Summary
Neurologic sequelae of human immunodeficiency virus (HIV) infection have been and remain a significant problem. Monocytes and macrophages in humans and monkeys are susceptible to infection by HIV and simian immunodeficiency virus (SIV) and are considered to be a main mechanism by which the central nervous system (CNS) is infected. Within the infected CNS, perivascular macrophages and, in some cases, parenchymal microglia are infected as are multi-nucleated giant cells (MNGC) when present. While neurons are not themselves directly infected, neuronal damage occurs within the infected CNS. Despite the success of anti-retroviral therapy (ART) in limiting virus in plasma to non-detectable levels, neurological deficits persist. This review discusses the continued neurological dysfunctions that persist in the era of ART, focusing on the roles of monocyte and macrophage as targets of continued viral infection and as agents of pathogenesis in what appears to be emergent macrophage-mediated disease resulting from long-term HIV infection of the host. Data discussed include the biology of monocyte/macrophage activation with HIV and SIV infection, traffic of cells into and out of the CNS with infection, macrophage-associated biomarkers of CNS and cardiac disease, the role of antiretroviral therapy on these cells and CNS disease, as well as the need for effective adjunctive therapies targeting monocytes and macrophages.
Keywords: monocytes/macrophages, immunodeficiency diseases, AIDS
Introduction
Human immunodeficiency virus (HIV) infection of the central nervous system (CNS) has been and continues to be a significant problem. Monocytes and macrophages in humans and monkeys are susceptible to infection by HIV and simian immunodeficiency virus (SIV) and are considered to be a main mechanism by which the CNS is infected (1). Within the infected CNS, perivascular macrophages and, in some cases, parenchymal microglia are infected as are multi-nucleated giant cells (MNGC) when present (2). While neurons are not themselves directly infected, neuronal damage occurs within the infected CNS. This damage leads to neuronal damage and loss, glial cell and macrophage activation, and neurological impairment. Early studies using anti-retroviral therapy (ART), with successful reduction of plasma virus, demonstrated a rapid and profound reversal of HIV-associated dementia (HAD) (3). Unfortunately, over the years it has become clear that while successful ART can limit virus in plasma to non-detectable levels, neurological deficits persist (4–7). Overall, the incidence of HAD has decreased, but the prevalence of neurological deficits have increased. This increase in neurological defects is due in part to people living longer with virus, but the patients with neurological defects have less severe neurological dysfunction than prior to ART (4, 5, 8–10). In this setting, it is becoming clear that patients on effective ART who have non-detectable plasma virus have persistent immune activation that includes monocytes and macrophages, accelerated immune aging and senescence, and other co-morbidities including an increased incidence of coronary atherosclerosis, kidney disease, osteoporosis, and liver disease. These phenomena may be due in part to the effects of anti-retroviral agents used, but they also are likely driven by chronic infection and activation of innate immunity. This review discusses the continued neurological dysfunctions that persist in the era of ART, focusing on the roles of monocyte and macrophage as targets of continued viral infection and on agents of pathogenesis in what appears to be emergent macrophage-mediated disease resulting from long-term HIV infection of the host. Data discussed include the biology of monocyte/macrophage activation with HIV and SIV infection, traffic of cells into and out of the CNS with infection, macrophage-associated biomarkers of CNS and cardiac disease, the role of antiretroviral therapy on these cells and CNS disease, as well as the need for effective adjunctive therapies targeting monocytes and macrophages.
The status of neurological impairment with HIV infection and ART – incomplete viral suppression with immune activation drives disease
Antiretroviral therapy has had a dramatic beneficial impact on the incidence and prevalence of severe forms of HIV-associated neurocognitive impairment (3). Because of this, HIV-associated dementia (HAD) has become rare. The estimated prevalence of HAD in the pre-ART era was between 10 to 15% of HIV infected individuals, and in the current era this has decreased by over 50% (4, 5, 9, 10). While there is a decreased incidence of HAD, in the setting of chronic suppressive treatment, minor forms of neurocognitive impairment have emerged (11). Current research nosology defines the broad spectrum termed HIV-associated neurological disorders (HAND) with graded classifications based on abnormal neuropsychological (NP) testing (a battery of 7 tests) and the patient’s functional limitation related to cognitive impairment. The three classifications of HAND are asymptomatic neurocognitive impairment (ANI), mild neurocognitive impairment (MND), and HIV-associated dementia (HAD) (11). ANI is defined as acquired impairment in at least two cognitive domains without perceived impact on daily function. MND requires impairment in two domains with perceived interference of daily functioning. HAD requires at least 2 impaired domains with severe impairment, typically in multiple domains, and marked impact on daily function (11).
Thus, HIV-associated CNS injury and neurocognitive impairment continues to be clinically significant in the current era of ART (5). Overall, studies demonstrate NP benefits of instituting ART in patients with HAND (3, 6, 7, 12), but in many cases HAND persists (11, 13–15). The persistence of HAND is likely mediated by continual immune activation that includes monocytes and macrophages. Another likely cause for continued neurologic deficits is that many ART-treated patients have some level of viral replication above non-detectable levels (< 50 copies/mL) (4, 16). In addition to continued detectable HIV in plasma, CSF virus can also persist (4, 16–19), which might also play a role in CNS disease. It is very possible that extended survival with incomplete viral suppression plays a role in the persistence of HAND.
Early neuronal injury with HIV and SIV infection, with and without ART
The mechanisms of HAND persistence in the ART era are not well understood. It is likely that events occurring early in the CNS set the stage for a future neurological disease that emerges during chronic infection with or without effective ART. Neuroimaging studies in monkeys and humans have revealed lower N-acetyl aspartate (NAA) levels, indicative of neuronal injury, within weeks of HIV and SIV infection (20–22). This observation is consistent with studies demonstrating early infection of the CNS days after infection (1, 23–26). Recently, it has been determined that the resting cerebral blood flow reductions that occur soon after HIV infection are reflecting early neuronal or vascular injury among HIV-positive individuals who do not yet manifest neuropsychological impairment (27). These imaging studies suggest that there exist early metabolite and blood flow changes, even though these changes do not result in significant clinical NP changes at those early time points.
Early initiation of ART: treatment within 1 year of infection effectively reduces innate immune activation
Early initiation of ART, before CD4+ T cells decline, may have long-term cognitive benefits and help achieve a better neurological outcome. Our studies (28) using plasma soluble CD163 (sCD163) as a marker of monocyte macrophage activation have shown that for individuals with early HIV infection (≤1 year in duration), effective ART resulted in decreased sCD163 to levels that were comparable to normal HIV-seronegative individuals (29). However, in chronically infected HIV patients, sCD163 levels decreased in parallel with HIV RNA levels but did not return to HIV-seronegative levels, suggesting the presence of residual monocyte/macrophage activation even with plasma viral loads below the limit of detection (29). These data suggest that early initiation of ART, in addition to preserving CD4+ T cells and decreasing viral load, would also dampen monocyte/macrophage activation. In addition to the studies described above, studies of patients on scheduled ART interruption during early HIV infection found that sCD163 and plasma virus levels spiked but rapidly returned to baseline with re-initiation of ART. These data suggest that not only early initiation but also continued treatment is necessary for effective inhibition of HIV, monocyte activation, and CD4+ T-cell numbers (29).
Biomarkers/legacy markers
Legacy markers are defined as markers of events happening early in HIV infection that may lead to neurocognitive impairment in later stages of the disease and, in some cases, are considered to be predictors of disease (5, 30). Thus, patients with a history of early severe immunosuppression may have an increased risk of the development of HAND, even after effective ART and immune recovery. It is possible that advanced immunosuppression reflected by low CD4+ T-cell nadir is a legacy event that may have occurred early and whose neurologic consequences may persist. The risk of neurocognitive impairment is lowest in patients whose CD4+ T-cell counts were never allowed to fall to low levels before ART, and these patients were relatively protected from NP impairment (5, 30). These observations reiterate the importance of early initiation of ART in reducing the risk of neurocognitive impairment as well as the importance of identifying HIV-seropositive patients early and encouraging ART use to prevent later complications (30). Unfortunately, epidemiologic data from the Centers for Disease Control (CDC) indicate that it is often difficult to identify HIV infection in its early stages; at least 34% of patients in the US have a CD4+ T-cell counts below 200 cells/μl at diagnosis (31). In addition to CD4+ T-cell nadir, others have looked at viral set point as an early predictor of HAND (32). Such legacy markers are likely markers of differential immune responses that reflect levels of immune activation and predict future HAND among HIV-infected individuals.
There is no biomarker of HAND that is used currently in the clinical setting that correlates to HAND in the era of ART. Early work in monkeys and humans indicated that CSF markers including HIV RNA (33) and immune activation factors [β2-microglobulin (34, 35), neopterin(36, 37), sCD14 (38, 39) and CCL2/MCP-1 (40–43)] were diagnostic of HAD (33, 44). Unfortunately, these markers are not as effective in patients on ART (33, 45). Our work has shown that NP impairment in ART treated and virologically suppressed HIV-infected individuals is associated with a sustained elevation of the monocyte/macrophage activation marker sCD163 in plasma (46, 47). Interestingly, CD163 is made solely by cells of the myeloid lineage and shed by them with immune activation. Our data (described above) demonstrate that plasma sCD163 is an early marker that predicts HIV/SIV pathogenesis (29). We reported elevated levels of sCD163 in patients with impaired global deficit scores (GDS) and in patients with HAND with persistent monocyte/macrophage activation in HIV-related NP impairment despite virologic suppression(46, 47). These data underscore the utility of sCD163 as a plasma marker of neurocognitive impairment that correlates to HAND in individuals on ART. We did not find correlations of sCD163 with NP impairment, unlike other biomarkers described previously(46, 47). Whether sCD163 levels in plasma are predictive of NP impairment will require studies with larger numbers of patients.
The observations above underscore the importance of monocyte activation and NP impairment with HIV infection, even with effective ART. We, along with others, have implicated monocyte activation and traffic not only as a source of inflammatory cells in the CNS and a key mechanism for neuroinvasion but also as a requirement for CNS pathogenesis (48–53). Such a perspective includes the notion that monocyte traffic to the CNS, which occurs normally in non-diseased brains, occurs with acute and chronic infection, and the accumulation of these cells as transient or resident macrophages contributes to immune dysregulation and disease (48–53) (discussed below). To this end, we found that differences with sCD163 in monkeys infected with SIV, as early as 8 days post infection and consistently by 21 days post infection, that predicted how rapidly these monkeys would develop AIDS and how severe CNS pathogenesis would be (53). sCD163 in these monkeys directly correlated with the magnitude of monocyte turnover in the bone marrow. Taken together, these data point to a link between early innate and adaptive immune response and future CNS pathology.
Mechanisms of HIV CNS disease pathogenesis: monocyte traffic and accumulation as transient or resident brain macrophages
Exact mechanisms for neurological impairment caused by HIV and SIV infection remain elusive. Many factors have been hypothesized including the virus itself, which does not infect neurons but does interact with neuronal cells, immune cells and glial cells by virtue of their viral proteins (reviewed in 54). Additionally, factors released from activated glia, including parenchymal microglia (the resident macrophage in the CNS), and inflammatory macrophages that are recruited also likely play a role (reviewed in 55). Early literature suggested the presence of virus alone was the best correlate of neuronal injury and dysfunction(56), but more recent evidence points to the accumulation of macrophages as the histopathologic correlate of HIV-associated neuronal dysfunction (57, 58). This seems also to be true in the era of ART, although less extensive histopathologic data exists. Consistent across all of the possible mechanisms is a prominent role for activated monocytes and macrophages.
Expansion and turnover of monocyte blood populations
The earliest evidence of the link between activated monocytes and HAD came from Drs. Pulliam and McGrath (59), who showed a correlation between the presence and number of CD14+CD16+ monocytes and HAD in HIV-infected men. Others have observed a similar expansion of CD14+CD16+ monocytes in HIV-infected humans (49, 60, 61) and SIV-infected monkeys (48, 62). The population of CD14+CD16+ monocytes are of interest with regard to HIV infection, because they are more susceptible to HIV infection (49, 62–64) and have an immune phenotype and possible functions that are more similar to mature macrophages than monocytes found in blood (1, 49). Whether this maturation accounts for their enhanced ability to replicate HIV is unknown. In HIV and SIV infections, there are also increased numbers of activated monocytes (1, 49, 59, 62, 65) and turnover of monocyte/macrophages in tissues (53, 66). Kuroda and colleagues first demonstrated in SIV-infected monkeys, using bromodeoxyuridine (BrdU), an analogue of thymidine that is incorporated in DNA during replication, that an increased turnover of monocytes from the bone marrow is a better marker of progression to AIDS than CD4+ T-cell count and plasma viral load (66). Extending those observations, Burdo et al. (53), also using BrdU incorporation, found that the magnitude of BrdU+ monocytes in blood of SIV-infected monkeys predicted how rapidly they would progress to AIDS. The more BrdU+ monocytes that were found in the blood of the animals (indicative of increased turnover), the more severe the brain histopathology (macrophage accumulation and productive viral infection) (53). Rappaport and colleagues (60) made similar observations, finding an expansion of CD16+CD163+ monocytes in blood that may replace or take up residence in the CNS of HIV-infected individuals. It is likely that this same increased monocyte turnover and accumulation of macrophages in the CNS is occurring in other organs, including the heart, within HIV-infected individuals even with durable ART.
Several recent reports have described an activated monocyte phenotype in HIV-infected patients with cardiovascular disease that is positive for CD14, CD16, and tissue factor (TF) that is associated with activation of coagulation factors and that correlates with reduced intima of cardiac vessels, increased vulnerable non-calcified plaque, and macrophage accumulation in the ascending aorta (67–74). In addition, there is evidence of elevated C-reactive protein (CRP), d-dimer, platelets, and microparticles underscoring chronic and sustained immune activation and activation of coagulation factors that can contribute to cardiac and CNS inflammation and dysfunction (75–79). It is important to note that accumulation of CD68+ macrophages that are also CD163+ has been demonstrated in cardiac tissues of SIV-infected monkeys (80, 81) as well as in the CNS (53, 60, 82–84). It is also noteworthy that elevated sCD163 cleaved from activated monocytes in HIV-infected humans with coronary atherosclerosis (69) and neuroAIDS (46, 47, 53) correlates with the number of inflammatory macrophages in hearts (85), HIV activity prior to and with effective ART (29), and neurocognitive deficits (46, 47). Thus, activated monocytes, and sCD163 as a marker of them, are minimally a biomarker of cardiac and CNS disease in HIV-infected patients, and macrophages in the heart and CNS are likely effector cells with regard to immune and physiologic responses to HIV infection. This observation suggests that in addition to developing therapies that target HIV, it would be important to also target monocyte/macrophage activation and traffic to affected organs.
CNS macrophage phenotypes
Our group and others (1, 49, 53, 60, 62, 83, 84, 86, 87) have demonstrated that populations of CNS macrophages can be distinguished by a combination of their anatomic location in the CNS and immunophenotypic markers. With regard to HIV and SIV infection, we have studied the resident parenchymal microglia, perivascular macrophages, and inflammatory macrophages that are only present in the CNS with inflammation (50, 88, 89). Perivascular macrophages are CD14+CD16+CD163+ and have high levels of CD45 (1, 83, 84, 86, 90, 91). Parenchymal microglia have low to non-detectable CD14 and CD16. These two cells types are the major constituents of HIV and SIV encephalitic lesions (1, 52, 83, 86, 92), where productive viral replication is often found. Perivascular macrophages are also found in small lesions along CNS vessels where they are infected (1, 83, 93). Another monocyte/macrophage cell type is present with inflammation. They are distinguished from perivascular macrophages and parenchymal microglia by being positive for MAC387 (84, 94). Comparing the numbers of MAC387+ cells in the CNS of (i) chronically SIV-infected monkeys with and without SIVE, (ii) rapid progressing animals with SIVE, and (iii) CD8+ lymphocyte-depleted animals with SIVE, we find MAC387+ cells are present in early developing lesions that are still immune active. This finding is similar to what has been found in multiple sclerosis (84). Interestingly, in our experiments using BrdU to examine cell trafficking to the CNS we find that, 24–48 h post BrdU pulse, the majority of the BrdU+ monocyte/macrophages in the CNS are MAC387+ cells. This finding is consistent with the notion that MAC387 is the earliest marker found on macrophages as they enter the CNS (53). In fact, when we compared the ratio of MAC387+ monocyte/macrophages with the ratio of CD68+CD163+ macrophages, we found greater numbers of MAC387+ cells than CD68+CD163+ cells in lesions that are early and active. Conversely, a greater number of CD68+CD163+ cells than MAC387+ cells were present in chronic established lesions (84). We have identified a similar change in the ratio of MAC387+ and CD68+CD163+ cells in the CNS tissues of HIV-infected humans (84). CD68+CD163+CCR2+ perivascular macrophages have an M2-polarized alternatively activated macrophage phenotype, while the MAC387+ cells are CD163−CCR2−, similar to classically activated M1 macrophages, which are thought to amplify immune and inflammatory responses and are associated with higher suppression of HIV-1 replication (95). It is tempting to suggest that the early lesions made up M1 macrophages represent an ongoing immune response to infection, while the later more chronic lesions made up of M2 macrophages may indicate attempts to downregulate immune responses (84, 96). In an extension of this notion, one could envision CD163+ macrophages at the lesion edge functioning to contain the damaging immune responses within the lesion from the rest of the CNS.
Monocyte/macrophage turnover in the CNS
It is well established in rodents, nonhuman primates, and humans that monocytes traffic to the CNS occurs normally and in disease (97–101). Early classic studies demonstrated that parenchymal microglia are present embryologically from yolk sac macrophages and are distinct from other macrophage populations (102–104). The parenchymal microglia remain a relatively stable population with a low level of turnover within the CNS (105–107). These cells appear to remain tethered within a reticular network within the CNS and are able to scan their respective territories acting as sentinels protecting against damage, invading pathogens, and tumors (108). Macrophages of the choroid plexus and meninges, as well as perivascular macrophages, are bone marrow-derived and are repopulated throughout life (100, 105, 109, 110). These cells are more likely to serve as carriers for viruses and bacteria entering and perhaps exiting the CNS. Studies in rodents and humans have established that the perivascular macrophages are continuously repopulated from bone marrow (100, 105, 109). Using nonhuman primates, who were irradiated and reconstituted with retroviral transduced, autologous CD34+ hematopoietic stem cells, we demonstrated turnover of perivascular macrophages that corresponded to the rate of engraftment of the stem cells in bone marrow and monocyte precursors in blood (111). Whether such cells could be engineered in a way that would make them not susceptible to HIV infection is not known, but it is an exciting possibility (112, 113).
Viral sequence in the CNS
The phenotype of CNS macrophages is relatively well established and critical with regard to which cells come to the brain and when and to which cells are important in terms of viral infection. Sequence analysis supports the notions that (i) virus found in the CNS is macrophage-tropic, meaning it replicates well in macrophages (114–116), (ii) virus in the CNS can be compartmentalized compared to other infected organs, but macrophage-tropic sequences in the CNS do match that of other organs, and (iii) there is evidence of early seeding of the meninges of the CNS, and then other brain tissues, suggesting successive waves or spread of virus, likely from infected monocytes that have become macrophages in the CNS (25, 58, 93, 116, 117). Importantly, evidence in the monkey model can support that notion that the CNS receives waves of viral seeding from the periphery that is not a one-time event but likely occurs throughout the course of infection (114, 116, 118). All of the above underscore the importance of myeloid cells as targets of infection outside and inside the CNS.
Drug treatment to stop virus and/or macrophage traffic: studies from SIV-infected macaques
Evidence from our laboratory and others using ART, minocycline, and antibodies directed against monocyte traffic to the CNS provides compelling evidence that continued monocyte activation in the periphery and traffic to the CNS, throughout infection drive the neuropathogenesis of HIV/SIV infection. Early studies by our group used a combination of 2 anti-retroviral agents, 9-R-2-phosphonomethoxypropyl adenine (PMPA) and racinavir (RCV), in an accelerated model of AIDS neuropathogenesis involving CD8+ cell depletion where we demonstrated large decreases of NAA/Cr by MR spectroscopy consistent with neuronal injury at 28 days post infection (119). In CD8-depleted animals treated daily with PMPA and RCV, neither of which significantly penetrate the CNS, the decreased NAA/CR ratios reversed within 2 weeks on ART and returned to within 90% of pre-infection levels by 4 weeks post treatment (119). Within the CNS of these animals, there was little-to-no productive viral infection and few inflammatory macrophages in perivascular cuffs (119). The most significant marker that correlated with the reversal of neuronal injury and the paucity of productive infection and inflammatory cells in the CNS of treated animals was the lack of a second peak of expansion of activated monocytes in the blood of these animals (119). More recently, we investigated the use of the antibiotic minocycline, which has been shown to have anti-inflammatory properties, using the same CD8+ cell depletion model and MR spectroscopy imaging studies (120, 121). Again, animals received minocycline daily beginning at day 28-post infection, a time point when we found significant loss of NAA/Cr and known active CNS inflammation. Minocycline treatment did not cause a restoration of NAA/Cr toward normal levels but did prevent subsequent declines in NAA/Cr, which we think is indicative of neuronal protection (120, 121). Moreover, minocycline treatment also resulted in decreased monocyte activation in blood, lack of traffic in an ex vivo transwell assay, reduced recruitment of macrophages to lymph nodes, and an absence of detectable inflammation and active viral replication in the CNS (120). Because neither of the studies used agents to prevent traffic of monocytes into the CNS, we performed a third study using an anti-VLA-4 antibody, and treated CD8+ lymphocyte-depleted animals at the time of infection, once a week for 3 weeks, or at 28 days post infection and sacrificed animals one week after the final anti-VLA-4 treatment. This treatment resulted again in reversal of neuronal injury as measured by MR spectroscopy, immunohistochemistry, and neuropathological assessment in animals treated beginning 28 days post infection (122). Interestingly, in the animals that received treatment starting at the time of infection, not only was traffic of monocyte/macrophages and virus to the CNS blocked, but we also observed greatly diminished viral infection and traffic of monocytes to the gut (122). These data therefore not only demonstrate that blocking monocyte and lymphocyte traffic to the CNS results in a reversal of CNS injury but that such treatment results in lack of seeding of the CNS with virus and less virus in the gut. This may be a critical short-term treatment to consider for individuals who engage in high-risk behavior. It could be used with ART to not only keep viral replication in check and spare the immune system (123) but also to potentially stop the seeding of important cellular reservoirs of virus in the CNS and gut. Additional data on the impact of changes in viral distribution and tropism on HIV/SIV pathogenesis come from recent work using the highly virulent molecular clone SIVmac239, in which two amino acids in the transmembrane protein were deleted (124). This virus, termed SIVmac239ΔGY, replicated to wildtype levels during acute infection, but only transiently infected mucosal sites such as the gut and caused little or no acute depletion of mucosal CD4+ T cells and little if any infection of macrophages; CNS lesions were also absent (124). While the data noted above support the importance of monocyte/macrophages contributing to CNS infection and the neuropathogenesis of AIDS, similar targeted therapies using different outcome measures have not been successful. Namely, minocycline used in HIV-infected patients did not have a favorable impact on cognitive function (125, 126). Whether minocycline used in conjunction with ART might be beneficial is not yet known.
Synergistic effects of chronic immune activation, metabolic syndrome, acute phase proteins, cardiovascular disease, and CNS pathology during HIV infection
The above discussion underscores the importance of monocytes as major players and biomarkers of macrophage-mediated disease in HIV infection, including the pathogenesis of cardiac and CNS disease and NP deficits. Clearly, there are other comorbidities associated with HIV disease progression and HAND, including other infections and the likely emergence of degenerative diseases as HIV-infected populations age (e.g. Alzheimer’s disease, Parkinson’s disease, cardiovascular disease) (127). In this regard, it is important to note that HIV affects cholesterol metabolism by monocyte/macrophages, which has been described as contributing to fatty macrophages in the vasculature in HIV-infection (128). Additionally, HIV-infected patients have accelerated senescence of the immune system for reasons that are not clearly defined (129, 130). In addition to HIV-associated CD8+ T-cell senescence, recent work has shown CD8-independent age-related changes in monocytes (129) and increases in markers of immune activation, sCD163 and CXCL10, in plasma (131). These changes suggestive of innate immune dysfunction and age-related immune senescence persist despite viral suppression (129). Several of these changes may be a result of generalized immune activation associated with microbial translocation (132), but other as yet unknown factors also probably contribute. A recent example of this is that rhesus macaques infected with SIVmac239ΔGY do not show any evidence of microbial translocation, and yet they develop immune activation and slow disease progression (124).
The lipopolysaccharide (LPS) and other microbial products from the gut drain into the liver, where the liver releases proteins associated with the acute phase response such as CRP, d-dimer, and IL-6. Additionally, platelet production and coagulation factors are increased, as well as activation of monocytes/macrophages and enhanced sCD163 shedding. In addition to chronic immune activation, which clearly affects monocyte/macrophages in HIV-infected patients, it is likely also due to secondary infection, residual low-level viral infection, and the effects of viral proteins on the immune system (133–135). There are reported correlations with body mass ratio, cardiovascular disease, and dementia with and without HIV (127, 131). All in all, it is clear that persistent macrophage activation is at least a marker of disease and CNS pathology, if not a contributor.
In this regard, it is interesting to consider our studies using CD8+ cell-depleted SIV-infected monkeys. We found a linear correlation between the number of BrdU+ monocytes in the blood 24 h after BrdU administration and the level of plasma sCD163 (53). The differences in the absolute number and percent of BrdU+ monocytes in blood and the levels of sCD163 among monkeys that distinguished rapid versus slow progressor could be detected and used to differentiate between animals by 21 days post infection, despite that fact that it was weeks to months longer before the animals developed AIDS (53). Moreover, we have made similar observations in HIV-infected individuals with sCD163. Chronically infected patients had high levels of sCD163 that correlated with their CD14+CD16+ monocytes, which were reduced with effective ART, but not to the level found in uninfected patients (29). In contrast, sCD163 levels in the early infected patients were lower than that of the chronic patients, and with 3 months of effective ART, it decreased to the same level found in uninfected individuals (29). These data with the observations discussed earlier that sCD163 correlates with the percentage of non-calcified plaque in HIV-infected patients (69), the number of macrophages in the ascending aorta (71) and HAND (46, 47) demonstrate two points: one, it is best to treat with ART early after infection to essentially spare chronic, reversible monocyte/macrophage activation, and two, chronic immune activation of monocytes, as measured by sCD163, is a good marker of HIV activity and macrophage inflammation in HIV cardiac disease and HAND (29, 46, 69–71).
Mechanisms accounting for persistence of neurological disease with ART
It is clear that despite the advent of ART, which is capable of driving peripheral viral load to undetectable levels, neurological deficits persist. This is supported not only by the increasing incidence of HAND but also by histologic examination of tissues where inflammation is still evident, as are signs of immune activation. The reasons for persistent neurological disorders can be many, including the fact that ART is not absolutely effective in all patients and that residual chronic viral replication outside the CNS in various tissues, including the intestinal track, has been described (136, 137). Additionally, CNS and CSF viral replication is found and can persist, even if there is non-detectable or low level replication in plasma (4, 16–19). Letendre and others (138) have suggested considering the ability of antiretrovirals to penetrate the CNS and effectively decrease replication in the CNS when developing ART regimens by utilizing a formula called CNS-penetration-effectiveness (CPE). A caveat of this work in general is the relative inefficiency with which ART functions on macrophages (139–141). Also, it assumes that only CNS virus drives neuropathology. Others have suggested that the antiretroviral drugs themselves contribute to neuronal injury and several comorbidities, including cardiovascular disease (142, 143). Lastly, effects of comorbidities, such as drug abuse and co-infections with viruses such as hepatitis C virus (HCV), cytomegalovirus (CMV), and polyomavirus (JC virus, progressive multifocal leukoencepalopathy), as well as other coinfections, such as toxoplasmosis, also likely play a role.
In addition to the factors listed above, it is clear that ART has allowed people to live much longer with HIV infection. These aging HIV-infected populations will have an increasing incidence of degenerative neurological diseases, which will impact and be impacted by the neuropathologic effects of HIV infection. Common to HIV infection and degenerative neurologic diseases such as Alzheimer’s and Parkinson’s disease is glial cell activation and low-level chronic inflammation with activated monocytes and macrophages, which are likely to have synergistic negative consequences for neurologic function (144). Lastly, in addition to CNS diseases associated with aging, is the increasing role of cardiovascular disease, increased body mass index, and vascular dementia as they all likely relate to HIV neuropathogenesis. Similar to the CNS, macrophage accumulation in the heart and cardiac vasculature has been associated with tissue damage (128). In addition, emerging data point to altered coagulation and lipid metabolism that contributes to cardiovascular disease, which would also affect CNS vasculature and complicate HAND. All of these observations underscore the importance of adjunctive therapies to target immune activation in general and activated monocyte/macrophages in particular with regard to neurologic and cardiovascular disease.
Future therapeutic approaches to HIV and macrophage-mediated diseases
It is clear that future research should focus on modulating activation of monocyte/macrophages, which seems to be central to the longer term degenerative aspects of HIV infection in addition to the control of viral replication by ART. Few pharmacologic agents that modulate monocyte/macrophage activation are currently available. Minocycline, an antibiotic with anti-inflammatory properties, has been unsuccessful in clinical trials of HIV-infected individuals in treating HIV-associated cognitive disorders (125, 126). It is not clear why minocycline has not been used with patients in combination with ART. Minocycline has potential as an adjunctive therapy for HIV-1-associated cognitive disorders. Statins are clearly one class of drugs that have reported immune suppressive effects that might be beneficial in HIV-infected patients. Similarly, dimethyl fumarate, which has been shown to be an immune modulator and inducer of antioxidant response that suppresses HIV replication and macrophage-mediated neurotoxicity (145), might also be of benefit. Work in our laboratory using a newly developed oral form of MGBG, termed PA300, has been effective in SIV-infected monkeys and resulted in decreased monocyte/macrophage accumulation and prevented SIV encephalitis in CD8 animals treated at day 28 post infection for 4 weeks (146). The same drug, in a dose dependent manner, decreased inflammation in the hearts of infected monkeys and decreased damage to the cardiac tissues.
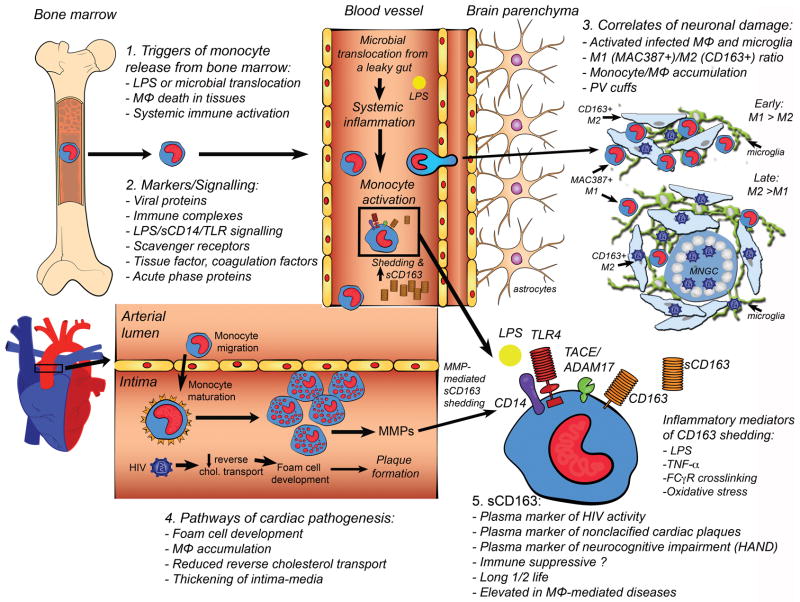
Fig. 1. Pathways and mechanisms of expanded monocyte/macrophage activation, turnover and accumulation in parenchymal tissues with HIV infection.
1) Monocyte egress from bone marrow in response to elevated LPS, macrophage death in tissues (lymph node and parenchymal), and systemic immune activation. 2) Markers and signaling molecules involved in augmented monocyte activation and turnover, including viral proteins, acute phase proteins, immune complexes, sCD163 and signaling through LPS/CD14/TLR4 and macrophage scavenger receptor (SR-A), and expression of tissue factor (TF) and CD163. 3) Correlates of neuronal damage in the central nervous system include: activated and infected macrophages and parenchymal microglia, M1 (MAC387)/M2 (CD163) ratio, monocyte/macrophage (MΦ) accumulation, and perivascular (PV) cuffs. MNGC= multi-nucleated giant cell. 4) Pathways of cardiac pathogenesis including development of foam cells, macrophage accumulation in the intima, reduced reversed cholesterol transport, thickening of the intima-media. 5) sCD163 is a marker of HIV activity, plasma marker of non-calcified (vulnerable) cardiac plaques, and neurocognitive impairment. sCD163 has immune-suppressive functions in vitro, is elevated in macrophage mediated diseases, has a long half-life, and is therefore a stable biomarker.
Acknowledgments
This work was partially supported by National Institute of Health grants, NS082116 (T.B.), NS040237 (K.W.), RR000164 and RR021309.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Williams KC, et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell JE. The neuropathology of adult HIV infection. Rev Neurol. 1998;154:816–829. [PubMed] [Google Scholar]
- 3.Schmitt FA, Bigley JW, McKinnis R, Logue PE, Evans RW, Drucker JL. Neuropsychological outcome of zidovudine (AZT) treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1988;319:1573–1578. doi: 10.1056/NEJM198812153192404. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra CM, Lockhart D, Zunt JR, Perrin M, Coombs RW, Collier AC. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology. 2003;60:1388–1390. doi: 10.1212/01.wnl.0000058768.73358.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson KR, et al. Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr. 2004;36:562–566. doi: 10.1097/00126334-200405010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS. 1997;11:1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Sacktor N, et al. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 10.Mocroft A, et al. Immunological, virological and clinical response to highly active antiretroviral therapy treatment regimens in a complete clinic population. Royal Free Centre for HIV Medicine. AIDS. 2000;14:1545–1552. doi: 10.1097/00002030-200007280-00010. [DOI] [PubMed] [Google Scholar]
- 11.Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra CM, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology. 2006;66:1447–1450. doi: 10.1212/01.wnl.0000210477.63851.d3. [DOI] [PubMed] [Google Scholar]
- 14.Robertson KR, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 15.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurol. 2011;17:176–183. doi: 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- 16.Heaton RK, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 17.Eden A, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canestri A, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin INfect Dis. 2010;50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 19.Peluso MJ, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lentz MR, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lentz MR, et al. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez RG, et al. New insights into the neuroimmunity of SIV infection by magnetic resonance spectroscopy. J Neuroimmune Pharmacol. 2006;1:152–159. doi: 10.1007/s11481-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti L, et al. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 24.Hurtrel B, Chakrabarti L, Hurtrel M, Maire MA, Dormont D, Montagnier L. Early SIV encephalopathy. Journal of medical primatology. 1991;20:159–166. [PubMed] [Google Scholar]
- 25.Davis LE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 26.An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ances BM, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73:702–708. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller HJ. Soluble CD163. Scand J Clin Lab Invest. 2011;72:1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 29.Burdo TH, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis RJ, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spudich SS, Ances BM. Neurologic complications of HIV infection. Top Antiviral Med. 2012;20:41–47. [PMC free article] [PubMed] [Google Scholar]
- 32.Childs EA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 33.Letendre S, Ellis R. Viral and Cellular Biomarkers during Antiretroviral Therapy. In: Gendelman HE, et al., editors. The Neurology of AIDS. 3. Oxford: Oxford University Press; 2012. pp. 825–846. [Google Scholar]
- 34.Elovaara I, et al. CSF and serum beta-2-microglobulin in HIV infection related to neurological dysfunction. Acta Neurol Scand. 1989;79:81–87. doi: 10.1111/j.1600-0404.1989.tb03717.x. [DOI] [PubMed] [Google Scholar]
- 35.McArthur JC, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 36.Sonnerborg AB, von Stedingk LV, Hansson LO, Strannegard OO. Elevated neopterin and beta 2-microglobulin levels in blood and cerebrospinal fluid occur early in HIV-1 infection. AIDS. 1989;3:277–283. doi: 10.1097/00002030-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Brew BJ, Dunbar N, Pemberton L, Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J Infect Dis. 1996;174:294–298. doi: 10.1093/infdis/174.2.294. [DOI] [PubMed] [Google Scholar]
- 38.Ryan LA, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184:699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 39.Ancuta P, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cinque P, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Conant K, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 43.Zink MC, Clements JE. A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J Neurovirol. 2002;8 (Suppl):42–48. doi: 10.1080/13550280290101076. [DOI] [PubMed] [Google Scholar]
- 44.Mankowski JL, Queen SE, Clements JE, Zink MC. Cerebrospinal fluid markers that predict SIV CNS disease. J Neuroimmunol. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 45.Sun B, Abadjian L, Rempel H, Calosing C, Rothlind J, Pulliam L. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol. 2010;16:115–124. doi: 10.3109/13550280903559789. [DOI] [PubMed] [Google Scholar]
- 46.Burdo TH, Weiffenbach A, Woods S, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 is a marker of neurocognitive impairment in HIV infection. AIDS. 2013 doi: 10.1097/QAD.0b013e32836010bd. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burdo T, Weiffenbach A, Woods S, Letendre S, Ellis R, Williams K. Elevated sCD163 is a marker of neurocognitive impairment in HIV-infected individuals on effective ART. 19th Conference on Retroviruses and Opportunistic Infections (CROI); Washington State Convention Center, Seattle Washington. 2012. [Google Scholar]
- 48.Williams KC, Hickey WF. Traffic of hematogenous cells through the central nervous system. Curr Top Microbiol Immunol. 1995;202:221–245. doi: 10.1007/978-3-642-79657-9_15. [DOI] [PubMed] [Google Scholar]
- 49.Fischer-Smith T, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 50.Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74:650–656. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- 51.Clay CC, et al. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol. 2007;81:12040–12048. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burdo TH, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 56.Cinque P, et al. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 58.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 59.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 60.Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008;24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratto-Kim S, et al. Expression of monocyte markers in HIV-1 infected individuals with or without HIV associated dementia and normal controls in Bangkok Thailand. J Neuroimmunol. 2008;195:100–107. doi: 10.1016/j.jneuroim.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim WK, et al. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol. 2010;87:557–567. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 64.Ellery PJ, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 65.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 66.Hasegawa A, et al. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayne E, et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquired Immun Def Syndromes. 2012;59:340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Funderburg NT, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burdo TH, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pereyra F, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Subramanian S, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arildsen H, Sorensen KE, Ingerslev JM, Ostergaard LJ, Laursen AL. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med. 2013;14:1–9. doi: 10.1111/j.1468-1293.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 73.Funderburg NT, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellacosa C, et al. Carotid intima media thickness changes, endothelial activation and inflammatory markers in advanced naive HIV patients starting antiretroviral therapy. J Int AIDS Soc. 2012;15:18153. doi: 10.7448/IAS.17.4.19545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Armah KA, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55:126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duprez DA, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ford ES, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuller LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez M, San Roman J, Estrada V, Vispo E, Blanco F, Soriano V. Endothelial Dysfunction in HIV Infection - The Role of Circulating Endothelial Cells, Microparticles, Endothelial Progenitor Cells and Macrophages. AIDS Rev. 2012;14:223–230. [PubMed] [Google Scholar]
- 80.Yearley JH, Pearson C, Shannon RP, Mansfield KG. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res Hum Retrovirus. 2007;23:515–524. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- 81.Kelly KM, et al. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- 82.Roberts ES, Masliah E, Fox HS. CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE) J Neuropathol Exp Neurol. 2004;63:1255–1264. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- 83.Kim WK, et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soulas C, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker J, et al. Elevated numbers of CD163+ macrophages in hearts of SIV+ rhesus macaques with cardiac disease are decreased using PA300. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 86.Williams K, et al. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 88.Williams KC, Hickey WF, Burdo TH, Soulas C. Mononuclear phagocytes. In: Gendleman HEGI, Everall IP, Fox HS, Gelbard HA, Lipton SA, Swindells S, editors. The Neurology of AIDS. Oxford: Oxford University Press; 2012. [Google Scholar]
- 89.Kim WK, Avarez X, Williams K. The role of monocytes and perivascular macrophages in HIV and SIV neuropathogenesis: information from non-human primate models. Neurotox Res. 2005;8:107–115. doi: 10.1007/BF03033823. [DOI] [PubMed] [Google Scholar]
- 90.Borda JT, et al. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol. 2008;172:725–737. doi: 10.2353/ajpath.2008.070848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fabriek BO, et al. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51:297–305. doi: 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- 92.Westmoreland SV, Halpern E, Lackner AA. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- 93.Lane JH, et al. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- 94.Esiri MM, Morris CS. Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 2. Non-neoplastic diseases. J Neurol Sci. 1991;101:59–72. doi: 10.1016/0022-510x(91)90018-3. [DOI] [PubMed] [Google Scholar]
- 95.Cassol E, Cassetta L, Alfano M, Poli G. Macrophage polarization and HIV-1 infection. J Leukoc Biol. 2010;87:599–608. doi: 10.1189/jlb.1009673. [DOI] [PubMed] [Google Scholar]
- 96.Bruck W, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- 97.Whitelaw DM. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 1972;5:311–317. doi: 10.1111/j.1365-2184.1972.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 98.Stossel TP. Phagocytosis (third of three parts) N Engl J Med. 1974;290:833–839. doi: 10.1056/NEJM197404112901506. [DOI] [PubMed] [Google Scholar]
- 99.van Furth R, Raeburn JA, van Zwet TL. Characteristics of human mononuclear phagocytes. Blood. 1979;54:485–500. [PubMed] [Google Scholar]
- 100.van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]
- 101.Ohgami M, Doerschuk CM, Gie RP, English D, Hogg JC. Monocyte kinetics in rabbits. J Appl Physiol. 1991;70:152–157. doi: 10.1152/jappl.1991.70.1.152. [DOI] [PubMed] [Google Scholar]
- 102.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 103.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 104.Ransohoff RM. Microglia and monocytes: ‘tis plain the twain meet in the brain. Nat Neurosci. 2011;14:1098–1100. doi: 10.1038/nn.2917. [DOI] [PubMed] [Google Scholar]
- 105.Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 106.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 107.Unger ER, Sung JH, Manivel JC, Chenggis ML, Blazar BR, Krivit W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J Neuropathol Exp Neurol. 1993;52:460–470. doi: 10.1097/00005072-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 108.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 109.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 110.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 111.Soulas C, Donahue RE, Dunbar CE, Persons DA, Alvarez X, Williams KC. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am J Pathol. 2009;174:1808–1817. doi: 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.An DS, et al. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc Natl Acad Sci USA. 2007;104:13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poles MA, Elliott J, Taing P, Anton PA, Chen IS. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol. 2001;75:8390–8399. doi: 10.1128/JVI.75.18.8390-8399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salemi M, Lamers SL, Yu S, de Oliveira T, Fitch WM, McGrath MS. Phylodynamic analysis of human immunodeficiency virus type 1 in distinct brain compartments provides a model for the neuropathogenesis of AIDS. J Virol. 2005;79:11343–11352. doi: 10.1128/JVI.79.17.11343-11352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burkala EJ, He J, West JT, Wood C, Petito CK. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS. 2005;19:675–684. doi: 10.1097/01.aids.0000166090.31693.aa. [DOI] [PubMed] [Google Scholar]
- 116.Strickland SL, et al. Efficient transmission and persistence of low frequency SIVmac251 variants in CD8-depleted rhesus macaques with different neuropathology. J Gen Virol. 2012;93:925–938. doi: 10.1099/vir.0.039586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lamers SL, Gray RR, Salemi M, Huysentruyt LC, McGrath MS. HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infect Genet Evolution. 2011;11:31–37. doi: 10.1016/j.meegid.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Strickland SL, et al. Significant genetic heterogeneity of the SIVmac251 viral swarm derived from different sources. AIDS Res Hum Retroviruses. 2011;27:1327–1332. doi: 10.1089/aid.2011.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williams K, et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Campbell JH, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ratai EM, et al. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS ONE. 2011;5:e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Campbell J, et al. VLA-4 Treatment Blocks Virus Traffic to the Gut and Brain Early, and Stabilizes CNS Injury Late in Infection. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 123.Walker BD, Hirsch MS. Antiretroviral therapy in early HIV infection. N Engl J Med. 2013;368:279–281. doi: 10.1056/NEJMe1213734. [DOI] [PubMed] [Google Scholar]
- 124.Breed MW, et al. Loss of a tyrosine-dependent trafficking motif in the simian immunodeficiency virus envelope cytoplasmic tail spares mucosal CD4 cells but does not prevent disease progression. J Virol. 2013;87:1528–1543. doi: 10.1128/JVI.01928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sacktor N, et al. Minocycline treatment for HIV-associated cognitive impairment: Results from a randomized trial. Neurology. 2011;77:1135–1142. doi: 10.1212/WNL.0b013e31822f0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakasujja N, et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology. 2013;80:196–202. doi: 10.1212/WNL.0b013e31827b9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roberts RO, et al. Cardiac Disease Associated With Increased Risk of Nonamnestic Cognitive Impairment: Stronger Effect on Women. JAMA Neurol. 2013:1–9. doi: 10.1001/jamaneurol.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crowe SM, Westhorpe CL, Mukhamedova N, Jaworowski A, Sviridov D, Bukrinsky M. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010;87:589–598. doi: 10.1189/jlb.0809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hearps AC, Angelovich TA, Jaworowski A, Mills J, Landay AL, Crowe SM. HIV infection and aging of the innate immune system. Sex Health. 2011;8:453–464. doi: 10.1071/SH11028. [DOI] [PubMed] [Google Scholar]
- 130.Smith R, de Boer RJ, Brul S, Budovskaya Y, van der Spek H. Premature and accelerated aging: HIV or HAART? Frontiers Genetics. 2012;3:1–10. doi: 10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin GE, et al. Age-Associated Changes in Monocyte and Innate Immune Activation Markers Occur More Rapidly in HIV Infected Women. PLoS One. 2013;8:e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 133.Parrinello CM, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012;205:1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sacre K, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS. 2012;26:805–814. doi: 10.1097/QAD.0b013e328351f780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naeger DM, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ling B, et al. The large intestine as a major reservoir for simian immunodeficiency virus in macaques with long-term, nonprogressing infection. J Infect Dis. 2010;202:1846–1854. doi: 10.1086/657413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Veazey RS, Lackner AA. Impact of antiretroviral therapy on intestinal lymphoid tissues in HIV infection. PLoS Med. 2006;3:e515. doi: 10.1371/journal.pmed.0030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Letendre S, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aquaro S, Calio R, Balzarini J, Bellocchi MC, Garaci E, Perno CF. Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir. Antiviral Res. 2002;55:209–225. doi: 10.1016/s0166-3542(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 140.Perno CF, Svicher V, Schols D, Pollicita M, Balzarini J, Aquaro S. Therapeutic strategies towards HIV-1 infection in macrophages. Antiviral Res. 2006;71:293–300. doi: 10.1016/j.antiviral.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 141.Smurzynski M, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fitch KV, et al. Increased coronary artery calcium score and noncalcified plaque among HIV-infected men: relationship to metabolic syndrome and cardiac risk parameters. J Acquir Immune Defic Syndr. 2010;55:495–499. doi: 10.1097/QAI.0b013e3181edab0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lo J, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- 145.Cross SA, et al. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol. 2011;187:5015–5025. doi: 10.4049/jimmunol.1101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burdo T, Blitzer J, McKearn J, Miller A, McGrath M, Williams K. Treatment of SIV+ Monkeys with a Polyamine Biosynthesis Inhibitor, PA300 that Decreases Monocyte Activation and Infection, Blocks AIDS and SIVE. 19th Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, Washington: Washington State Convention Center; 2012. [Google Scholar]