continued preservation of photoreceptors. This could be most useful when retinal–RPE separation might continue for extended periods of time, such as in neovascular age-related macular degeneration or tractional retinal detachment caused by diabetes or proliferative vitreoretinopathy.
Patients often have retinal detachments for an unknown duration of time before they seek care from an ophthalmologist. In our model, delay of the initial subretinal injection of exogenous IL-6 by 2 weeks from the creation of the detachment still allowed significantly greater preservation of photoreceptors 1 month after detachment compared with controls, suggesting that IL-6 may still be useful in preserving photoreceptors despite the delayed presentation of patients. Additional studies to evaluate the time window of effectiveness of subretinal IL-6 administration are needed. Interestingly, the effect of a single IL-6 injection 2 weeks after retinal-RPE separation only lasted for 2 weeks, not 4 weeks as might have been expected from the results seen with IL-6 injection at the time of creation of the detachment. This suggests that other controllers of photoreceptor apoptosis may override the effect of a single IL-6 injection as the duration of detachment lengthens.
With regard to the mechanism by which IL-6 acts as an antiapoptotic factor, IL-6 is known to be a strong activator of the Janus kinase (JAK)/STAT pathway.10,23 In our model, retinal–RPE separation potently activates STAT1 and STAT3. In general, STAT1 is associated with tumor suppression and proapoptotic activity, whereas STAT3 is predominantly associated with cellular proliferation and is considered to be antiapoptotic.23,28-31 Our data suggest that the IL-6 effect is mediated predominantly through STAT3 and not STAT1. Modulation of apoptotic pathways by STATs may be through the downstream transcriptional regulation of factors that trigger cell death, such as FAS and caspases, or factors that promote cell survival, such as Bcl-xL and FLICE (FADD (Fas-associated death domain)-like interleukin-1β-converting enzyme) inhibitory protein (FLIP).31,32 FLIP is an enzymatically inactive homologue of caspase-8 that can compete with caspase-8 for recruitment to death-inducing signaling complexes (DISCs) and thus acts as a dominant-negative inhibitor of apoptosis.33 IL-6 may stabilize protein levels of FLIP because FLIP is more rapidly degraded in IL-6−/− mice.34 We cannot conclusively state, based on our data, whether IL-6 acted directly on the photoreceptors or secondarily through an effect on a second cell type. Further work is required to define the IL-6 source and target cells.
Studies have shown that IL-6 may protect against retinal degeneration induced by a range of insults, including ischemia–reperfusion injury,16 NMDA toxicity,17 pressure-induced death,19 and, in our study, retinal detachment. The therapeutic potential of IL-6 is tempered by evidence suggesting that elevated levels of IL-6 in the vitreous have been documented in proliferative vitreoretinopathy,35 inflammation,36 diabetic and branch retinal vein occlusion-related macular edema,37,38 and proliferative diabetic eye disease.39 IL-6 has also been implicated in ocular neovascularization.11,40 It is unclear, however, whether elevated IL-6 in these conditions is the cause of pathology or whether IL-6 is upregulated in response to the retinal disease. Our data provide compelling proof-of-principle for the photoreceptor-protective role of IL-6 in the context of retinal–RPE separation and suggest that this may be a valuable point of therapeutic intervention for improving the visual outcome in patients with this type of retinal injury.
Acknowledgments
Supported by National Eye Institute Grant K08-124-14705 (DNZ), the International Retinal Research Foundation, Inc. (DNZ), Research to Prevent Blindness, Inc. (DNZ), and Departmental Core Center for Vision Research Grant EY-07003.
Footnotes
Disclosure: D.Y. Chong, None; C.S. Boehlke, None; Q.-D. Zheng, None; L. Zhang, None; Y. Han, None; D.N. Zacks, None
References
- 1.Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36(6):990–996. [PubMed] [Google Scholar]
- 2.Hisatomi T, Sakamoto T, Goto Y, et al. Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr Eye Res. 2002;24(3):161–172. doi: 10.1076/ceyr.24.3.161.8305. [DOI] [PubMed] [Google Scholar]
- 3.Zacks DN, Hanninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller JW. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2003;44(3):1262–1267. doi: 10.1167/iovs.02-0492. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Bula D, Arroyo JG, Chen DF. Preventing retinal detachment-associated photoreceptor cell loss in Bax-deficient mice. Invest Ophthalmol Vis Sci. 2004;45(2):648–654. doi: 10.1167/iovs.03-0827. [DOI] [PubMed] [Google Scholar]
- 5.Burton TC. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc. 1982;80:475–497. [PMC free article] [PubMed] [Google Scholar]
- 6.Ross WH, Kozy HW. Visual recovery in macula-off rhegmatogenous retinal detachments. Ophthalmology. 1998;105(11):2149–2153. doi: 10.1016/S0161-6420(98)91142-3. [DOI] [PubMed] [Google Scholar]
- 7.Hassan TS, Sarrafizadeh R, Ruby AJ, Garretson BR, Kuczynski B, Williams GA. The effect of duration of macular detachment on results after the scleral buckle repair of primary, macula-off retinal detachments. Ophthalmology. 2002;109(1):146–152. doi: 10.1016/s0161-6420(01)00886-7. [DOI] [PubMed] [Google Scholar]
- 8.Zacks DN, Han Y, Zeng Y, Swaroop A. Activation of signaling pathways and stress-response genes in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2006;47(4):1691–1695. doi: 10.1167/iovs.05-1209. [DOI] [PubMed] [Google Scholar]
- 9.John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9(1):10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebrahem Q, Minamoto A, Hoppe G, Anand-Apte B, Sears JE. Triamcinolone acetonide inhibits IL-6- and VEGF-induced angiogenesis downstream of the IL-6 and VEGF receptors. Invest Ophthalmol Vis Sci. 2006;47(11):4935–4941. doi: 10.1167/iovs.05-1651. [DOI] [PubMed] [Google Scholar]
- 12.Gadient RA, Otten UH. Interleukin-6 (IL-6)–a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52(5):379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 13.Schobitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur J Neurosci. 1993;5(11):1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Sotozono C, Ikeda T, Kinoshita S. Interleukin-6 (IL-6) production by cytokine-stimulated human Muller cells. Curr Eye Res. 2001;22(5):341–347. doi: 10.1076/ceyr.22.5.341.5498. [DOI] [PubMed] [Google Scholar]
- 15.Benson MT, Shepherd L, Rees RC, Rennie IG. Production of interleukin-6 by human retinal pigment epithelium in vitro and its regulation by other cytokines. Curr Eye Res. 1992;11(suppl):173–179. doi: 10.3109/02713689208999529. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez RN, Chan CK, Garg S, et al. Interleukin-6 in retinal ischemia reperfusion injury in rats. Invest Ophthalmol Vis Sci. 2003;44(9):4006–4011. doi: 10.1167/iovs.03-0040. [DOI] [PubMed] [Google Scholar]
- 17.Inomata Y, Hirata A, Yonemura N, Koga T, Kido N, Tanihara H. Neuroprotective effects of interleukin-6 on NMDA-induced rat retinal damage. Biochem Biophys Res Commun. 2003;302(2):226–232. doi: 10.1016/s0006-291x(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 18.Mendonca Torres PM, de Araujo EG. Interleukin-6 increases the survival of retinal ganglion cells in vitro. J Neuroimmunol. 2001;117(1-2):43–50. doi: 10.1016/s0165-5728(01)00303-4. [DOI] [PubMed] [Google Scholar]
- 19.Sappington RM, Chan M, Calkins DJ. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest Ophthalmol Vis Sci. 2006;47(7):2932–2942. doi: 10.1167/iovs.05-1407. [DOI] [PubMed] [Google Scholar]
- 20.Zacks DN, Boehlke C, Richards AL, Zheng QD. Role of the fas-signaling pathway in photoreceptor neuroprotection. Arch Ophthalmol. 2007;125(10):1389–1395. doi: 10.1001/archopht.125.10.1389. [DOI] [PubMed] [Google Scholar]
- 21.Guerin CJ, Lewis GP, Fisher SK, Anderson DH. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci. 1993;34(1):175–183. [PubMed] [Google Scholar]
- 22.Lewis GP, Matsumoto B, Fisher SK. Changes in the organization and expression of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest Ophthalmol Vis Sci. 1995;36(12):2404–2416. [PubMed] [Google Scholar]
- 23.Samardzija M, Wenzel A, Aufenberg S, Thiersch M, Reme C, Grimm C. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006;20(13):2411–2413. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- 24.La Heij EC, Van De Waarenburg MP, Blaauwgeers HG, et al. Levels of basic fibroblast growth factor, glutamine synthetase, and interleukin-6 in subretinal fluid from patients with retinal detachment. Am J Ophthalmol. 2001;132(4):544–550. doi: 10.1016/s0002-9394(01)01125-4. [DOI] [PubMed] [Google Scholar]
- 25.Bakunowicz-Lazarczyk A, Sulkowski S, Moniuszko T. Comparative studies of morphological changes and interleukin concentration in subretinal fluid of patients with retinal detachment. Ophthalmologica. 1999;213(1):25–29. doi: 10.1159/000027389. [DOI] [PubMed] [Google Scholar]
- 26.Heinrich PC, Graeve L, Rose-John S, et al. Membrane-bound and soluble interleukin-6 receptor: studies on structure, regulation of expression, and signal transduction. Ann N Y Acad Sci. 1995;762:222–236. doi: 10.1111/j.1749-6632.1995.tb32328.x. [DOI] [PubMed] [Google Scholar]
- 27.Curnow SJ, Scheel-Toellner D, Jenkinson W, et al. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173(8):5290–5297. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson DS, Horvarth CM. A roadmap for those who don’t know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 29.Stephanou A, Latchman DS. STAT-1: a novel regulator of apoptosis. Int J Exp Pathol. 2003;84(6):239–244. doi: 10.1111/j.0959-9673.2003.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephanou A, Latchman DS. Opposing actions of STAT-1 and STAT-3. Growth Factors. 2005;23(3):177–182. doi: 10.1080/08977190500178745. [DOI] [PubMed] [Google Scholar]
- 31.Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2(4):381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- 32.Haga S, Terui K, Zhang HQ, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112(7):989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6(3):196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- 34.Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of antiapoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem. 2001;276(28):26605–26613. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto H, Hayashi H, Uchida H, Kato H, Oshima K. Increased soluble interleukin-6 receptor in vitreous fluid of proliferative vitreoretinopathy. Curr Eye Res. 2003;26(1):9–14. doi: 10.1076/ceyr.26.1.9.14251. [DOI] [PubMed] [Google Scholar]
- 36.Wakefield D, Lloyd A. The role of cytokines in the pathogenesis of inflammatory eye disease. Cytokine. 1992;4(1):1–5. doi: 10.1016/1043-4666(92)90028-p. [DOI] [PubMed] [Google Scholar]
- 37.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 38.Noma H, Minamoto A, Funatsu H, et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2006;244(3):309–315. doi: 10.1007/s00417-004-1087-4. [DOI] [PubMed] [Google Scholar]
- 39.Mocan MC, Kadayifcilar S, Eldem B. Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Can J Ophthalmol. 2006;41(6):747–752. doi: 10.3129/i06-070. [DOI] [PubMed] [Google Scholar]
- 40.Izumi-Nagai K, Nagai N, Ozawa Y, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170(6):2149–2158. doi: 10.2353/ajpath.2007.061018. [DOI] [PMC free article] [PubMed] [Google Scholar]