Abstract
Systemic Lupus Erythematosus (SLE) is an autoimmune disease characterized by loss of tolerance to self nucleic acids. The source of autoantigen that drives disease onset and progression is unclear. A candidate source of autoantigen is the Neutrophil Extracellular Trap (NET), which results in the release of nucleic acids into the extracellular environment, generating a structure composed of DNA coated with antimicrobial proteins. Based on in vitro and patient correlative studies, several groups have suggested NETs may provide lupus autoantigens. The observation that NET release (NETosis) relies on activity of the phagocyte NAPDH oxidase (Nox2) in neutrophils of both humans and mice provided a genetic strategy to test this hypothesis in vivo. To do so, we have crossed an X-linked nox2 null allele onto the lupus-prone MRL.Faslpr genetic background and assessed immune activation, autoantibody generation, and SLE pathology. Strikingly, and counter to the prevailing hypothesis, Nox2-deficient lupus-prone mice have markedly exacerbated lupus, including increased spleen weight, increased renal disease, and elevated and altered autoantibody profiles. Intriguingly, heterozygous female mice, which have Nox2-deficiency in 50% of neutrophils, also had exacerbated lupus and altered autoantibody patterns, suggesting that failure to undergo normal Nox2-dependent cell death may result in release of immunogenic self-constituents that stimulate lupus. Our results indicate that NETosis does not contribute to SLE in vivo, and rather that Nox2 acts to inhibit disease pathogenesis.
Introduction
SLE is characterized by production of autoantibodies against DNA, RNA, and associated proteins. This targeted response against nucleic acids depends on Toll-like Receptor (TLR)7 and TLR9 (1, 2). In a lupus-prone genetic background, TLR9 deletion prevents appearance of antibodies to double-stranded DNA, while TLR7 deficiency prevents formation of antibodies to RNA-containing antigens, such as Smith antigen (Sm) (1). Though these discoveries demonstrated the pivotal role of nucleic acid recognition in lupus, the predominant source of nuclear self-antigen remains unknown.
Clues have come from the observation that mutations that impair the clearance of dying cells correlate with increased SLE pathology (3–7). Clearance defects increase the quantity of antigen that is “visible” to the immune system, as do elevated rates of cell turnover, such as the increased levels of neutrophil cell death that have been reported in SLE patients (8). The immunogenic quality of cellular debris may increase via several types of alterations. Failure to cleave and properly dispose of DNA from apoptotic cells may create autoantigens with which the immune system is poorly equipped to deal. Post-translational modifications, which can be regulated by inflammatory signals, affect immunogenicity of “self” (9). In addition, protein antigens typically associated with DNA, if cleaved by caspases or other proteolytic enzymes, can become preferred targets of lupus autoantibodies (10, 11).
The type of death a cell undergoes affects the quality and quantity of its contents that are available to the immune system and conditions responding cells. Apoptosis is typically thought to be anti-inflammatory, but apoptotic cells that die and fail to be rapidly cleared undergo secondary necrosis (12), releasing proinflammatory mediators. Pyroptosis is a proinflammatory form of macrophage cell death in which cellular contents and IL-1β are rapidly released (13). Necroptosis is a recently described form of RIP-kinase dependent programmed cell death involving both reactive oxygen species (ROS) and elements of the autophagy pathway; the implications of this mode of death for immunogenicity of self are unknown (14). Finally, of particular relevance is a form of neutrophil cell death termed “NETosis” in which DNA coated with antimicrobial proteins is released into the extracellular environment, forming a neutrophil extracellular trap (NET) (15, 16). This form of cell death has also been described in mast cells (17) and eosinophils (18), but most studies have been carried out in neutrophils.
Neutrophils are an attractive candidate for the source of autoantigen that drives SLE pathology. They are abundant and have a short half-life under non-inflammatory conditions. Indeed it is estimated that in humans 109 neutrophils die each day per kg of body mass (19). Two separate publications (20, 21) have made the observation that NET DNA delivered to pDCs in vitro has a proinflammatory effect, resulting in the production of type I interferon (IFN) through TLR-dependent signaling. A third group found that NETs derived from blood neutrophils of a subset of SLE patients are protected from degradation by DNase I; such patients had an increased predisposition to nephritis (22). These papers have attempted to link NET formation to the source of autoantigen in lupus as well as directly to pathogenesis. This view has gained wide acceptance (23, 24).
Stimuli that lead to NET formation in vitro include whole pathogens, Phorbol 12-myristate 13-acetate (PMA), and hydrogen peroxide (H2O2). The purpose of NET formation is believed to be pathogen killing; however, this idea remains controversial with several groups reporting conflicting results (15, 16, 25, 26). The details of the signaling cascade leading to NETosis are not fully defined, but ROS production is required for NET formation. Specifically, in the absence of ROS produced by Nox2, NETs are not generated (15, 16, 27).
We took advantage of known defective NET formation in Nox2-deficient mice, which lack the X-linked gp91 component of the phagocyte NADPH oxidase, to directly test the hypothesis that NETs play a key role in SLE pathogenesis. We crossed Nox2-deficient mice to the MRL.Faslpr genetic background and assessed immune activation and target organ damage. The MRL.Faslpr model is an ideal system in which to study this question, as it develops rapid, severe, and genetically penetrant spontaneous lupus that meets the diagnostic criteria defined by the American College of Rheumatology (28), including an autoantibody profile closely matching that of human lupus; interstitial and glomerular nephritis; and skin disease.
Contrary to expectations, in Nox2-deficient MRL.Faslpr animals we found markedly exacerbated disease characterized by severe glomerulonephritis. Furthermore, there was a shift in autoantibody profiles toward RNA-containing autoantigens, with the appearance of a cytoplasmic anti-nuclear antibody (ANA) pattern. Interestingly, nearly all of the phenotypes, including autoantibody patterns, were evident in heterozygous females, in which 50% of neutrophils cannot undergo NETosis. These results are surprising in light of the prevailing hypothesis that NETs drive the anti-self response in SLE. On the other hand, our data are consistent with anecdotal reports linking Nox2 deficiency with an increase in SLE and autoimmunity in humans (29–31), underscoring the relevance of our results and the applicability of the models we used.
Results
Assessment of effects of Nox2-deficiency on neutrophils
As reported for other mouse strains and humans (15, 16, 27), we confirmed that in the absence of Nox2, neutrophils from MRL.Faslpr bone marrow failed to release DNA following stimulation with PMA, but did release DNA in response to hydrogen peroxide, a signaling mediator in the NETosis pathway that is downstream of Nox2 (Fig. S1A). Limited experiments revealed no difference in the propensity of Balb/c, Balb/c.Faslpr, and MRL.Faslpr mice to form extracellular traps, thereby excluding a major effect of the lpr mutation on NET formation (Fig S1B). We also determined that Nox2 deficiency had no effect on neutrophil numbers or percentages in the spleens of fully backcrossed animals (Fig S1C).
Effects of Nox2-deficiency on spleen cell populations in lupus-prone mice
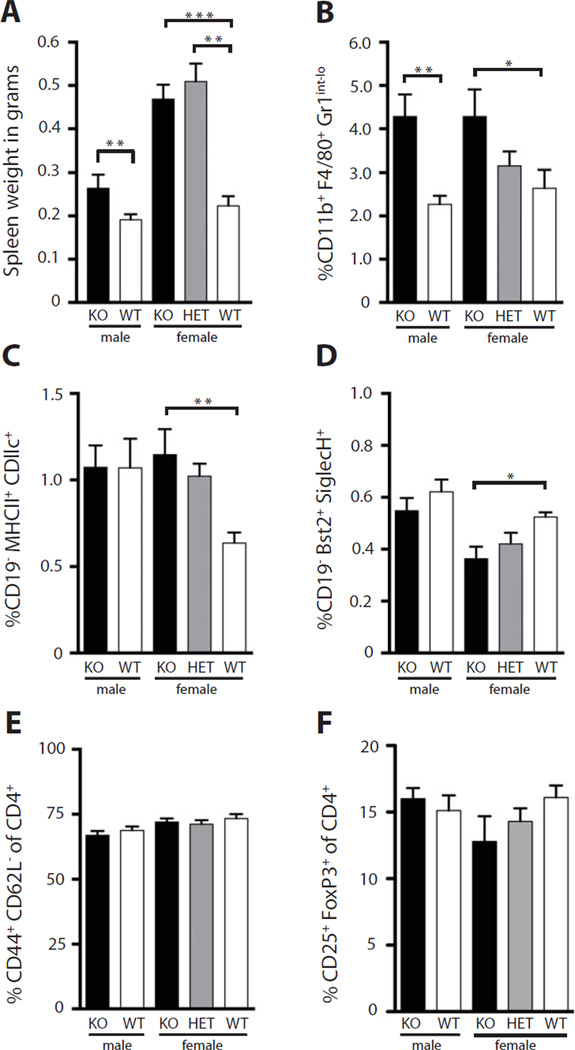
In order to determine the effect of Nox2 deficiency on the progression of SLE pathology, we generated two cohorts of Nox2-deficient mice: an F2 cohort and a fully backcrossed cohort, analyzed at 16 and 14 weeks of age respectively. Both the F2 cohort (Fig. S2) and the fully backcrossed cohort had significantly increased spleen weight in Nox2-deficient males and females (Fig. 1A). Interestingly, heterozygous female mice also had significant splenomegaly. Nox2-deficient mice had expanded percentages (Fig. 1B) of splenic macrophages. We observed the same trend in our F2 cohort (Fig. S2). Similarly, we saw an increase in conventional dendritic cells in Nox2-deficient mice, which only affected females (Fig. 1C). Heterozygous females had an intermediate phenotype that was not statistically significant when compared to fully sufficient mice. Despite increased spleen weight in Nox2-deficient animals, percentages of splenic plasmacytoid dendritic cells (pDCs) were significantly reduced in Nox2-deficient female mice (Fig. 1D). There were no differences in the percentages of activated T cells or Tregs in the spleens of Nox2-deficient animals (Fig. 1E and F).
Fig. 1.
Increased spleen weight and expanded myeloid compartment in Nox2-deficient mice. (A) Spleen weight in grams as a function of nox2 genotype and sex (B–D) FACS analysis of percentage of CD11b+, F4/80+, Gr1int-lo macrophages (B), MHC II+, CD11c+ conventional dendritic cells (C), and Bst+, SiglecH+, pDCs (D) in the spleens of mice with the indicated genotype, reported as percentage of live. (E–F) FACS analysis of CD44+, CD62L−, activated CD4 T cells (E), and CD25+, FoxP3+ T cells (F) in the spleens of mice with the indicated genotype. Percentages are reported as a percentage of CD4+, TcRβ+ cells. Statistical analyses were performed with the one-tailed Mann Whitney test. Five or more mice were analyzed per genotype at 14 weeks of age.
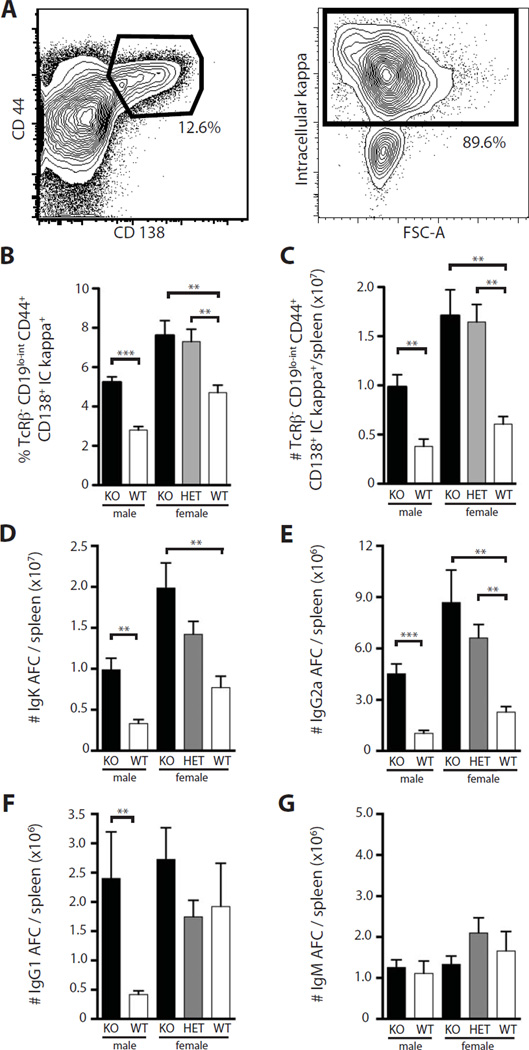
Increased antibody forming cells (AFCs) in the absence of Nox2
By flow cytometry we defined AFCs as TCRβ−, CD19lo-int, CD44+, CD138+, intracellular kappa+ (Fig. 2A) cells. We noted an increase in percentages (Fig. 2B) and total number (Fig. 2C) of AFCs in Nox2-deficient males and females. Heterozygous female mice also exhibited elevated numbers of AFCs, comparable to levels seen in deficient females. Commensurate with the flow cytometric data, we observed increases in kappa-producing AFCs, as measured by ELISpot in male and female Nox2-deficient mice; again heterozygous females had an intermediate phenotype though the differences did not reach significance compared to Nox2-sufficient animals (Fig. 2D). There was a similar pattern for IgG2a AFCs, with heterozygous females having statistically significant elevations (Fig. 2E). IgG1 AFCs were also elevated in Nox2-deficient animals, though it reached significance only in the male mice (Fig. 2F). There were no changes in IgM AFC numbers among the groups (Fig. 2G). Thus, by both flow cytometry and ELISPot analysis, Nox2-deficient animals had substantially higher frequencies and numbers of AFCs, with heterozygous females often showing a phenotype almost as strong as the homozygous knockout mice.
Fig. 2.
Increased antibody forming cells in the absence of Nox2. (A) Gating strategy for AFCs in the spleen. Cells were initially gated on live, TcRβ− cells. CD44+ CD138+ cells (left) were then gated on intracellular kappa+ (right) to identify AFCs. (B–C) FACS quantification of TcRβ−, CD19low-int, CD44+, CD138+ intracellular kappa+ cells as a function of genotype for both percentage of live (B) and total numbers (C) in the spleen. (D–G) AFCs per spleen as determined by ELISPOT for IgK (D), IgG2a (E), IgG1 (F), and IgM (G). Statistical analyses were performed with the one-tailed Mann Whitney test. Five or more mice were analyzed per genotype. Mice were analyzed at 14 weeks of age.
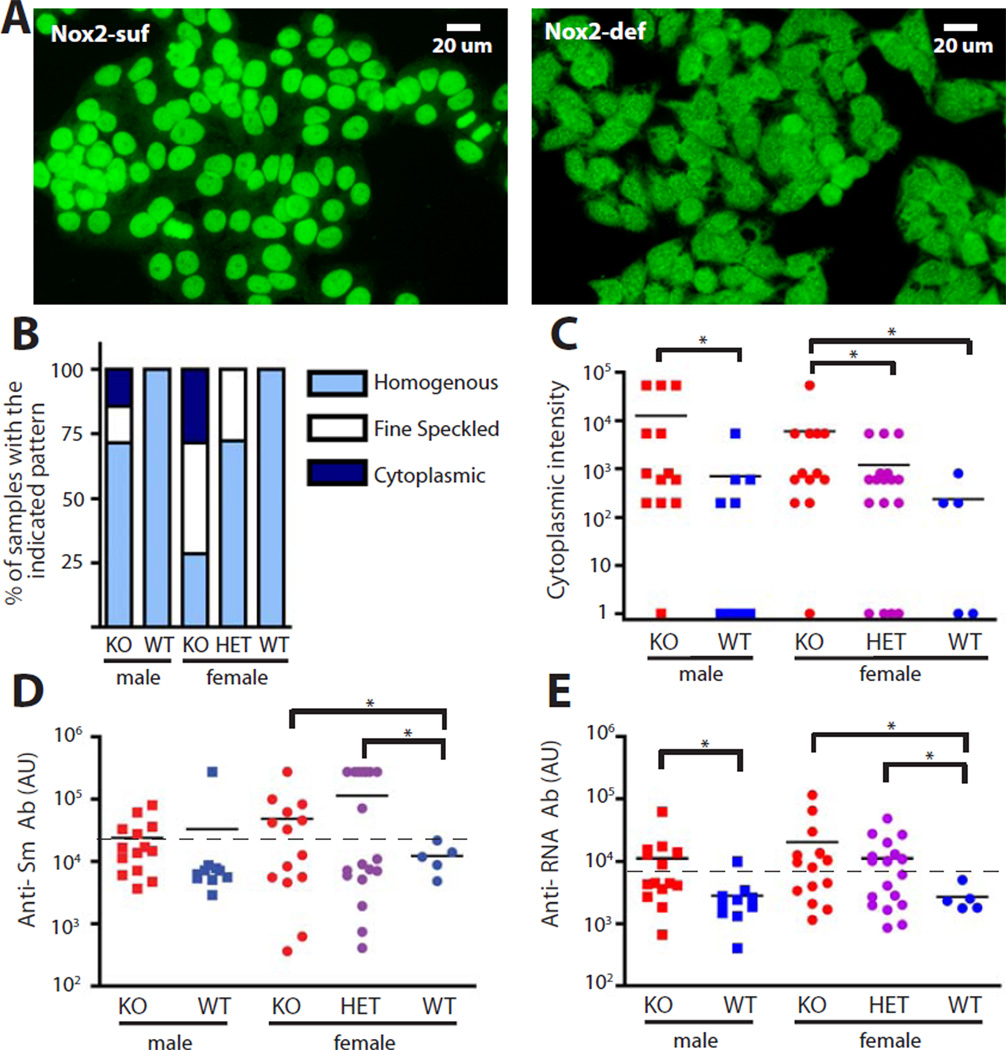
Nox2 influences the anti-self response
To determine the effect of Nox2 on the nature of the anti-self response, we characterized the dominant ANA patterns seen in Nox2-sufficient vs. deficient mice. Plasma from Nox2-sufficient mice produced a homogenous staining pattern (Fig. 3A, left). Homogenous staining patterns were also produced by some Nox2-deficient mice, but a considerable fraction of samples displayed a cytoplasmic staining pattern in both the fully backcrossed (Fig. 3A right, 3B) and F2 cohort (Fig. S3). In addition, a fraction of plasma samples from the Nox2-deficient animals demonstrated a speckled nuclear pattern (Fig. 3B). Neither of these two patterns was observed as a dominant pattern in plasma from Nox2 wild-type mice. Accordingly, cytoplasmic staining intensity was increased in Nox2-deficient males and females relative to their sufficient counterparts, though in this case heterozygous females did not differ from fully sufficient females (Fig. 3C). Similar results were also observed in our F2 cohort (Fig. S3). Speckled patterns are associated with anti-RNP and Sm autoantibodies; we assessed the latter by ELISA. Both the Nox2-deficient and heterozygous females in the backcrossed cohort were more likely to make a high titer anti-Sm response, based on a Chi-Squared analysis (Fig. 3D). Anti-Sm was also prominent in males and females of the F2 cohort, again with a heterozygous phenotype (Fig. S3). Anti-RNA antibodies were also increased in Nox2-deficient males and females (Fig 3E). Again, the heterozygous females showed significantly elevated anti-RNA antibodies as compared to fully sufficient females. No statistically significant changes were noted in anti-nucleosome or rheumatoid factor autoantibodies in fully backcrossed mice ( Fig. S4), nor did we see any differences in IFNα levels (Fig S4). The shift in the nature of the ANA response and the appearance of a cytoplasmic pattern indicates the character of the autoimmune response is altered in the absence of Nox2. Moreover, it is particularly notable that these shifts affected heterozygous females, arguing for a dominant effect of Nox2-deficiency.
Fig. 3.
Altered anti-self response in the absence of Nox2. (A) Representative ANA staining patterns from plasma of Nox2-sufficient (left) and Nox2-deficient animals (right). (B) Dominant ANA pattern quantitated for each genotype by dilution. (C) Intensity of cytoplasmic HEp-2 staining quantitated for each genotype by the dilution at which the dominant pattern disappeared. Statistical analysis was performed with the one-tailed Mann-Whitney test. (D–E) ELISA assessment of Anti-Sm antibodies (D) and anti-RNA antibodies (E) in the plasma of fully backcrossed mice of the indicated genotype. A one-tailed Chi-Squared test was used to determine statistical significance. Threshold positivity (indicated by dashed line) was set at 25,000 AU for Anti-Sm (D) or 8,000 AU for Anti-RNA (E). Samples were taken from 14-week old mice.
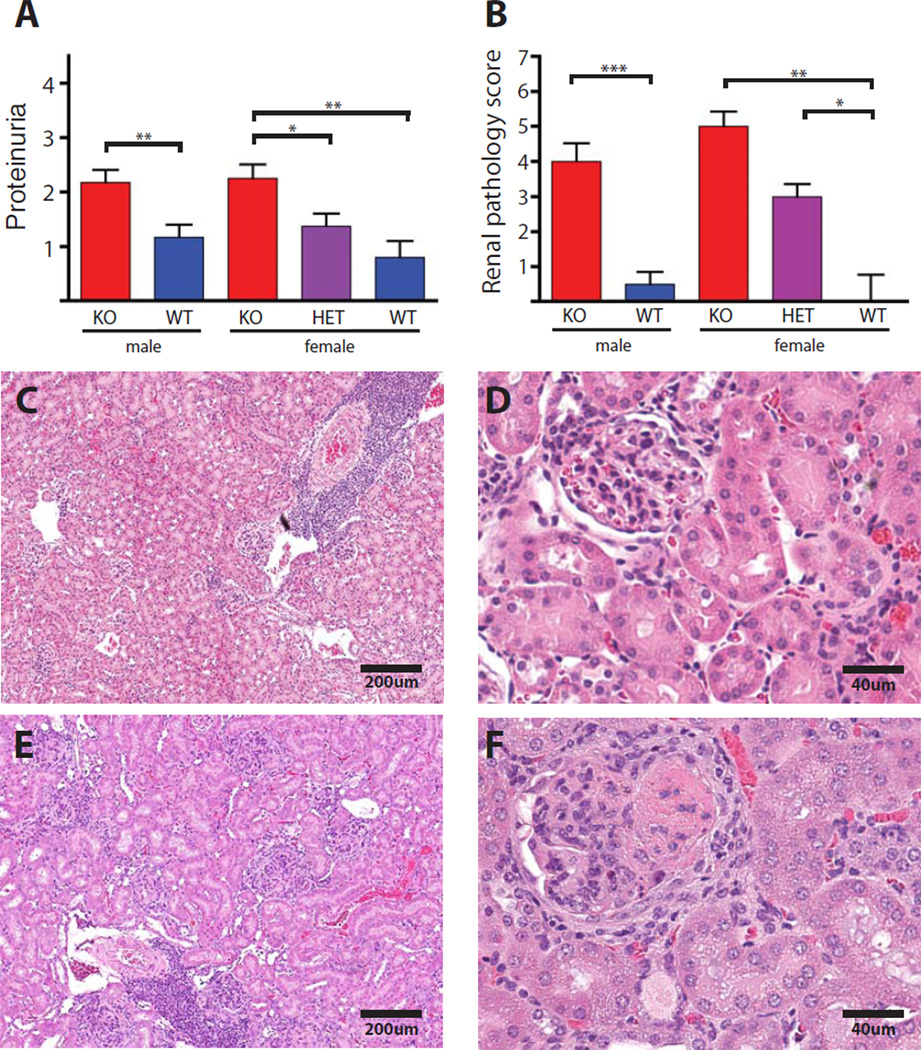
Renal disease is increased in the absence of Nox2
Proteinuria was markedly elevated in Nox2-deficient males and females compared to controls. As with several of the other phenotypes, there was a trend toward elevated proteinuria in the heterozygous females though this did not reach significance compared to Nox2-sufficient animals (Fig. 4A). Commensurate with these functional alterations, males and females deficient in Nox2 had more severe histologic glomerular disease as compared to Nox2-sufficient controls. In addition, heterozygous females showed increased glomerular pathology relative to their sufficient littermates (Fig. 4B–F). Exacerbated glomerular pathology was also observed in Nox2-deficient mice in the F2 cohort (Fig. S5). Severe glomerulonephritis in Nox2-deficient animals was characterized by enlarged, hypercellular glomeruli with proteinaceous deposits and surrounding fibrocellular crescent formation (Fig. 4F and Fig S5).
Fig. 4.
Increased renal disease in the absence of Nox2. (A) Proteinuria was assessed using Siemens Albustix(R). (B) Glomerulonephritis was scored blindly by M.K. on a scale of 1 to 6, and is represented as a function of Nox2 genotype in backcrossed mice. (C–F) Example of a Nox2-sufficient kidney (C and D) and deficient kidney (E and F) at 4X (left panels) and 10X magnification (right panels). Proteinuria assessment and histological analysis was performed on mice that were 14 weeks of age.
Discussion
The idea that NETs are a source of autoantigen that drives SLE pathogenesis has been proposed by several groups (20–22), based on in vitro data and correlative in vivo observations. We took advantage of NET dependence on Nox2-generated ROS to test this novel concept in vivo, using a model of murine SLE. Contrary to the prevailing hypothesis, we found that SLE could proceed without NET formation. In fact, disease was exacerbated in mice lacking the ability to generate extracellular traps.
One potential concern is that, because of implicit immunodeficiency associated with Nox2-deficiency, exacerbated disease was due to infection rather than autoimmunity. This is unlikely for several reasons. First, and most critically, there was a significant disease phenotype in heterozygous female mice; yet, these heterozygous mice are immunocompetent. Second, all experimental animals in our fully backcrossed cohort were given continuous broad-spectrum antibiotics in specific pathogen-free housing, minimizing the chance of clinically relevant infection. Finally, the disease that we observed, including the increase in autoantibodies, the character of the renal pathology, and the shift in ANA pattern toward an RNA-containing Ag were all pathognomonic of SLE and not consistent with underlying infection.
The observation that Nox2-dependent NET generation is not a significant driver of disease pathology is a conclusion of fundamental importance to the understanding of lupus pathogenesis as well as the potential role of NETs. Beyond this, and equally important, the finding that disease was markedly exacerbated highlights the unexpected regulatory role of Nox2 and identifies Nox2 as an important target of research aimed at understanding and ameliorating SLE. Nox2 could be playing a role in reducing pathology that is NET-independent, NET-dependent, or both.
There are several ways that Nox2 could regulate disease pathology independent of NETosis. One possibility is that, since the immunosuppressive enzyme IDO requires superoxide produced by Nox2 as a cofactor for proper function, it does not function efficiently in Nox2-deficient animals and that the lack of its suppressive properties is responsible for exacerbated immunopathology (32, 33). However, several studies of patients with chronic granulomatous disease (CGD), in which Nox2 activity is impaired or absent, have shown no defects in IDO activity (34, 35). Nox2-deficiency could also promote disease by impairing effective phagocytosis (36). In the absence of Nox2, cellular debris may be ineffectively cleared, leading to an increase in the amount of antigen driving the anti-self response. Finally, Nox2 has been shown to shift the balance between Treg and Th17 cells. After fungal infection, nox2−/− B6 mice had deficient Treg and excessive Th17 cells, associated with pathological hyperinflammation. A similar picture was also observed in B6 Nox2-deficient mice that were reported with spontaneous arthritis (37). We found no differences in Tregs (Fig. 1F) or IL-17 production (data not shown), arguing that such T cell shifts do not play a large role in our system.
It is also possible that NETosis is inherently anti-inflammatory. The phenomenon is evolutionarily conserved (38), suggesting a homeostatic function. We speculate that NETosis is a physiological form of cell death that facilitates efficient, non-inflammatory clearance of neutrophils. In the absence of NETosis, neutrophils may undergo a form of cell death that is more inflammatory and which releases excessive nucleic acids and proteins, possibly in an altered and thus more immunogenic form.
It is notable that mice heterozygous for the targeted nox2 allele had alterations in autoantibody patterns and exacerbated nephritis similar to that seen in fully Nox2-deficient animals. These results are particularly intriguing given that mothers of boys with X-linked CGD show an increased predisposition to SLE (29–31), thus validating the results of our experimental model. Because nox2 is on the X-chromosome, in heterozygotes 50% of neutrophils cannot die via NETosis, with the Nox2-deficient fraction of neutrophils potentially serving as a source for immunogenic self-components. The heterozygous phenotype suggests that Nox2-deficiency acts in a dominant manner to promote disease pathology. In particular, the alteration in autoantibody specificities, even when only 50% of neutrophils lack Nox2, suggests that there is a shift in the nature of the autoantigen driving the anti-self response. We thus propose that altered cell death in the absence of Nox2 leads to an increase in immunogenic cellular debris that in turn dominantly breaks self-tolerance.
The exacerbated disease in the absence of Nox2 and NETosis implies neutrophil cell death is an important determinant of lupus. How then might neutrophils die in the absence of Nox2 and what could be the consequences for quantity and quality of available autoantigen? Caspases are inhibited by active Nox2, preventing apoptotic cell death (39, 40); hence neutrophil apoptosis is expected to be more prominent in the absence of Nox2. In addition, ROS inhibits serine proteases, which are abundant in neutrophil granules. This inhibition of proteases, along with the tethering of proteases in the NET itself, may normally limit the extracellular damage such enzymes could inflict. Rosen and colleagues were among the first to point out that lupus autoantigens are often cleaved by serine proteases (41). When Nox2-deficient neutrophils die, releasing more activated proteases, they may also create more neo- and cleaved autoantigens, with enhanced immunogenicity. It is also possible that Nox2-deficient neutrophils undergo a more proinflammatory form of cell death, such as necroptosis or pyroptosis. Both of these can lead to more caspase activation and IL-1β release—itself associated with SLE (42)—and both can be regulated by ROS.
Though the importance of dead cell clearance has long been recognized in SLE (4), our work implicates Nox2 as a critically important player in protection from the immunogenic burden of dying cells, in particular neutrophils. Further support for the importance of Nox2 comes from a recent study showing that a mutation in neutrophil cytosolic factor 2 (NCF2), which confers substantially increased SLE risk, results in a reduction of Nox2 activity and ROS production (43). This observation further bolsters the relevance of our results to human disease.
Often in genetically predisposed individuals, an inciting event seems to initiate or restimulate an anti-self response. Infections will induce Nox2 activity in neutrophils and macrophages. As infection is associated with excess cell death, we suggest that optimal Nox2 activity may be needed in order to prevent release of cellular material either too great in quantity or too immunogenic in quality. Mutations that impair components of this pathway thus could be a liability in the context of infection, and would be candidates for a context-dependent genetic predisposition linking infection to the development of frank autoimmune disease (44).
Materials and Methods
Mice
F1 offspring were generated by crossing Nox2-deficient with MRL.Faslpr mice, which were then intercrossed to generate an F2 cohort. Faslpr homozygotes were analyzed for disease pathology as described below. Fully backcrossed mice were crossed onto the MRL.Faslpr background between 8 and 11 times. These mice were intercrossed to produce an experimental cohort. SLE pathology was analyzed at 16 weeks for F2 mice and 14 weeks for backcrossed mice due to severe SLE disease that limited the lifespan of the mutant animals. Mice were housed under specific-pathogen-free (SPF) conditions and backcrossed animals received continuous Sulfatrim. All work was approved by Yale University’s Institutional Animal Care and Use Committee.
Evaluation of SLE pathology
Proteinuria was quantified using Albustix. Plasma was obtained by cardiac puncture. Left kidneys were removed, bisected, formalin-fixed, paraffin embedded, and H&E stained. Kidneys were scored for glomerulonephritis in a blinded manner by M.K. Details regarding nephritis scoring are in the supplemental methods.
Flow Cytometry
Spleens were homogenized and red blood cells were lysed. Cells were resuspended in Phosphate Buffered Saline (PBS) with 3% calf serum and the FcR-blocking antibody 2.4G2. Live/dead discrimination was performed with ethidium monozaide bromide (Invitrogen). Surface staining antibodies are listed in the Supplemental Methods. Cells were fixed in 1% paraformaldehyde (Electron Microscopy Sciences). A FoxP3 staining kit (eBioscience) was used for intracellular FoxP3 staining. Data were obtained using a LSRII (BD) with FACS DIVA software and analyzed using FlowJo.
ANA
Plasma was diluted in 6 steps and each dilution applied to HEp-2 slides (Antibodies Incorporated) and staining detected using goat anti-mouse IgG-FITC (Southern Biotech). The cytoplasmic and nuclear staining was scored on coded samples and endpoint titers determined as the dilution at which the pattern disappeared, with the last pattern to disappear being the dominant pattern. If nuclear and cytoplasmic patterns disappeared at the same dilution, the dominant pattern was characterized as cytoplasmic. Images were acquired with a wide-field microscope (Nikon Eclipse Ti) and a CCD camera (QImaging Retiga 2000R) with NIS Elements software.
ELISA and ELISpot assays
Anti-Sm, anti-nucleosome, and rheumatoid factor ELISAs and ELISpot assays were performed as previously described (2). Briefly, the anti-RNA assay was performed by coating plates first with poly-L-lysine and after washing with 15ug/mL of RNA from S. cerevisae (Sigma). Plates were blocked with PBS with 1% BSA and 0.05% sodium azide and serially diluted plasma was applied. Anti-RNA antibody in plasma was detected with goat anti-mouse IgG-AP (Southern Biotech). The anti-IFNα ELISA was performed by coating Immulon-4 plates with rat anti-mouse IFNα PBL Labs). Plates were blocked as above and plasma was applied at a 1:4 dilution. After washing, IFNα was detected with rabbit polyclonal anti-mouse IFNα (PBL Labs) and goat anti-rabbit IgG-AP (Southern Biotech).
Statistics
Statistical analysis was performed using Prism. Bar graphs display the means of at least 5 mice per group. Error bars indicate standard error of the mean unless otherwise specified in the figure legend. Scatter plots display the medians of each group. Each dot in a scatter plot corresponds to one mouse. Statistical tests used are listed in the captions of each figure.
*indicates p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Supplementary Material
Acknowledgments
We thank Yale Animal Resources Center for outstanding animal husbandry and Jaime Cullen for expert technical help. We thank Ann Marshak-Rothstein, Marko Radic, Joe Craft, Lino Teichmann, and Noah Capurso for critical reading of the manuscript. Funding: Support was provided by R01-AI084958 and P01-AR050256 (M.J.S.) , NIH 2T32GM07205 (A.M.C) and NIH Ruth L Kirschstein F30 DK091993-02 (A.M.C.)
Footnotes
Author contributions: A.M.C. Performed the experiments, analyzed the data, and wrote the manuscript. M.K. analyzed renal pathology. M.J.S. supervised the study, analyzed the data, and wrote the manuscript.
Competing interests: We declare no competing interests.
References and Notes
- 1.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 5.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 6.Shin HD, Park BL, Cheong HS, Lee HS, Jun JB, Bae SC. DNase II polymorphisms associated with risk of renal disorder among systemic lupus erythematosus patients. J Hum Genet. 2005;50:107–111. doi: 10.1007/s10038-004-0227-3. [DOI] [PubMed] [Google Scholar]
- 7.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, M�r�y T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 8.Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2888–2897. doi: 10.1002/art.11237. [DOI] [PubMed] [Google Scholar]
- 9.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol. 2004;16:753–758. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Darrah E, Rosen A. Granzyme B cleavage of autoantigens in autoimmunity. Cell death and differentiation. 2010;17:624–632. doi: 10.1038/cdd.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall JC, Casciola-Rosen L, Rosen A. Altered structure of autoantigens during apoptosis. Rheumatic diseases clinics of North America. 2004;30:455–471. vii. doi: 10.1016/j.rdc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Molinaro C, Johnson N, Casiano CA. Secondary necrosis is a source of proteolytically modified forms of specific intracellular autoantigens: implications for systemic autoimmunity. Arthritis Rheum. 2001;44:2642–2652. doi: 10.1002/1529-0131(200111)44:11<2642::aid-art444>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Fink S, Cookson B. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 14.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The Role of the Kinases RIP1 and RIP3 in TNF-Induced Necrosis. Sci. Signal. 2010;3 doi: 10.1126/scisignal.3115re4. re4- [DOI] [PubMed] [Google Scholar]
- 15.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von K�ckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 18.Mihalache CC, Yousefi S, Conus S, Villiger PM, Schneider EM, Simon HU. Inflammation-associated autophagy-related programmed necrotic death of human neutrophils characterized by organelle fusion events. J Immunol. 2011;186:6532–6542. doi: 10.4049/jimmunol.1004055. [DOI] [PubMed] [Google Scholar]
- 19.Athens JW, Haab OP, Raab SO, Mauer AM, Ashenbrucker H, Cartwright GE, Wintrobe MM. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA-Peptide Complexes in Systemic Lupus Erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proceedings of the National Academy of Sciences. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craft JE. Dissecting the immune cell mayhem that drives lupus pathogenesis. Sci Transl Med. 2011;3:73ps9. doi: 10.1126/scitranslmed.3002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Menegazzi R, Decleva E, Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood. 2012;119:1214–1216. doi: 10.1182/blood-2011-07-364604. [DOI] [PubMed] [Google Scholar]
- 26.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 27.Ermert D, Urban C, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 29.Cale LMDG CM. Cutaneous and other lupus-like symptoms in carriers of X-linked chronic granulomatous disease: incidence and autoimmune serology. Clinical and Experimental Immunology. 2007;148:79–84. doi: 10.1111/j.1365-2249.2007.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzi S, Urbach AH, McCune AB, Altman HA, Kaplan SS, Medsger TA, Ramsey-Goldman R. Systemic lupus erythematosus in a boy with chronic granulomatous disease: Case report and review of the literature. Arthritis & Rheumatism. 1991;34:101–105. doi: 10.1002/art.1780340116. [DOI] [PubMed] [Google Scholar]
- 31.Schaller J. Illness Resembling Lupus Erythematosus in Mothers of Boys with Chronic Granulomatous Disease. Annals of Internal Medicine. 1972;76:747–750. doi: 10.7326/0003-4819-76-5-747. [DOI] [PubMed] [Google Scholar]
- 32.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti M, Grohmann U, Segal B, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 33.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 34.Jürgens B, Fuchs D, Reichenbach J, Heitger A. Intact indoleamine 2,3-dioxygenase activity in human chronic granulomatous disease. Clin Immunol. 2010;137:1–4. doi: 10.1016/j.clim.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Ravin SS, Zarember KA, Long-Priel D, Chan KC, Fox SD, Gallin JI, Kuhns DB, Malech HL. Tryptophan/kynurenine metabolism in human leukocytes is independent of superoxide and is fully maintained in chronic granulomatous disease. Blood. 2010;116:1755–1760. doi: 10.1182/blood-2009-07-233734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Boyanapalli R, McPhillips KA, Frasch SC, Janssen WJ, Dinauer MC, Riches DW, Henson PM, Byrne A, Bratton DL. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, Won HY, Bae MA, Hong J-H, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proceedings of the National Academy of Sciences. 2011;108:9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 39.Fadeel B, Ahlin A, Henter J-I, Orrenius S, Hampton MB. Involvement of Caspases in Neutrophil Apoptosis: Regulation by Reactive Oxygen Species. Blood. 1998;92:4808–4818. [PubMed] [Google Scholar]
- 40.Wilkie RP, Vissers MCM, Dragunow M, Hampton MB. A Functional NADPH Oxidase Prevents Caspase Involvement in the Clearance of Phagocytic Neutrophils. Infect. Immun. 2007;75:3256–3263. doi: 10.1128/IAI.01984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrade F, Casciola-Rosen L, Rosen A. Apoptosis in systemic lupus erythematosus. Clinical implications. Rheumatic diseases clinics of North America. 2000;26:215–227. v. doi: 10.1016/s0889-857x(05)70136-8. [DOI] [PubMed] [Google Scholar]
- 42.Nagahama M, Nomura S, Ozaki Y, Yoshimura C, Kagawa H, Fukuhara S. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity. 2001;33:85–94. doi: 10.3109/08916930108995993. [DOI] [PubMed] [Google Scholar]
- 43.Jacob CO, Eisenstein M, Dinauer MC, Ming W, Liu Q, John S, Quismorio FP, Reiff A, Myones BL, Kaufman KM, McCurdy D, Harley JB, Silverman E, Kimberly RP, Vyse TJ, Gaffney PM, Moser KL, Klein-Gitelman M, Wagner-Weiner L, Langefeld CD, Armstrong DL, Zidovetzki R. Lupusassociated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci U S A. 2012;109:E59–E67. doi: 10.1073/pnas.1113251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doria A, Canova M, Tonon M, Zen M, Rampudda E, Bassi N, Atzeni F, Zampieri S, Ghirardello A. Infections as triggers and complications of systemic lupus erythematosus. Autoimmun Rev. 2008;8:24–28. doi: 10.1016/j.autrev.2008.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.