Abstract
Background
The Wnt/β-catenin signaling pathway plays an important role in regulating cellular differentiation, proliferation, and polarity.
Methods
We used bleomycin to induce lung fibrosis in a transgenic Wnt reporter mouse to characterize the expression pattern of cyclin Dl, MMP-7, and TGF-β in conjunction with the Wnt/β-catenin signaling pathway. LacZ expression reveals the Wnt/β-catenin signaling pathway through the activated (nuclear) β-catenin and coactivation of LEF/TCF transcription factors. X-gal staining and immunohistochemical staining of β-catenin, cyclin Dl, MMP-7, and TGF-β were assessed after bleomycin administration.
Results
We observed LacZ expression in bronchiolar proliferative lesions and the epithelium in remodeled cystic and fibrotic areas at both 1 and 3 weeks. Nuclear β-catenin staining was prominent in epithelial cells of remodeled and fibrotic areas at 3 weeks. MMP-7 was faint in basement membranes of airways and matrix zones in fibrotic areas at 3 weeks. Cyclin Dl was observed in alveolar macrophages (AM), alveolar epithelium, and fibrotic areas consistent with rapid cell turnover in these areas at both 1 and 3 weeks. TGF-β was faintly staining in alveolar macrophages and epithelial cells at 3 weeks.
Conclusion
The Wnt/β-catenin pathway is activated in bleomycin-induced lung fibrosis, and downstream genes were localized in AM, alveolar epithelium, and interstitium.
Keywords: pulmonary fibrosis, beta-catenin, Wnt pathway
Introduction
Idiopathic pulmonary fibrosis (IPF), a progressive fibroproliferative lung disease, is characterized initially by alveolar epithelial cell injury followed by exaggerated fibroblast migration, activation, and proliferation with extracellular matrix remodeling.1–3 Alveolar epithelial cell injury by oxidants followed by hyperplasia, loss of subepithelial basement membrane, and Import of macrophage and fibroblast growth factors leads to mesenchymal cell proliferation.3 The histologic hallmarks of IPF are fibroblastic foci, the early active areas of fibroblastic proliferation, which represent the “leading edge” of the interconnected reticulum and progressive fibrotic process.3–5 Lysophosphatidic acid receptor links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leaks.6 The source of the myofibroblast may be a bronchoalveolar progenitor cell that has migrated into the injured interstitium or from the circulation or bone marrow, or it could be through epithelial-to-mesenchymal transition (EMT).7–9 Understanding the molecular events leading to expansion of myofibroblasts may present unique therapeutic strategies.10 Alveolar epithelial cells can undergo EMT to produce myofibroblasts in IPF patients or even in vitro under TGF-β1 treatment.11 TGF-β activation is implicated in EMT and fibrosis through Stimulation of α-smooth muscle actin, desmin, and procollagen mRNA that localized in myofibroblasts in active fibrotic lesions.12 The highly coordinated interaction of TGF-β and Wnt signaling pathways, an important morphogenetic pathway, have been widely observed in developmental and metastatic processes.13,14
Wnt proteins play important roles in regulating cellular differentiation, proliferation, and polarity by signaling through at least three known pathways: Wnt/β-catenin pathway (Canonical pathway), Wnt/Ca++ pathway and planar cell-polarity pathway.13,15,16 In the Wnt/β-catenin pathway, β-catenin is a key effector. In the absence of Wnt ligands, the cytoplasmic levels of β-catenin are kept low through the phosphorylation by a protein complex composed of axin, adenomatous Polyposis coli protein (APC), and glycogen synthase kinase-3 (GSK-3) and degradation by proteasome. Activation of Wnt signaling inhibits GSK-3 activity through the interacting of Wnt proteins with their Frizzled (Fzd) receptors. Hypophosphorylated β-catenin accumulates in the cytoplasm and then translocates to the nucleus. There, β-catenin binds to the T-cell factor/lymphoid enhancer factor (Tcf/Lef) family and regulates the expression of downstream genes. Activation of specific gene transcription by β-catenin is also coordinated by TGF-β signaling and its downstream effectors (Smads) in the nucleus.13 In development, activities of Smads, TCF/LEF and β-catenin appear to synergize to increase gene transcription such that any one of the proteins alone activates transcription at a base level, which increases to maximum when all three proteins are involved.13 For example, TGF-β3 signaling through Smads 2, 4 activates transcription of the LEF1 gene to induce EMT, instead of through β-catenin/LEFl, and close the palatal seam, preventing torus palatinus in the mouse.14 Changes in gene expression from microarray evaluation of IPF lung tissue have been noted for several Wnt or growth-related genes: Wnt homologs, the frizzled receptor, TGF-β3, LEF1, and insulin-like growth factor I.1 Insulin-like growth factor binding protein 5 (IGF-BP5) is expressed in alveolar epithelial cells with α-smooth muscle actin in a fibrosis mouse model consistent with EMT, and IGF-BP3 is increased in IPF.17,18 Increased TGF-β expression, decreased bone morphogenetic protein-2(BMP), and active BMP inhibition by gremiin favor an EMT environment in IPF lungs.1,19 The Wntl-inducible signaling protein 1 (WISP 1) and the secreted frizzled-related protein 2 are increased in IPF compared to hypersensitivity Pneumonitis.20
Deregulation of this pathway has been implicated in tumorigenesis of several organs, including GI epithelia and skin.21,22 Several recent studies of the pathogenesis of IPF have shown that the Wnt/β-catenin pathway is involved in fibrogenesis in both humans and mice.23,24 Chilosi et al. found extensive accumulation of β-catenin indicating activation of the Wnt/β-catenin pathway in proliferative bronchiolar lesions, including bronchial epithelial cells, basal cell hyperplastic cells, and areas of bronchiolization and honeycombing.24 Nuclear β-catenin was found in myofibroblasts in fibroblast foci in 16 out of 20 IPF lung specimens. Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) for components of the Wnt signaling pathway found β-catenin to be increased in 12 IPF lungs compared to 12 transplant donor lungs, and further, immunohistochemicallocalization of Wntl, Wnt3a, β-catenin, and GSK-3β was to the alveolar and bronchial epithelium.25 β-catenin mRNA was increased in isolated alveolar epithelial cells from IPF lungs. Two downstream targets of β-catenin, matirilysin (MMP-7) and cyclin-Dl were also found to be prominent in many of the same fibroproliferative lesions.26 MMP-7, a family member of matrix metalloproteinases (MMPs), mediates matrix degradation and plays multifunctional roles, including the induction of epithelial cell migration, apoptosis, and metaplastic conversion. Microarray analysis identified MMP-7 as a major mediator of fibrosis in both murine and human lung fibrosis.26 The protection of MMP-7 knockout mice from bleomycin-induced fibrosis further confirms that this metalloproteinase plays an important role in the development of pulmonary fibrosis. Cyclin Dl is a critical cell cycle regulator that drives the cell cycle from the Gl to the S phase and has a lymphocyte-enhancing factor (LEF-1) binding site on its promoter.27 Inhibitors of β-catenin activation, wild-type adenomatous Polyposis coli, axin, and the cytoplasmic tail of Cadherin suppresses cyclin Dl promoter activity in Cancer cells.27 TGF-β/Smad signaling targets the LEF-1 gene in the Wnt/β-catenin pathway.28 In this study, we used bleomycin to induce lung fibrosis with a transgenic Wnt reporter mouse line TOPGAL and studied the roles of Wnt/β-catenin signaling in lung fibrosis by immunohistochemical analysis of gene expression in specific cell compartments. We hypothesized that, similar to Cancer, where Wnt signaling is involved in the loss of cell-cell adhesion and cancer induction and progression, increased Wnt signaling in pulmonary inflammation/fibrosis will contribute to EMT and exaggerated proliferation of myofibroblasts.
Methods of Procedure
Mice and bleomycin induce lung fibrosis. The transgenic Wnt reporter mouse TOPGAL were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in a specific pathogen-free environment.29 The 6–8 week old mice were anesthetized with isoflurane. Fifty microliters of bleomycin Solution (BLM, 0.5 U/100 g body weight in 50 μL sterile saline) or sterile saline was injected as a Single dose directly into the trachea. Mice were sacrificed at time points of 1 day, 1 week, and 3 weeks after BLM administration. There were n = 3−6 mice in each group, and representative examples are shown. The mouse strain was CD1, and equal females and males were used. The experimental protocol had approval of the local IUCAC committee.
Histology and Immunohistochemistry
At various time points, mice were sacrificed for paraffin section or cryosection and processed as follows. For cryosection, lungs were perfused with ice cold PBS and inflated 1:1 with OCT with 30% sucrose in PBS before dissection. After rinsing with ice cold PBS, the whole lungs were immersed in 15% sucrose overnight followed by 30% sucrose for 3–4 h at 4°C. Then, lung samples were embedded in OCT and cryosectioned at 8–10 μm. To identify the Wnt/β-catenin signaling active cells (LacZ expressing cells), frozen sections were subjected to X-gal staining by incubating sections with X-gal staining Solution 0.1% 4-chloro-5-bromo-3-indolyl β-D-galactopyranoside (X-gal), 2 mM MgCl2, 5 mM EGTA, 0.02% Nonidet P-40, and 5 mM K4Fe(CN)6·3 H20 at 37°C overnight. Lungs from were inflation-fixed at 25 cm H20 with zinc fixative (Sigma) and later paraffin embedded for histologic analysis. Tissue sections were stained with H&E for histology.
Immunohistochemistry was performed on 5 μm sections from paraffin-embedded lung blocks. The sections were deparaffinized and incubated in 6% hydrogen peroxide in methanol to inactivate endogenous peroxidases and then 0.1% pepsin for antigen retrieval. After blocking with protein blocking Solution, the sections were incubated with the following antibodies: β-catenin (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), cyclin Dl (1:60, Santa Cruz Biotechnology), MMP-7(1:60, Santa Cruz Biotechnology), and TGF-β(l:60, Santa Cruz Biotechnology). Biotinylated secondary antibodies and a streptavidin-biotin-peroxidase detection System (Vector Laboratories, Burlingame, CA) were used according to manufacturer's Instructions. The sections were counterstained with hematoxylin. The primary antibody was replaced by nonimmune serum for negative control slides. Ten randomly selected fields were analyzed for each section.
Results
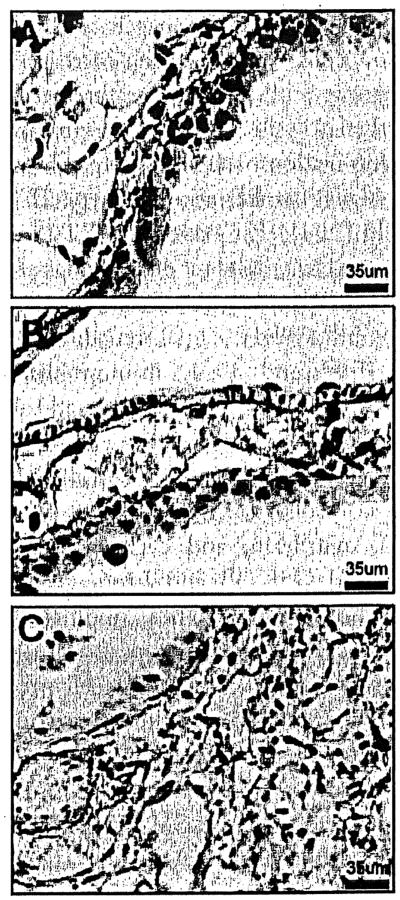
Wnt/β-catenin signaling pathway was active in BLM-induced lung fibrosis. The TOPGAL mice were Wnt reporter mice that carry the lacZ gene under the control of an artificial promoter: Tcf-optimal-promoter (TOP). TOP consists of three consensus lymphoid enhancer binding factor 1/transcription factor 3 (LEF/TCF)-binding motifs upstream of a minimal c-fos promoter. Therefore, LacZ expression reveals active Wnt/β-catenin signaling through the activated (nuclear) β-catenin and coactivation of LEF/TCF transcription factors.29 We compared normal saline to bleomycin-treated mice at 1 day, 1 week, and 3 weeks (3–7 mice/ group). In normal saline control mice, LacZ expression (blue cells) were observed in bronchial epithelium and some cells in alveolar areas (Figs. 1, 2A, and 2B). At 1 day after bleomycin, there were alveolar and interstitial inflammatory cells but no fibrosis or remodeling. Beginning at 1 week and increasing at 3 weeks, the epithelial cells lining honeycomb cysts and mesenchymal cells in fibrotic areas expressed lacZ in BLM-treated mice compared to normal saline controls (Figs. 1C–1F and 2C–2F). Extensive collagen (yellow) was observed in fibrotic areas by picrosirius red staining (Fig 2D and 2F).
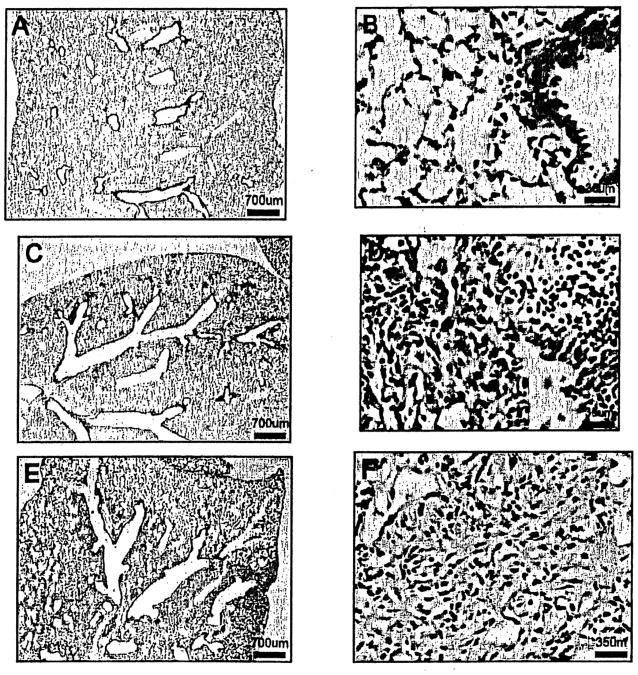
Figure 1.
Hematoxylin and Eosin stained lung sections of TOPGAL mice. (A, B) No abnormal histological changes were observed in the NS-treated control mice. (C, D) A typical patchy pattern of inflammatory (predominant) and fibrotic lesions around distal bronchioles and subpleural areas were observed in 1 week BLM-treated mice. (E, F) Extensive fibrotic and inflammatory lesions through the whole lung were observed in 3 week BLM-treated mice. Original magnification 20× for (A, C, E) and 400× for (B, D, F).
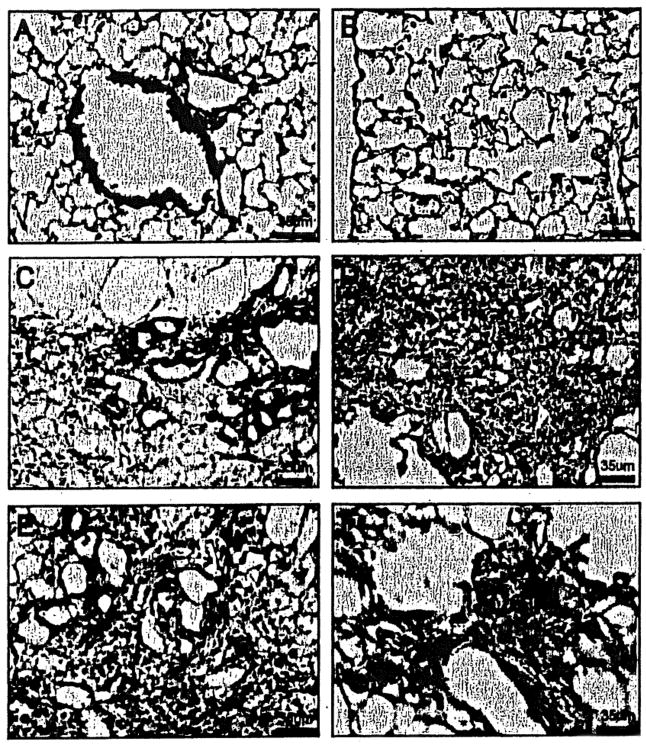
Figure 2.
X-gal/Sirius Red-stained lung sections of TOPGAL mice. (A, B) LacZ expression (blue cells) was observed in bronchial epithelium and scatted cells in alveoli in NS-treated control mice. (C-F) At 1 week/3 weeks in BLM-treated mice, LacZ was expressed in epithelial cells lining honeycomb cysts and mesenchymal cells (blue). Extensive collagen (yellow) was observed in fibrotic areas. Original magnification 400×.
β-catenin was observed in die cell membrane of bronchial epithelial cells in normal saline-treated control mice, and in the subepithelial membranes (Fig. 3A). After 1 week of bleomycin, there was increased β-catenin in cytoplasmic and nuclear areas of respiratory bronchioles in an increasing fibroproliferative lesion (Fig. 3B) that became more intense at 3 weeks (Fig. 3C). The nuclear staining of β-catenin, the key effecter in the Wnt/β-catenin signaling pathway, was increased in respiratory bronchioles, mesenchymal cells, hyperplastic type 2 alveolar epithelial cells, interstitial macrophages, and epithelium overlying fibroblastic areas at 3 weeks in BLM-treated mice (Figs. 3C–3E).
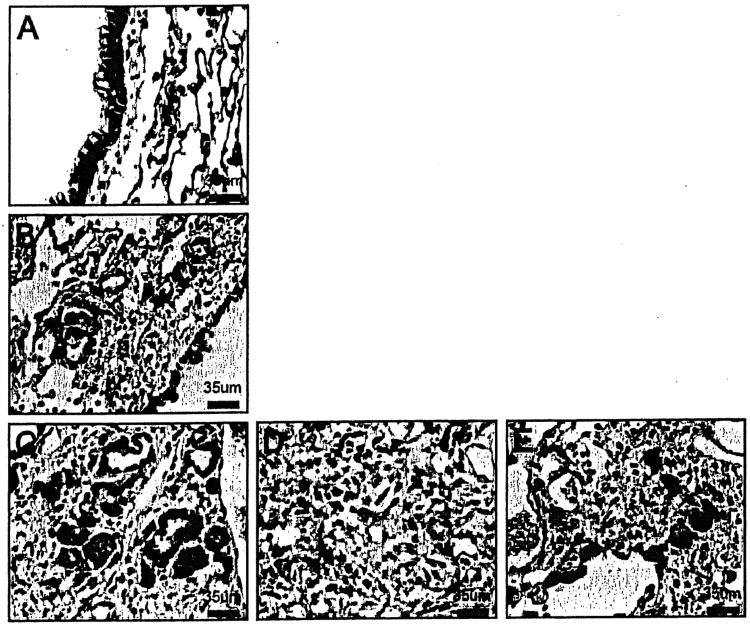
Figure 3.
β-catenin immunostained lung sections of TOPGAL mice. (A) β-catenin was observed in the membrane of bronchial epithelial cells in NS-treated control mice. (B) β-catenin at 1 week BLM-treated mice was increased both in the membrane and cytoplasm of bronchial epithelial cells. (C-E) β-catenin at 3 weeks in BLM-treated mice was further increased in both the nucleus and cytoplasm of respiratory bronchioles (C). There was increased β-catenin in mesenchymal cells (D). Hyperplastic type 2 alveolar epithelial cells, interstitial macrophages, and epithelium overlying fibroblastic areas were prominent (E). Original magnification 400×.
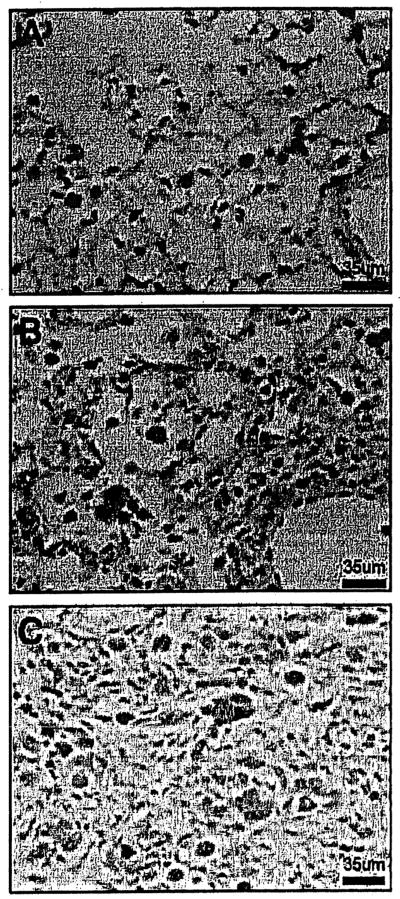
Target genes Cyclin Dl, matrix metalloproteinase 7 (Matrilysin), and transforming growth factor-β are upregulated in Wnt/β-catenin pathway. After normal saline, Cyclin Dl was minimally immunostained in bronchial epithelial cells over the time course of Observation from 1 day to 3 weeks (Fig. 4A). Both cytoplasmic and nuclear immuno-staining of cyclin Dl were observed in bronchial epithelium at 1 week in BLM-treated mice (Figs. 4B and 4C). At 3 weeks in BLM-treated mice, there was increased intensity of immunostaining in bronchial and bronchiolar areas, yet only minimal to modest immunostaining in mesenchymal cells and macrophages in the fibrotic areas (Fig. 4D and 4E). The Wnt/β-catenin signaling pathway target gene, MMP-7, was minimally expressed in basement membranes of airways in normal saline-treated mice at all time points (Fig. 5A), and the immunostaining of the basement membranes of the airways was increased after 1 week of BLM (Fig. 5B). At 3 weeks after BLM-treated mice, there was minimal MMP-7 immunostaining in extracellular matrix in the fibrotic areas (Fig. 5C). TGF-β immunostaining was minimal in alveolar macrophages throughout the time course in normal saline-treated mice (Fig. 6A). TGF-β was upregulated in alveolar macrophages in BLM-treated mice and immunostaining increased from 1 to 3 weeks in addition to modest increases in mesenchymal cells at 3 weeks (Figs. 6B and 6C).
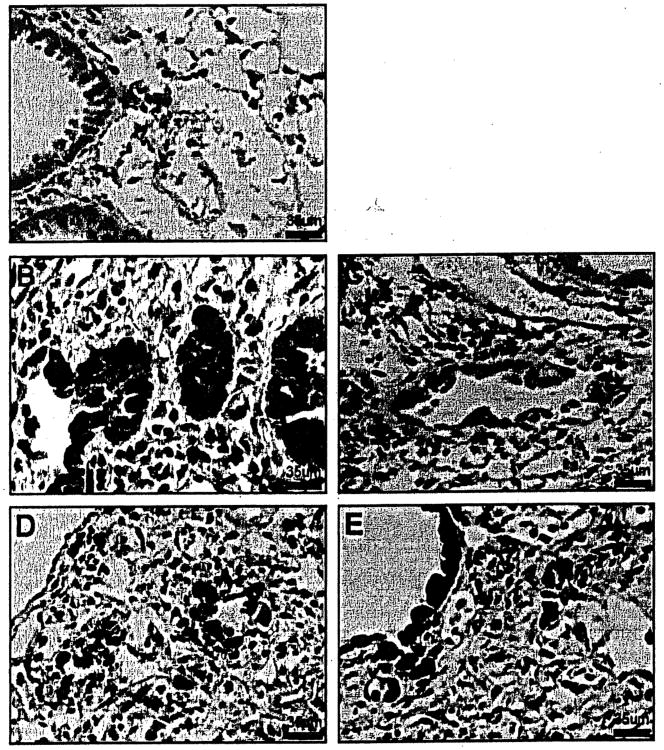
Figure 4.
Cyclin Dl immunostained lung sections of TOPGAL mice. (A) Minimal cytoplasmic immunostaining of cyclin Dl was observed in bronchial epithelial cells in NS-treated control mice. (B, C) Both cytoplasmic and nuclear immuno-staining of cyclin Dl were observed in bronchial epithelium in 1 week BLM-treated mice. (D, E) At 3 weeks in BLM-treated mice, there was increased intensity of immunostaining in bronchial and bronchiolar areas. Cyclin Dl was also increased in mesenchymal cells and in macrophages. 400×.
Figure 5.
MMP-7 immunostained lung sections of TOPGAL mice. (A) Minimal staining of MMP-7 in basement membranes of airways was observed in NS-treated control mice. (B) At 1 week in BLM-treated mice, MMP-7 immunostaining was seen in basement membranes of airways. (C) At 3 weeks in BLM-treated mice, there was minimal MMP-7 immunostaining of extracellular matrix in the fibrotic areas. Original magnification 400×.
Figure 6.
TGF-β immunostained lung sections of TOPGAL mice. (A) NS-treated control mice had few alveolar macrophages that were TGF-β positive. (B, C) The main TGF-β positive cells, alveolar macrophages, progressively increased through the time course (1 and 3 weeks) in BLM-treated mice. At 3 weeks in BLM-treated mice, there was increased TGF-β immunostaining in mesenchymal cells and extracellular matrix in the fibrotic areas in (C). Original magnification 400×.
Discussion
We studied the Wnt/β-catenin signaling pathway and the downstream target genes: cyclin Dl and MMP-7 in the bleomycin-induced lung fibrosis model. We demonstrated significant upregulation of expression by genes in this pathway within bronchiolar fibroproliferative lesions, myofibroblasts, basement membranes, and alveolar macrophages that are also noteworthy in human IPF. The TOPGAL mice that we used are on an outbred strain, CD-1 background, and have similar BLM-sensitive features as the BLM-sensitive strain C57BL/6. A single intratracheal administration of BLM resulted in patchy inflammatory and fibrotic lesions around distal bronchioles and subpleural areas at 1 week followed by extensive fibrotic lesions at 3 weeks after BLM treatment. This followed the common BLM model paradigm that postulates an initial alveolar injury, which triggers an inflammatory response, and subsequently, fibrosis. β-catenin, the key effector in the Wnt pathway. The downstream proteins, cyclin Dl and MMP-7, showed immunoreactivity in normal lung, demonstrating that the Wnt/β-catenin pathway is involved in the maintenance of adult lung homeostasis. In the BLM model at 1 and 3 weeks, LacZ expression (activation of the Wnt pathway) and β-catenin/cyclinDl were increased in the same cell compartments: bronchial epithelium, hyperplastic type II pneumocytes, and mesenchymal cells in fibrotic areas. Importantly, 3 weeks after BLM, β-catenin was localized both in the cytoplasm and nucleus of bronchial epithelial cells, hyperplastic type II alveolar epithelial cells, and interstitial macrophages. Cyclin Dl is a target gene of β-catenin since it has LEF-1 binding site on its Promoter.27 The results demonstrated that the Wnt/β-catenin pathway plays an integral role in developing lung fibrosis. The role of MMP-7 in basement membranes and TGF-β in alveolar macrophages in fibrotic areas was more limited, and posed an interesting role for this pathway. β-catenin activation induces osteopontin as well as MMP-7; osteopontin is elevated in microarrays of IPF lung tissue, is immunolocalized to alveolar epithelial type II cells along with MMP-7, and is significandy elevated in bronchoalveolar lavage from IPF patients.30 In addition, TGF- β is a very potent cytokine in the development of pulmonary fibrosis because of its stimulatory effect to synthesize extracellular matrix protein. The increase in TGF-β in alveolar macrophages suggests that they may play a role in directing matrix Stimulation especially myofibroblast proliferation. TGF-β overexpression with an adenovirus administered intratracheally in a tumor necrosis factor-α receptor knockout mouse that was fibrosis resistant, induced extensive inflammation and fibroproliferative disease.31 Hinz and colleagues focused on the resident fibroblast as the origin of the myofibroblast. They suggested that TGF-β, specialized ECM components, such as fibronectin, and mechanical stress were essential for the developing myofibroblast consistent with the fact that α-SMA in stress fibers confers to the myofibroblast a twofold stronger contractile activity compared to fibroblasts in culture.5 The main myofibroblast inducer TGF-β upregulates expression of fibronectin and its integrin receptors in lung fibroblasts and links to activation of focal adhesion kinase necessary for myofibroblast differentiation.5,32 Monocyte chemoattractant protein-1 (CCL2) has been reported to induce TGF-β and procollagen gene expression by fibroblasts.8 These targets have been further characterized by gene microarray studies of the bleomycin lung injury model at 7 days and the bleomycin lung fibrosis model at 21 days.33 CCL2 was elevated 4.8–3.6-fold in bleomycin lung injury and fibrosis respectively, and the extracellualr matrix, TGF-β and Wnt signaling pathways were all significandy increased. The Latent TGF-β binding protein was increased, and Wispl and Wnt7b were significandy elevated.33 These genes were specific to the bleomycin lung injury and fibrosis model because they were not increased in bacterial infection or allergy models. Interestingly, short-form, inhibitory C/EBPβ may keep fibroblasts in their undifferentiated State, and inflammation may repress this and activate the long form of C/EBPβ.5 In a TCF reporter mouse, Cheon et al. found that β-catenin and TCF transcriptional activity were elevated in fibroblasts during the proliferation phase of healing and that fibroblasts from inducible β-catenin transgenic mice exhibited increased fibroblast proliferation and motility.34 Mice developed aggressive fibromatoses, hyperplastic gastrointestinal polyps, and hyperplastic healing of cutaneous wounds. In hypertrophic scars and keloids, immunohistochemistry showed elevated β-catenin protein levels and data from fibroblast cell lines derived from hypertrophic scars showed that TGF-β activated β-catenin via the Smad3 and p38 MAP Kinase pathways.35
Chilosi et al. reported that in IPF/UIP patients the number of cells expressing nuclear β-catenin was highly increased, especially in areas with abnormal remodeling of lung architecture.24 Myofibroblasts were intensely immunostained in the nucleus, and α-smooth muscle actin and tenascin were also clearly identified on serial sections. The increase in cyclin D may correlate with the proliferative nature of the lesions, and matrilysin with matrix remodeling. The nuclear accumulation of β-catenin may be the signal for the excessive proliferation of type II pneumocytes seen in IPF/UIP. Quantitative RT-PCR of IPF lungs compared to transplant donor lungs showed increases for Wntl, 7b, 10b, the Wnt receptors Frizzled 2 and 3, the intracellular signal transducers GSK-3β, β-catenin, Tcf3 and Lefl; immunohistochemical localization was mosdy to alveolar and bronchial epithelium for Wntl, β-catenin, and GSK-3β.25 Evaluation of downstream genes from 6 IPF lungs and donor controls showed significant increases for fibronectin, matrilysin, and cyclin Dl. Functional phosphorylated GSK-3β and Lrp6 were increased in IPF lung homogenates. These findings suggest β-catenin may regulate alveolar type 2 cell hyperplasia and increased bronchial epithelial cell proliferation in IPF. Immunofluorescent staining has shown some IPF cells to coexpress prosurfactant protein-C, a type II alveolar epithelial cell marker, and N-cadherin, a mesenchymal Cadherin.32 α-SMA was colocalized to alveolar epithelial cells with AEC type II cell markers prosurfactant-B and/or thyroid transcription factor-l.11 An abnormal increase of matrilysin expression in IPF/UIP has been noted in gene expression arrays.26 Chilosi et al. suggested that β-catenin was involved in EMT by nuclear localization in bronchiolar lesions with matrilysin increasing basal cell mobility and promoting bronchiolization and tissue remodeling.24 BLM treatment of an α-smooth muscle actin-Cre/R26R mouse found pulmonary βgal staining and α-SMA staining in a small subset of bronchial epithelial cells consistent with airway EMT.36 EMT has been documented by the accumulation of βgal positive cells after TGF-β expression in mice with alveolar epithelial cells expressing lacZ; furthermore, alveolar epithelial cells exposed to fibronectin and fibrin underwent EMT after integrin-dependent activation of TGF-β .32 TGF-β treatment of alveolar epithelial cells for 12 days increased expression of mesenchymal markers α-SMA, type I collagen, desmin, and vimentin.11 Douglas et al. evaluated the noncanonical Wnt pathway in the reparative remodeling response to a hyperoxic acute lung injury model finding increased whole lung β-catenin, TCF-1 and -3, cyclin Dl, and integrin-linked kinase-1.23 A novel role for Wnt signaling was demonstrated by Lobov et al., where Wnt7b was essential in hyaloid vessels where adjacent macrophages initiate apoptosis in vascular endothelium promoting vascular regression in eye development.37 Macrophage TGF-β was increased which is a cross-talk gene to the Wnt pathway possibly providing a link between programmed cell death and cell cycle entry. In the liver model, inflammatory macrophages promote myofibroblast proliferation and apoptosis as a mechanism of fibrosis followed by resolution and repair, respectively.38 Macrophages were essential to both scar and healing mechanisms. In summary, we report activation of the Wnt/β-catenin pathway in the BLM mouse injury/fibrosis model contributing to the increased interstitial components including myofibroblasts.
Acknowledgments
This was supported by NIH, NIEHS (T32 ES007267).
References
- 1.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: Aberrant recapitulation of developmental programs? PLoS Med. 2008;5:0001–8. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE, Jr, Leinwand LA, Liotta L, Martin GR, Schwarte DA, Schulte GS, Wagner CR, Musson RA. Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute Working Group. Am J Respir Crit Care Med. 2002;166:236–46. doi: 10.1164/rccm.2201069. [DOI] [PubMed] [Google Scholar]
- 3.Noble PW. Idiopathic pulmonary fibrosis. New insights into Classification and pathogenesis usher in a new era in therapeutic approaches. Am J Respir Cell Mol Biol. 2003;29:S27–31. [PubMed] [Google Scholar]
- 4.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair. Am J Respir Crit Care Med. 2006;174:654–8. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz B, Phan SH, Thannickal VJ, Galli A, BochatonPiallat M, Gabbiani G. The myofibroblast: One function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, KarimiShah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 7.Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 8.Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res. 2007;24:819–41. doi: 10.1007/s11095-006-9216-x. [DOI] [PubMed] [Google Scholar]
- 9.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murfay LA, Xue YY, Belpetio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scotton CJ, Chambers RG. Moleculat targets in pulmonary fibrosis. The myofibroblast in focus. Chest. 2007;132:1311–21. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 11.Willis BC, Iiebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta 1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–32. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. Am J Pathol. 1994;145:114–25. [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and Cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nawshad A, Hay ED. TGF-β3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J Cell Biol. 2003;163:1291–301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon RT, Kolin AD, De Ferrari GV, Kaykas A. Wnt and β-Catenin signaling: Disease and therapies. Nature. 2004;5:689–99. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 16.Morrisey EE. Wnt signaling and pulmonary fibrosis. Am J Pathol. 2003;162:1393–6. doi: 10.1016/S0002-9440(10)64271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration. Am J Pathol. 2006;169:1633–42. doi: 10.2353/ajpath.2006.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aston C, Jagirdar J, Lee TC, Hur T, Hintz RL, Rom WN. Enhanced production of insulin-like growth factor molecules in idiopathic pulmonary fibrosis (EPF) Am J Respir Crit Care Med. 1995;151:1597–603. doi: 10.1164/ajrccm.151.5.7537587. [DOI] [PubMed] [Google Scholar]
- 19.Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:321–9. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity Pneumonitis. Am J Respir Crit Care Med. 2006;173:188–98. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon Cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 22.Polakis P, Hart M, Rubinfeld B. Defects in the regulation of beta-catenin in colorectal Cancer. Adv Exp Med Biol. 1999;470:23–32. doi: 10.1007/978-1-4615-4149-3_3. [DOI] [PubMed] [Google Scholar]
- 23.Douglas IS, Diaz del Valle F, Winn RA, Voelkel N. β-Catenin in the fibroproliferative response to acute hing injury. Am J Respir Cell Mol Biol. 2006;34:274–85. doi: 10.1165/rcmb.2005-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/β-Catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE. 2008;3:1–12. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Nad Acad Sci U S A. 2002;99:6292–7. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Nad Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and Wnt pathways. Proc Nad Acad Sci USA. 2000;97:8358–63. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 30.Pardo A, Gibson K, Cisneros JK, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS. 2005;2:0891–903. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JY, Sime PJ, Wu T, Warshamana GS, Pociask D, Tsai SY, Brody AR. Transforming growth factor-beta(l) overexpression in tumor necrosis factor-alpha receptor knockout mice induces fibroproliferative lung disease. Am J Respir Cell Mol Biol. 2001;25:3–7. doi: 10.1165/ajrcmb.25.1.4481. [DOI] [PubMed] [Google Scholar]
- 32.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Nati Acad Sci U S A. 2006;103:13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis CC, Yang JYH, Huang X, Banerjee SK, Blackburn MR, Baluk P, McDonald DM, Blackwell TS, Nagabhushanam V, Peters W, Voehringer D, Erle DJ. Disease-specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med. 2008;177:376–87. doi: 10.1164/rccm.200702-333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Cleavers H, Alman BA. Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibrornatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99:6973–8. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato M. Upregulation of the Wnt/β-catenin pathway induced by Transforming Growth factor-β in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–7. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Yang L, Cai L, Zhang M, Cheng X, Yang X, Xu J. Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an α-smooth muscle actin-Cre transgenic mouse. Respir Res. 2007;8:1. doi: 10.1186/1465-9921-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey AP, McMahon AP, Karsenty G, Lang RA. Wnt7b mediates macrophage-induced progtammed cell death in patterning of the vasculature. Nature. 2005;437:417–42. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinet, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]