Abstract
Purpose
There is mounting evidence that retinal ganglion cells (RGCs) require a complex milieu of trophic factors to enhance cell survival and axon regeneration after optic nerve injury. The authors' goal was to examine the contribution of components of a combination of hormones, growth factors, steroids, and small molecules to creating a regenerative environment and to determine if any of these components modulated macroglial behavior to aid in regeneration.
Methods
Postnatal day 7 mouse retinal explants embedded in collagen were used as an in vitro model of neurite regeneration. Explants were treated with the culture supplements fetal bovine serum, N2, and G5 and a mixture of G5 and N2 components, designated enhanced N2 (EN2). Explants were evaluated for neurite outgrowth over 7 days in culture. The effects of each treatment were also evaluated on cultured RGCs purified by Thy1 immunopanning. Immunohistochemistry and qPCR analysis were used to evaluate differences in gene expression in the explants due to different treatments.
Results
EN2 stimulated significant neurite outgrowth from explants but not from purified RGCs. Elimination of hydrocortisone (HC) from EN2 reduced the mean neurites per explant by 37%. EN2-treated explants demonstrated increased expression of Gfap, Glul, Glt1, Cntf, Pedf, and VegfA compared with explants treated with EN2 without HC. Subsequent experiments showed that increased expression of Cntf and Glul was critical to the trophic effect of HC.
Conclusions
These data suggest that the HC in EN2 indirectly contributed to neurite outgrowth by activating macroglia to produce neurotrophic and neuroprotective molecules.
Hydrocortisone, as a part of a minimally complex trophic environment, increased neurite outgrowth from retinal explants indirectly by stimulating surrounding macroglia to produce neurotrophic and neuroprotective molecules.
Introduction
In contemporary theories of glaucoma, elevated intraocular pressure, a major risk factor for retinal ganglion cell (RGC) damage, initially damages RGC axons at the optic nerve (ON) head. This injury then, among other things, leads to the loss of signals to and from the visual centers of the brain, subsequent RGC soma death, and potentially irreversible blindness.1–4 Current glaucoma therapies that lower intraocular pressure can significantly slow disease progression but do not offer any recovery of lost vision. Since the pathways that control RGC axon degeneration and RGC soma death are distinct both mechanistically and temporally,4,5 neuroprotective strategies that focus on saving RGC somas from cell death after insult to the ON could leave behind a population of RGC somas without axons.6–16 However, saving cells is not enough to restore vision. For any treatment to be successful in restoration of function, rescued cell somas need to reestablish their connections to the brain by regenerating axons. Multiple strategies have been proposed to overcome the pathological growth inhibitory environment present in the damaged retina and ON and to activate axon outgrowth, guidance, and functional connectivity.7,11,16–22
The application of growth factors has been a popular, but ultimately disappointing, strategy for both neuroprotection and regeneration. Previous studies have shown that single growth factors, including basic fibroblast growth factor (FGF2), ciliary neurotrophic factor (CNTF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor, pigment epithelium–derived factor (PEDF), and vascular endothelial growth factor-A (VEGFA), were able to enhance RGC survival and/or axon regeneration only in a transient and limited manner in vivo and/or in vitro.23–31 Combinations of growth factors that support both soma survival and axonal regeneration have often generated improved results. BDNF and CNTF together, for example, induced increased axon regeneration,32 while other studies showed that supplementing growth factors with additional factors such as forskolin or insulin further improved axon regeneration.17,26,33–38 It appears then that a complex trophic environment is beneficial for the biological processes required for both RGC survival and regeneration.
The concept that a complex trophic environment will better support cell survival and growth has been tested in cell culture studies for several decades. Well-defined complex mixtures of small molecules and trophic factors were developed by Jane Bottenstein to support specific cell types in culture without the need for fetal calf serum.39,40 Among the cell culture supplements that she formulated was one to support neuronal cells in culture (N2) and one to support glial cells in culture (G5). Both of these supplements are currently available commercially.
It is important to note that in order to generate new axons, the RGCs not only must be correctly stimulated but also must be in an environment that is supportive to new outgrowth. RGCs are intimately associated with the macroglia (astrocytes and Müller cells) of the retina. The Müller cells especially are responsible for a variety of critical support processes in the retina ranging from production of trophic factors, to metabolic support, to structural support, and to neurotransmitter recycling, among others.41,42 Retinal injury results in both astrocytes and Müller cells' becoming reactive, and this disruption in normal macroglial activity is a hallmark of pathology.43 However, many studies have highlighted the importance of retinal macroglia in not just pathology but also normal development and regeneration of RGC axons.13,22,44–47 Ideally then, a complex trophic environment should achieve a balance of stimulatory factors for RGC axon regeneration and macroglia homeostatsis.
To study RGC axon regeneration in such a way that it is both high-throughput and also representative of tissue-level interactions, the retinal explant serves as a valuable in vitro model. Compared to isolated primary RGCs, the explant model has the benefit of maintaining the extracellular matrix and the support cells surrounding the RGCs and thereby gives a more complete picture of how a treatment will affect the whole tissue. In contrast to in vivo models, the explant model allows for many more samples to be tested from the same animal and examined by different histological and molecular techniques. Since explants are grown in vitro, the effect of different combinations of growth factors, hormones, and small molecules can be tested much more easily than in an in vivo model.
The goal of this study was to examine the ability of a minimally complex defined growth media to stimulate neurite outgrowth from postnatal day 7 (PN7) mouse retinal explants and to characterize the effects and mechanism of action of one component, hydrocortisone (HC), on neurite outgrowth. Previous studies have shown that HC can increase both survival and neurite outgrowth from isolated RGC48 and that HC can increase RGC survival and axon regeneration after ON axotomy.49 Here we show that HC contributes to a neuritogenic environment when administered in combination with growth factors and that it likely acts on the surrounding macroglia, rather than directly on RGCs. HC appears to stimulate production of potent neurotrophic factors from the macroglia as well to increase glutamate turnover to aid in RGC health and neuritogenic potential.
Materials and Methods
Handling of Animals and Retinal Explant Protocol
Animals were handled in accordance with the Association for Research in Vision and Ophthalmology Statement on the use of animals for research and approved by the University of Wisconsin Institutional Animal Care and Use Committee. An F1 hybrid strain, CB6F1 (generated from a cross of a BALB/c female and a C57BL/6 male), was used for all experiments.
Pups from interbred CB6F1 mice were aged to 7 days (PN7) and euthanized by decapitation. Whole heads were bathed in 7.5% betadine solution, after which the eyelids were cut away. Eyes were removed and enucleated, and the retinas were isolated and washed twice in Dulbecco's modified Eagle's medium (DMEM with 4.5 g/L glucose and L-glutamine, BioWhittaker, Walkersville, MD). Four relaxing cuts were made in the retina to flatten it. Strips were cut from the peripheral retina and cut into approximately 0.5-mm2 squares to make a total of eight explants from each retina. Measurements of digital images of explants confirmed that there was no significant difference in explant size among various groups (approximate 0.5 mm2, data not shown). Single explants were placed into individual wells of a 24-well plate (Becton Dickinson, Franklin Lakes, NJ) or a 96-well plate (Corning Incorporated, Corning, NY) each containing 400 μL or 50 μL, respectively, of a dilute neutralized collagen solution (1.3 mg/mL rat tail collagen Type I [BD Bioscience, Bedford, MA] neutralized with 100 nM Hepes [pH 7.3 in 2X phosphate-buffered saline [PBS] and diluted with DMEM). The collagen was allowed to polymerize at 37°C in 5% CO2 for 30 minutes, and then 500 μL or 150 μL of the appropriate supplemented media (see Supplements and Culture Conditions below) was added.
All explants were cultured for 4 or 7 days at 37°C in 5% CO2, and media were replaced every other day. The number of neurite outgrowths from each explant was counted every 24 or 48 hours under phase contrast optics using a Leitz DM IL microscope (Microsystems, Inc., Buffalo Grove, IL), and the mean (±SEM) number of neurites was determined for each supplement-treated group. To index neurite length, the 10 longest neurites were measured from each explant concurrently using an ocular micrometer, and the mean (±SEM) was determined for each supplement-treated group.50,51 After 4 or 7 days in culture, explants were fixed in 4% paraformaldehyde in PBS for 1 hour at room temperature for fluorescent labeling or washed for 10 minutes in PBS and then frozen at −80°C for RNA extraction.
Supplements and Culture Conditions
Supplements included fetal bovine serum (FBS; BioWhittaker), G5 (Invitrogen, Carlsbad, CA), and N2 (Invitrogen). Formulations are listed in Table 1. All supplements were diluted to 10% in DMEM with 1% penicillin-streptomycin mixture (PenStrep, BioWhittaker). To make enhanced N2 (EN2), unique components of G5 were added to 10% N2 (commercially formulated) based on concentrations reported by the manufacturer of G5 (Table 1). This included 1 μg/mL biotin (Invitrogen), 0.36 μg/mL HC (Sigma-Aldrich, St. Louis, MO), 0.5 μg/mL FGF2 (Invitrogen), and 1 μg/mL epidermal growth factor (EGF; Invitrogen). For inhibitor experiments, either 1000 nM mifepristone (Sigma-Aldrich), 1000 nM spironolactone (Sigma-Aldrich), or 1 μg/mL L-methionine sulfoximine (Sigma-Aldrich) was added to the listed supplemented media. Additional growth factors added to supplemented media included 1 μg/mL CNTF (PeproTech Inc., Rocky Hill, NJ), VEGF (Sigma-Aldrich), or PEDF (Sigma-Aldrich). Neutralizing antibodies added to supplemented media included 0.2 μg/mL chicken anti-CNTF (Pierce Biotechnology, Rockford, IL), goat anti-VEGF (R&D Systems, Minneapolis, MN), or goat anti-PEDF (Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Table 1.
Components of the Supplements Used in This Study
|
Supplement Name |
Source |
Components, μg/mL |
| N2 | Invitrogen/Gibco | Insulin (500) |
| Human transferrin (10,000) | ||
| Selenite (0.52) | ||
| Progesterone (0.63) | ||
| Putrascine (1611) | ||
| G5 | Invitrogen/Gibco | Insulin (500) |
| Human transferrin (5000) | ||
| Selenite (0.52) | ||
| Biotin (1) | ||
| Hydrocortisone (0.36) | ||
| bFGF (0.5) | ||
| EGF (1) | ||
| EN2 | Invitrogen/ Gibco (N2 only) | Insulin (500) |
| Human transferrin (10,000) | ||
| Selenite (0.52) | ||
| Progesterone (0.63) | ||
| Putrascine (1,611) | ||
| Biotin (1) | ||
| Hydrocortisone (0.36) | ||
| bFGF (0.5) | ||
| EGF (1) |
Commercially available supplements used are N2 (for neuronal cell cultures) and G5 (for glial cell cultures); both are available from Invitrogen/Gibco. Since N2 and G5 share some components, EN2 is made using commercial N2 with unique G5 components individually added.
Isolated RGCs
Primary RGCs were enriched and cultured as described previously.38,52 Briefly, retina tissue from P3 to P5 Sprague-Dawley rats were dissociated by papain digestion and gentle trituration. Macrophages were depleted from the cell mixture using CD11b/c (BD Biosciences Pharmingen)–coated magnetic beads (CELLection Dynabeads Invitrogen). RGCs were then enriched from the remaining cells using magnetic beads (CELLection Dynabeads Invitrogen) coated with an antibody against the surface protein Thy-1.1 (Millipore, Billerica, MA), whose expression in the retina is largely restricted to RGCs. Cells were released with DNase, washed, and then seeded at 5000 cells/well in 96-well culture plates (BD Bioscience) that had been coated with poly-D-lysine (0.01 mg/mL, Sigma-Aldrich) and laminin (0.01 mg/mL, Sigma-Aldrich). Cells were cultured in DMEM containing 1% PenStrep and either 10% FBS, N2, G5, EN2, or EN2 without HC. After 72 hours, cultures were stained with 5 μM Hoescht 33,342 (Invitrogen), 4 μM ethidium homodimer-1 (Invitrogen), and 10 μM calcein-AM (Invitrogen). Cell survival and neurite length were determined from images acquired using the Cellomics ArrayScan VTi (Thermo Scientific, Pittsburgh, PA) and analyzed with custom algorithms using the Cellomics Neuronal Profiling image-analysis software package.
ON Crush Procedure and Counting of Surviving RGCs
Cell survival after ON crush was evaluated from Nissl-stained retinal whole mounts of adult CB6F1 mice, as described previously.53,54 Mice were anesthetized with an intraperitoneal injection of ketamine (6 mg/mL) and xylazine (0.4 mg/mL), and the left eye was numbed with one drop of Alcain (Alcon Laboratories, Ft. Worth, TX) prior to both the injection and ON crush. Twenty-four hours prior to crush, the sclera of the left eye of each mouse was punctured with a 30-gauge needle, and 2 μL of a 10% solution of supplement (FBS, G5, N2, EN2, or EN2 without HC) diluted in balanced saline solution was injected through a glass micropipet inserted into the vitreous. A noninjected group was also included. The left eye then underwent ON crush the next day, as previously described.55 Briefly, the ON was exposed using an intraorbital approach, and the nerve was crushed for 3 seconds with self-closing curved N7 microforceps (Fine Science Tools, Vancouver, British Columbia, Canada). Mice were allowed to recover and then euthanized 2 weeks later.
Quantitative analysis of cell loss in the ganglion cell layer was performed on Nissl-stained retinal whole mounts, as described previously.53 Photographs of the RGC layer were taken in eight sample regions, two per quadrant at 40× magnification using an Olympus BX40 light microscope (Olympus, Mellville, NY) with a Pentax istD digital camera attachment (Pentax, Montvale, NJ). Cells in the sample photographs were counted and averaged using Image Pro Plus v4.5 (Media Cybernetics, Inc., Silver Springs, MD), and then crushed (left) eyes were compared with control (right) eyes to calculate the percentage cell loss after ON crush surgery. RGCs and amacrine cells could not be differentiated by Nissl staining; therefore, the resulting percentage cell loss takes into account the total cell population of the RGC layer and not solely the RGCs. Four to five mice were evaluated in each group.
Phalloidin Labeling
For phalloidin whole-mount labeling, explants (fixed in 4% paraformaldehyde in PBS for 1 hour at room temperature) were washed with PBS, 0.15 M glycine in PBS, PBS, and finally 0.02% Triton X-100 in PBS. All washes were for 10 minutes each at room temperature. After rinsing in PBS, explants were blocked in 4% bovine serum albumin (BSA; Fisher Scientific, Fair Lawn, NJ) in PBS for 48 hours at 4°C. Explants were stained with phalloidin-Alexa 488 (1:300 in 0.1% BSA in PBS, Invitrogen) for 2 hours at room temperature. Following staining, explants were washed in PBS three times for 10 minutes at room temperature, washed twice in distilled water for 5 minutes, mounted on glass slides, then covered in Immuno Mount (Thermo Scientific), and coverslipped.
Immunofluorescent Labeling
For sections, fixed explants were put into 0.4% paraformaldehyde in PBS overnight, equilibrated in 30% sucrose in PBS, and embedded in optimal cutting temperature compound (Fisher Scientific) in blocks and frozen at −80°C on dry ice. Frozen sections were cut at 5 μm. Slides were washed three times for 1 hour at room temperature in 0.1% Triton-X in PBS and then blocked for 1 hour at room temperature in 2% BSA in 0.1% Triton-X in PBS. Slides were incubated overnight at 4°C with rat monoclonal anti-mouse glial fibrillary acidic protein (GFAP; 1:100, gift from Albee Messing, University of Wisconsin-Madison, Madison, WI) and either rabbit polyclonal anti-mouse glutamate ammonia ligase (GLUL; 1:100, Abcam Inc., Cambridge, MA), chicken polyclonal anti-human CNTF (1:100, Pierce Biotechnology), goat polyclonal anti-mouse VEGF (1:100, R&D Systems), or goat polyclonal anti-human PEDF (1:00, Santa Cruz Biotechnology, Inc.). After washing three times in PBS at room temperature for 5 minutes, slides were incubated for 1 hour at room temperature in the dark with FITC-conjugated donkey anti-rat IgG (1:50, Sigma) and either rhodamine-conjugated rabbit anti-chicken IgG (1:300, Chemicon, Temecula, CA), Texas Red–conjugated rabbit-anti-goat IgG (1:300, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), or Texas Red–conjugated goat-anti-rabbit IgG (1:300, Jackson ImmunoResearch Laboratories, Inc.). All antibodies were diluted in 2% BSA in PBS. Slides were washed in PBS, incubated with 300 ng/mL 4′,6-diamidino-2-phenylindole (Pierce Biotechnology) in water for 10 minutes at room temperature in the dark, washed again with PBS, then covered with Immuno Mount and coverslipped.
Microscopy
All immunofluorescent photographs were acquired using a Zeiss Axioplan 2 Imaging microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY) with a digital camera and the multidimensional acquisition function. Images were flattened and merged by extended focus and analyzed using the Zeiss Axiovision Image Analysis software v4.6 (Carl Zeiss Microimaging, Inc.).
Quantitative Analysis of mRNA Abundance in Explants
Frozen retinal explants were pooled by treatment group, and RNA was extracted using TRI Reagent Solution (Applied Biosystems, Foster City, CA). For absolute quantification qPCR, 2 μg RNA per treatment group was used to synthesize cDNA using Moloney murine leukemia virus (MMLV) reverse transcriptase and random hexamer primers (Promega, Madison, WI).56 One microliter cDNA was added to diluted SYBR Green PCR master mix (Applied Biosystems) with primers to S16, Glul, Gfap, Thy1, or Nr3C1. Primer sequences are listed in Table 2. Each sample was represented in triplicate in each qPCR run, and three runs total were performed using an ABI 7300 Real Time PCR system (Applied Biosystems). The amplification conditions were 95°C (15 seconds) and 60°C (60 seconds) for 40 cycles with a dissociation step. Standard curves for all amplimers were used to determine absolute transcript quantities, and these were normalized to S16 ribosomal protein mRNA.57 Fold change was determined for EN2-treated pooled explants relative to EN2 without HC–treated pooled explants.
Table 2.
Primer Sequences for qPCR Analysis
|
Gene Name |
Primer Sequence 5′→3′ |
Size, bp |
|
| Aif1 | Fwd | TAGAGAGGTGTCCAGTGGCTCCG | 201 |
| Rev | CCCCACCGTGTGACATCCACCT | ||
| Bdnf | Fwd | GTCTTCTGTAGTCGCCAAGGTGG | 291 |
| Rev | GCCGCCTTCATGCAACCGAAG | ||
| Cntf | Fwd | ACCAGCTCACTTGTTTCCTGGGAC | 228 |
| Rev | AGCGATCAGTGCTTGCCACTGG | ||
| Egf | Fwd | CTCGAGAGAAGCGAGAGAAGCGG | 281 |
| Rev | CAGTCTATGGCCAGCCCAGACAC | ||
| Fgf2 | Fwd | GGGAGTGTGTGCCAACCGGT | 213 |
| Rev | ATGGCCTTCTGTCCAGGTCCC | ||
| Gfap | Fwd | CAAACTGGCTGATGTCTACC | 269 |
| Rev | AGAACTGGATCTCCTCCTCC | ||
| Glast | Fwd | GCAAGCGGACACTTCTGGCCA | 262 |
| Rev | GCCGCCATTCCTGTGACGAGAC | ||
| Glt1 | Fwd | CGCCGAGGCGCTAAAGGGCTTA | 203 |
| Rev | GCGCACTTCTACCTGCTTGGGC | ||
| Glul | Fwd | CCGCCTCGCTCTCCTGACC | 203 |
| Rev | CGGGTCTTGCAGCGCAGTC | ||
| Nr3C1 | Fwd | GGTGCTGACGTGTGGAAG | 284 |
| Rev | GGGTAAGCTGTGGCAGCG | ||
| Pedf | Fwd | CGCAACCACAGTTCCGGGATG | 232 |
| Rev | TGGCACTGGATCTCAGGCGGT | ||
| S16 | Fwd | CACTGCAAACGGGGAAATGG | 198 |
| Rev | TGAGATGGACTGTCGGATGG | ||
| Thy1 | Fwd | CTTGCAGGTGTCCCGAGGGC | 379 |
| Rev | CTGAACCAGCAGGCTTATGC | ||
| VegfA | Fwd | GCACTGGACCCTGGCTTTACTGC | 299 |
| Rev | TCCGCATGATCTGCATGGTGATGT | ||
All primers were designed to span an intron.
For relative quantification qPCR, 0.5 μg RNA per treatment group was used to synthesize cDNA using MMLV reverse transcriptase and random hexamer primers, and the resulting cDNA was further diluted 12.5-fold. Five microliters diluted cDNA was added to diluted SYBR Green PCR master mix (Applied Biosystems) with primers to a panel of growth factors consisting of Bdnf, Cntf, Egf, FGF2, Pedf, and VegfA and glial markers Aif1, Glast, and Glt1. Primer sequences are listed in Table 2. The amplification conditions were 95°C (15 seconds) and 60°C (60 seconds) for 40 cycles with a dissociation step. Relative quantification was determined based on the Pfaffl method after determining the amplification efficiency for each primer set (data not shown).58
Statistics
In general, at least 24 explants were evaluated for each treatment per experiment, representing eight retinal explants from six individual mice in all treatment groups. Experiments were repeated two to three times. Means are reported with the standard error of the mean (SEM). Statistical significance between two means was determined using Student's t-tests, P ≤ 0.05.
Results
Cell Culture Supplement Effects on Neurite Outgrowth in Explants
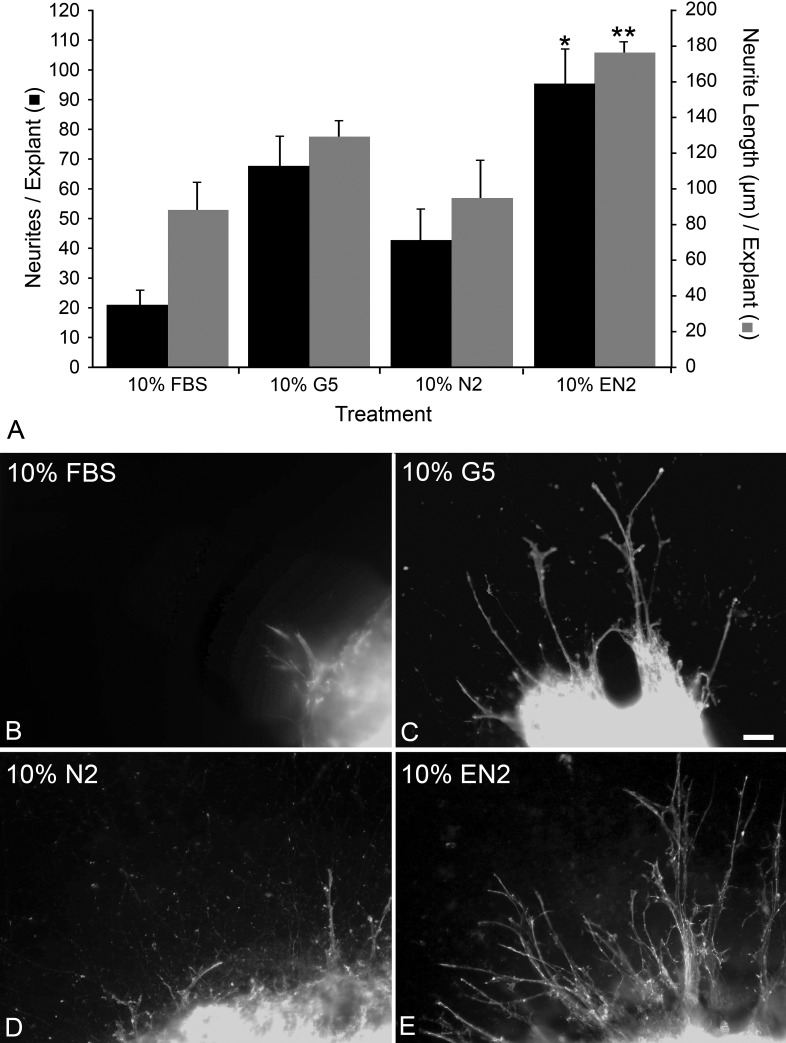
PN7 retinal explants were used to examine if commercial supplements (formulations are detailed in Table 1) stimulated neurite outgrowth. G5 yielded the greatest neurite outgrowth compared with any other treatment, generating an average of 75.95 ± 13.13 neurites per explant by 7 days in culture (Fig. 1A). This represented an increase of 373.6% over FBS (P = 8 × 10−5). There were nearly double the number of neurites in N2-treated explants compared with FBS-treated explants (P = 0.022), but N2-treated explants had only approximately half the number of neurites of 10% G5-treated explants (P = .027).
Figure 1.
EN2 stimulated the most neurite outgrowth and the longest neurites from retinal explants of PN7 CB6F1 mice after 7 days in culture. (A) Histograph showing the mean (±SEM) number of neurites per explant (▪) or the mean (±SEM) of the 10 longest neurites per explant (█) grown in DMEM containing different growth supplements after 7 days in culture. Treatment with 10% EN2 produced significantly more neurites than treatment with any other 10% cell culture supplement (*P < 0.05, compared with 10% FBS, N2, and G5), and the neurites were significantly longer (**P < 0.01, compared with either 10% FBS, N2, and G5). (B–E) Photomicrographs showing PN7 CB6F1 mouse retinal explants treated with 10% FBS (B), 10% G5 (C), 10% N2 (D), or 10% EN2 (E) for 7 days then stained with phalloidin-Alexxa 488. Neurites were more numerous, longer, and more highly branched in EN2-treated explants than other treatment groups. Size bar = 15 μm.
Since both N2 and G5 were able to stimulate improved neurite outgrowth from explants compared with FBS, we were interested in whether combining components of the two different supplements would create not only a more complex but also a better stimulatory environment. The supplements had several common components, including transferrin, selenite, and insulin. We created a new supplement by adding the unique components from G5 (FGF2, EGF, HC, and biotin) to N2 (producing EN2). Explants grown in EN2 were able to produce significantly more neurites, 94.92 ± 12.01, than either N2 (P = 0.002) or G5 (P = 0.046) alone (Fig. 1A).
As an index of neurite length, we determined the mean length of the 10 longest neurites per explant. This index was significantly higher in EN2-treated explants (177.4 ± 6.6 μm) relative to explants in FBS- (86.2 ± 16.6 μm, P = 0.0005), G5- (129.15 ± 9.2 μm, P = 0.0016), or N2- (93.86 ± 22.0 μm, P = 0.0024) treated explants (Fig. 1A).
Phalloidin-labeled explants highlighted that the processes in EN2-treated explants were more numerous, longer, and had more complex branching than those in explants treated with FBS, G5, or N2 (Figs. 1B–E). Phalloidin labeling demonstrated that neurites contained organized filamentous actin (f-actin) networks, and f-actin dense growth cones were also observed at the end of neurites. Neurites also were positive for βIII-tubulin and growth associate protein 43 (data not shown). These data were consistent with a previous report identifying neurite outgrowth from retinal explants as new axons.59 The combination of factors from the commercial cell culture supplements N2 and G5, designated EN2, was able to significantly increase the number and length of neurites from PN7 retinal explants, and these neurites were positive for markers of new axons.
Individual Component Analysis of EN2
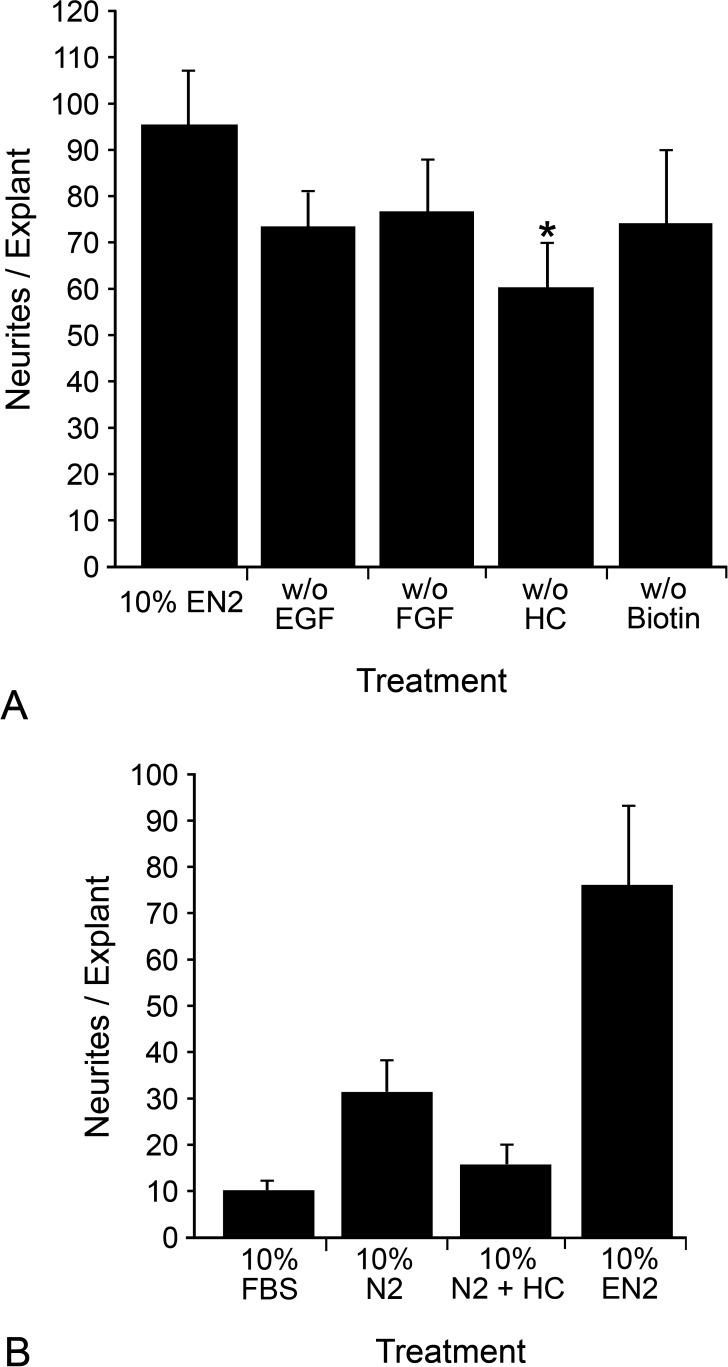
We then examined which unique G5 components in EN2 were most crucial to neurite stimulation by treating explants for 7 days with supplements in which a single unique component from G5 was left out (Fig. 2A). Explants treated with EN2 without EGF, FGF2, or biotin had approximately 25% fewer neurite outgrowths, which was not statistically significant (versus EN2, P = 0.068 for EN2 without EGF, P = 0.13 for EN2 without FGF2, P = 0.15 for EN2 without biotin). Removing HC, however, resulted in a significant 37% decrease in neurite outgrowth (versus EN2, P = 0.017).
Figure 2.
Removing HC from EN2 resulted in a significant decrease in neurite outgrowth from retinal explants, while adding HC alone to N2 did not increase neurite outgrowth. (A) Histograph of the mean (±SEM) number of neurites per explant for each treatment group. Explants were cultured in 10% EN2 that was either complete or missing a single component (EGF, FGF2, HC, or biotin). Removing EGF, FGF2, or biotin reduced neurite outgrowth compared with complete EN2 by approximately 25%, but this decrease was not significant (P > 0.05). HC removal, however, did significantly reduce neurite outgrowth compared with EN2 by 37%. *P < 0.02 (compared with complete EN2). (B) Histograph of the mean (±SEM) number of neurites per explant for each treatment group. Explants were cultured in 10% FBS, 10% N2, 10% N2 with 100 nM HC, or complete 10% EN2. Addition of HC alone to N2 did not increase neurite outgrowth from explants compared with treatment with FBS or N2 (P = 0.17 compared with FBS and P = 0.051 compared with N2). Explants treated with N2 with 100 nM HC had significantly less neurite outgrowth than EN2-treated explants (P = 0.0007).
To determine whether the addition of HC alone to N2 was sufficient to increase neurite outgrowth, we treated explants for 7 days with FBS, N2, N2 with 100 nM HC, or EN2 (Fig. 2B). Explants treated with N2 with 100 nM HC had significantly less neurite outgrowth than EN2-treated explants (P = 0.0007) and no significant difference in neurite outgrowth compared with explants treated with FBS or N2 (versus FBS, P = 0.17 and versus N2, P = 0.051). Since the exclusion of HC from EN2 resulted in significantly less neurite outgrowth from explants, but the addition of HC to N2 in the absence of other G5-derived factors did not increase neurite outgrowth, the trophic effects of this hormone may have been the result of combinatorial effects mediated by all the components present in EN2.
Cell Culture Supplement Effects on Isolated RGCs and Cell Survival after ON Crush
Of the media tested, EN2 supported the most neurite outgrowth from retinal explants. We also observed that EN2-treated explants appeared to have the best overall structural integrity after 7 days in culture (data not shown). Since the trophic effects of EN2 on total neurite outgrowth might have been the by-product of increased survival of RGCs, leaving more RGCs available to sprout neurites, we wanted to test the effects of the various supplements on individual RGCs and on RGC survival after injury in vivo.
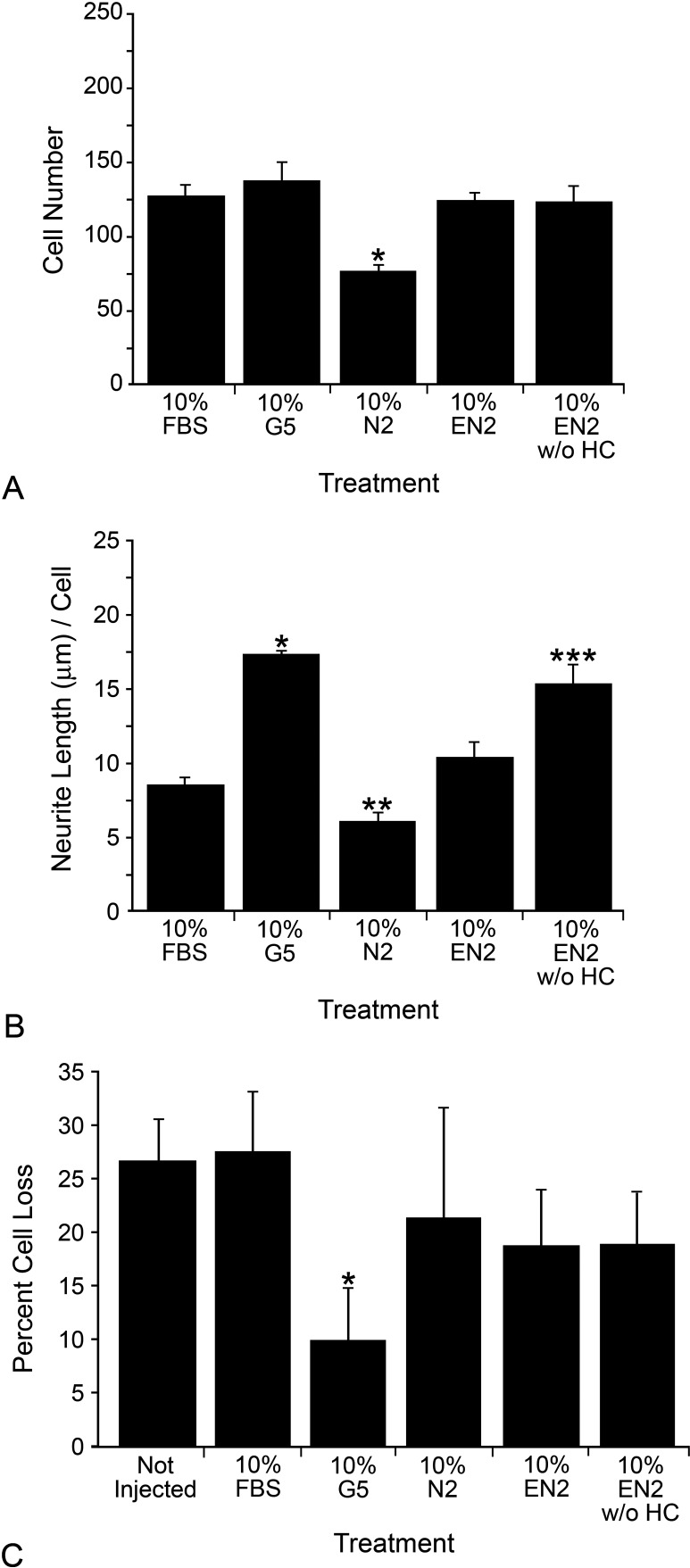
FBS, N2, EN2, and EN2 without HC (EN2 without HC) were tested on cultures of isolated RGC. Their ability to support RGC survival (Fig. 3A) and directly stimulate neurite outgrowth was assessed (Fig. 3B). FBS, EN2, EN2 without HC, and G5-treated RGCs exhibited no difference in cell survival after 7 days, while N2 yielded significantly fewer surviving cells (P = 0.0006; Fig. 3A). G5 (which contains HC) and EN2 without HC significantly increased neurite length from isolated RGCs compared with FBS (P = 1.53 × 10−6 and 0.001, respectively), while EN2 demonstrated a modest, but not statistically significant, increase (P = 0.087; Fig. 3B). N2-treated cells had decreased neurite length compared with FBS (P = 0.009). When treated with FBS only, 34.80% ± 4.47% of cells extended neurites. Compared to this, there was no difference in the number of cells extending neurites when treated with N2 (37.14% ± 4.98%, P = 0.26) or EN2 (38.2% ± 5.81%, P = 0.20), while significantly more cells were found to extending neurites when treated with G5 (49.39 ± 2.70, P = 0.001) or EN2-HC (54.39 ± 5.07%, P = 0.0006).
Figure 3.
EN2 did not significantly increase neurite outgrowth from isolated RGCs, nor did it attenuate RGC soma loss in the ganglion cell layer after optic nerve crush. (A) Histograph showing the mean number (±SEM) of isolated RGCs surviving in different treatments. *P < 0.05 (compared with FBS-treated cells). (B) Histograph showing the average neurite length (±SEM) from RGCs in different treatments. *P < 0.001 (compared with FBS-treated cells), **P < 0.01(compared with FBS-treated cells), ***P < 0.002 (compared with FBS-treated cells). (C) Histograph of the mean percentage cell loss (±SEM) is shown for 8 to 13 mice per group. Mice preinjected with 2 μL of 10% G5 show significantly less cell loss compared with either noninjected or FBS-injected eyes (which were no different than noninjected eyes). While both 10% EN2 and 10% EN2 without HC were able to modestly reduce cell loss, neither result was significant when compared with noninjected or FBS-injected mice. *P < 0.05 (compared with the noninjected group or FBS-treated group).
To test whether either EN2 or EN2 without HC was able to significantly attenuate cell loss after ON crush, we intravitreally injected solutions of FBS, G5, EN2, and EN2 without HC 24 hours prior to ON crush. The percentage loss of cells in the ganglion cell layer of crushed eyes, relative to control eyes of each mouse examined, was measured 2 weeks after crush surgery using the Nissl staining method (Fig. 3C). Both the noninjected and FBS-injected control groups exhibited approximately 27% cell loss after 2 weeks, which, assuming 60% RGCs and 40% amacrine cells in the RGC layer,55,60 would extrapolate out to a little more than half of the RGCs having died, conforming well with other previously published reports using this method.55 G5 exhibited the least cell loss relative to retinas from noncrushed eyes (approximately 10%), which was significantly less than either the noninjected or FBS-injected controls (P = 0.008 and 0.02, respectively). Injection of either EN2 or EN2-HC was able reduce soma loss to approximately 19%, but this was not significant when compared with either noninjected (P = 0.12 for both EN2 and EN2-HC) or FBS-injected controls (P = 0.10 and 0.27, respectively). These data suggest that it was unlikely that EN2 increased the number of neurites from explants simply by increasing the number of surviving RGCs available to grow neurites. In addition, these data suggest that the effect of HC as a component of EN2 was complex and likely involved interactions both with other components of EN2 and possibly with other cell types within the explant.
HC Receptor Analysis
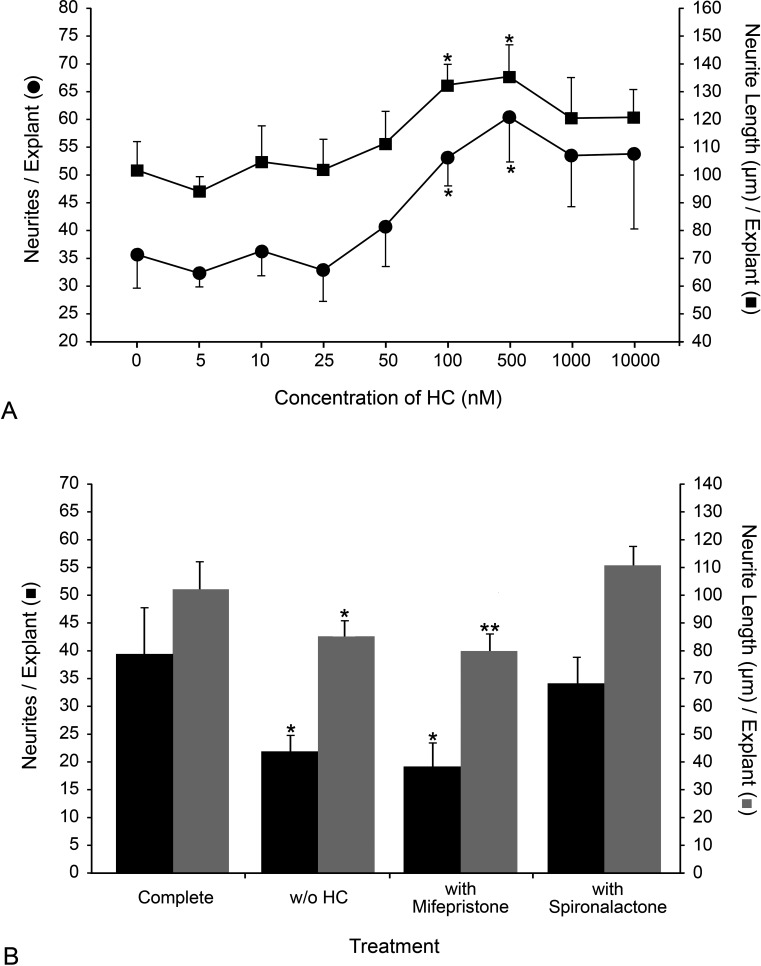
The findings that HC had no significant effect on RGC survival or neurite outgrowth from isolated RGCs but did have a significant impact on neurite outgrowth in explants suggested that a different cell type might be mediating the HC effect in PN7 mouse retinal explants. Retinal tissue primarily contains two high-affinity receptors for HC: the glucocorticoid receptor (NR3C1) and the mineralocorticoid receptor (NR3C2). Reported KDs for HC of these high-affinity receptors in rodents vary from 0.9 to 20.1 nM.61,62 We performed a dose-response experiment to determine the effective range of HC on neurite outgrowth, as well as to validate that HC was acting through the high-affinity receptors (Fig. 4A). EN2 in all prior experiments contained 100 nM HC. Peak neurite outgrowth was observed in EN2 containing between 100 and 500 nM HC (P = 0.016 and 0.01 vs. 0 nM HC, respectively). Peak neurite length also occurred between 100 and 500 nM HC (P = 0.012 and 0.027 vs. 0 nM HC, respectively).
Figure 4.
HC stimulated maximal neurite outgrowth at 100 nM, and neurite outgrowth stimulated by HC was inhibited by the glucocorticoid receptor antagonist mifepristone. (A) A dose-response curve is shown for both the average number of neurites per explant (•) and average length of neurites, in μm (▪) at increasing concentrations of HC (±SEM). The peak number of neurites/explants occurred between 100 and 500 nM HC. Peak average neurite length occurred between 100 and 500 nM HC. *P < 0.02 (100 and 500 nM HC compared with 0 nM HC). (B) Histograph of the mean number of neurites per explant (▪) and the average length of neurites, in μm (▪), for each treatment group (complete 10% EN2, 10% EN2 without HC, complete 10% EN2 with 1000 nM mifepristone, complete 10% EN2 with 1000 nM spironolactone) are shown (±SEM). Explants incubated in the presence of 1000 nM mifepristone produced significantly fewer and shorter neurites compared with explants in uninhibited EN2. Explants incubated in 10% EN2 containing 1000 nM spironolactone exhibited no change in neurite numbers or length compared with those incubated in uninhibited EN2. *P < 0.02 (compared with uninhibited EN2), **P < 0.05 (compared with uninhibited EN2).
We then used specific inhibitors to help define which receptor was mediating the neurite-stimulating effect of HC. The NR3C1 antagonist mifepristone (KD = 0.4 nM) and the NR3C2 antagonist spironolactone (KD = 14 nM) were used at 1000 nM in explants treated with complete EN2, which contained 100 nM HC (Fig. 4B). A 10-fold increase in the inhibitor concentration over the HC concentration was chosen to ensure receptor occupancy with the appropriate inhibitor. We found that 1000 nM mifepristone reduced both neurite number (versus complete EN2 without inhibitor, P = 0.019) and length (P = 0.037) to levels similar to 0 nM HC EN2 (versus EN2, P = 0.0096 for neurite number and P = 0.020 for neurite length). Spironolactone 1000 nM had no effect on either neurite number (versus complete EN2 without inhibitor, P = 0.28) or length (P = 0.24). These data indicated that the HC effect was likely mediated through NR3C1 and not NR3C2.
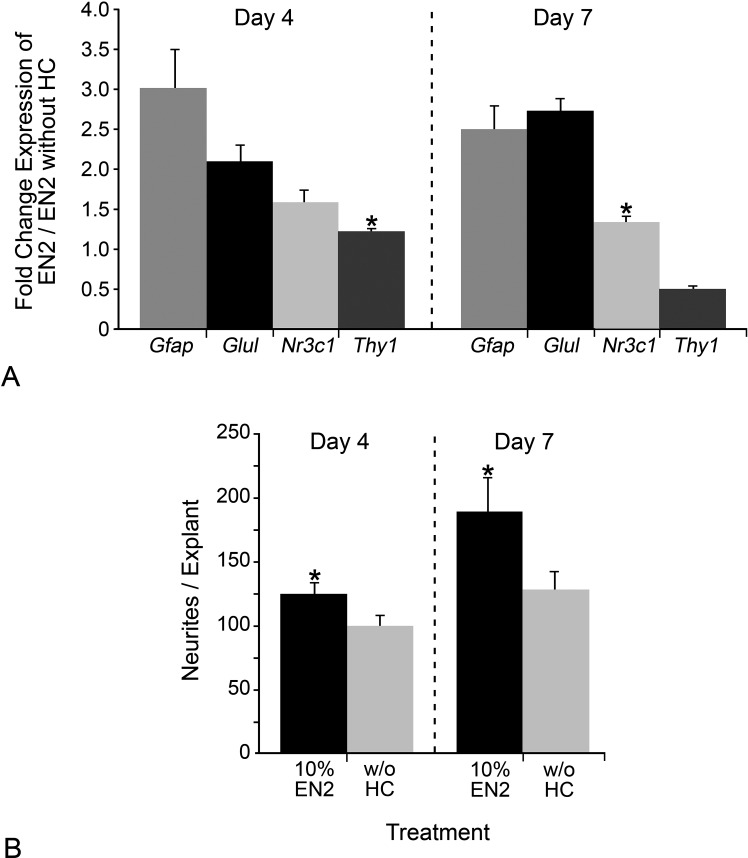
Glul, Gfap, Nr3C1, and Thy1 mRNA Abundance in Explants
NR3C1 has been localized in the retina predominately to Müller cells,63,64 indicating that the HC effect on neurite outgrowth was likely indirect and mediated through macroglia. Transcript levels of Glul, Gfap, Nr3C1, and Thy1 in explants treated with either EN2 or EN2 without HC at 4 and 7 days in culture were measured to see if HC had any differential effect on glial (Glul, Gfap, Nr3C1I) or RGC (Thy1) transcripts (Fig. 5A). At day 4 in culture, transcripts for Gfap, Glul, and NR3C1 were significantly increased in EN2-treated explants relative to EN2 without HC-treated explants (P = 0.046, 0.017, and 0.021, respectively). There was no difference in Thy1 transcript levels (P = 0.199). At day 7 in culture, transcripts for Gfap and Glul remaining significantly increased (P = 2.07 × 10−8 and 5.07 × 10−5, respectively), while there was no difference in Nr3c1 (P = 0.176) and a decrease in Thy1 (Fig. 5A; P = 0.003). At days 4 and 7 in culture, there was a significant increase in neurites in EN2-treated explants compared with explants treated with EN2 without HC (Fig. 5B; P = 0.026 and 0.028, respectively). The increased levels of macroglial-specific Glul and Gfap transcripts in the presence of HC indicated that the macroglia cells were responding to HC and may have been part of mediating the HC effect on neurite outgrowth from RGCs.
Figure 5.
Neurite outgrowth and expression of Gfap and Glul was increased at 4 and 7 days in culture in EN2-treated explants. (A, B) Histographs of the relative fold change (+SEM) in expression of Gfap, Glul, Nr3c1, and Thy1 at days 4 and 7 in culture in EN2- versus EN2 without HC–treated explants (A) and of the mean number of neurites per explant (+SEM) for 10% EN2- or 10% EN2 without HC–treated explants at days 4 and 7 in culture. At day 4, Gfap, Glul, and Nr3c1 transcripts were elevated in EN2-treated explants relative to EN2 without HC–treated explants, while there was no difference in Thy1 transcripts. At day 7, Gfap and Glul transcripts remain elevated in EN2-treated explants compared with EN2 without HC–treated explants, while Nr3c1 has no difference in transcript levels between the two treatments. Thy1 transcripts were decreased in EN2-treated explants compared with EN2 without HC–treated explants. *P > 0.05 for no difference in transcript levels.
Influence of Glial-Derived Proteins on Neurite Outgrowth
The increase in Gfap and Glul transcripts in EN2-treated explants, but not explants treated with EN2 without HC, suggests that HC is acting, at least in part, on macroglial cells. Maintaining glutamate homeostasis is a key role of retinal glial. We examined whether two glutamate transporters, Glast (expressed in Müller cells) and Glt1 (expressed in the astrocytes as well as cones and bipolar cells),65 were up-regulated in response to HC (Table 3). We also examined whether a marker of microglial activity (Aif1) was altered. In addition to maintaining glutamate homeostasis, glial cells can produce potent neurotrophins. We therefore examined the relative expression of a panel of growth factors implicated in RGC survival and neurite outgrowth (Bdnf, Cntf, Egf, Fgf2, Pedf, VegfA) from explants at day 4 in culture to further elucidate HC's mode of action.
Table 3.
Relative Quantification Values of a Panel of Growth Factors and Glial Markers
|
Gene Name |
RQ (RQ Min/RQ Max) |
| Bdnf | 0.0308 (0.0304/0.0311) |
| Cntf | 1.55 (0.68/3.57) |
| Egf | Not consistently detected |
| Fgf2 | 0.028 (0.027/0.029) |
| Pedf | 11.55 (7.63/17.49) |
| VegfA | 7.20 (6.37/8.14) |
| Glast | 1.09 (0.85/1.41) |
| Glt1 | 2.64 (2.55/2.74) |
| Aif1 | 0.036 (0.035/0.037) |
Relative quantification (RQ) was determined by the Pfaffl method58 along with the RQ minimum and maximum.
There was no increase in expression of Glast in explants treated with EN2 relative to explants treated with EN2 without HC but an almost threefold change in Glt1 expression (Table 3). There was a 33-fold decrease in Aif1 expression in EN2-treated explants compared with EN2 without HC–treated explants. Two growth factors, Bdnf and Fgf2, were decreased 33-fold in EN2-treated explants compared with EN2 without HC–treated explants after 4 days in culture, while Egf was not consistently detected in either sample. On the other hand, the transcript abundance for three growth factors, Cntf, Pedf, and VegfA, were found to be increased 1.55-, 11.55-, and 7.20-fold, respectively.
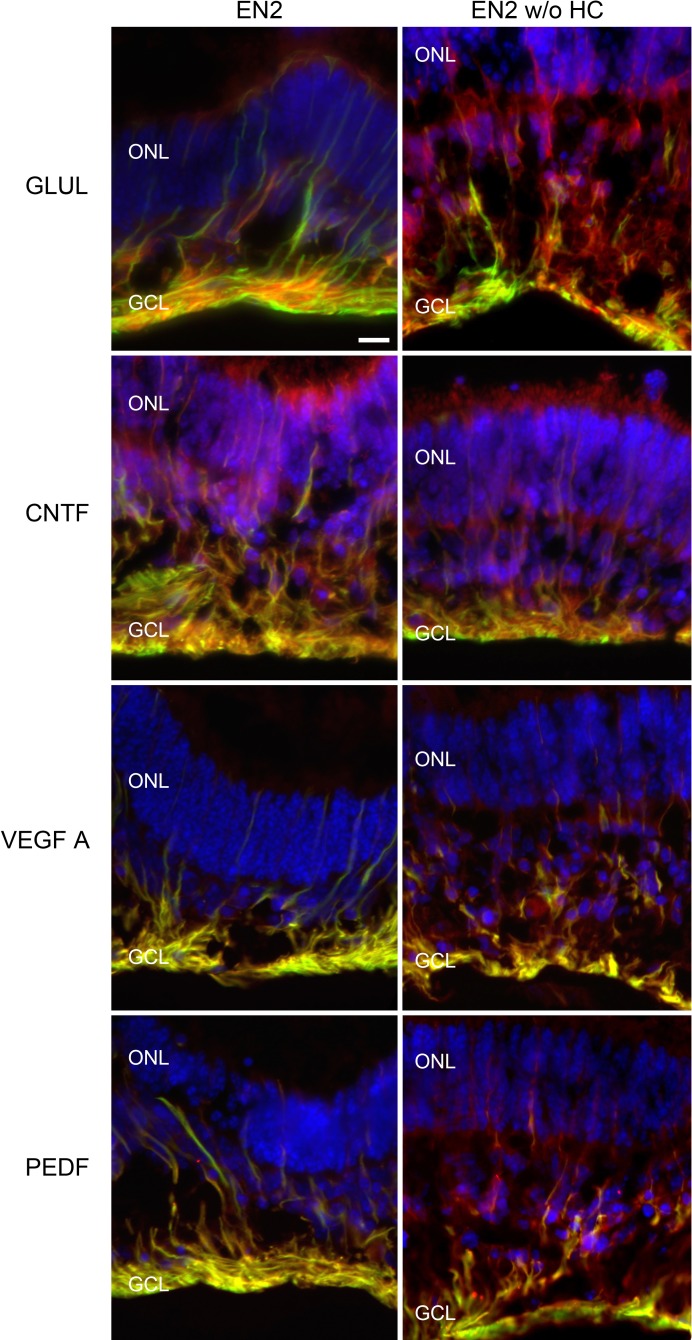
Sections of retinal explants at day 4 were immunolabeled for GFAP and either GLUL, CNTF, VEGF, or PEDF. These sections showed intense staining for GFAP in the ganglion cell layer, corresponding with astrocytes and Müller cell end feet in both EN2- and EN2 without HC–treated explants (Fig. 6, all panels; Supplemental Fig. 1, http://www.iovs.org/content/53/4/2046/suppl/DC1 ). Labeling for GFAP was also seen throughout all of the retinal layers corresponding with the bodies and processes of the Müller cells, but this staining in the Müller cells was more intense in the EN2-treated explants where GFAP immunoreactivity could be seen even in the outer limiting membrane (Supplemental Fig. 1). In EN2 without HC–treated explants, the GFAP in the Müller cells was clear only around the cell body and in the end feet near the ganglion cell layer (Supplemental Fig. 1). GLUL immunoreactivity was seen in both EN2- and EN2 without HC–treated explants, but labeling appeared to be localized more toward the Müller cell end feet in EN2 explants, while it was more diffuse in EN2 without HC explants (Fig. 6; Supplemental Fig. 1). GFAP and GLUL co-labeling was seen in the ganglion cell layer.
Figure 6.
Glial cells in explants produced GLUL, CNTF, VEGFA, and PEDF. Fluorescent digital micrographs showing sections of retinal explants at day 4 treated with EN2 (left column) or EN2 without HC (right column) labeled for DAPI (blue), GFAP (green), and either GLUL (first row, red), CNTF (second row, red), VEGFA (third row, red), or PEDF (fourth row, red). Size bar equals 20 μm.
A similar pattern was seen in sections co-labeled for GFAP and CNTF, VEGF, or PEDF. Sections of explants treated with EN2 had more intense labeling for the three growth factors, especially in the ganglion cell layer, and the growth factors appeared to co-localize with the GFAP. Sections of explants treated with EN2 without HC had a more diffuse and less intense labeling for the growth factors, and there was less apparent co-localization with GFAP. Positive growth factor labeling was apparent in both EN2- and EN2 without HC–treated explants, indicating that other factors in EN2 may also increase production of growth factors. The overlap in labeling of GFAP with the growth factors points to the macroglia as one source of the growth factors. Since other cell type–specific markers were not examined in these experiments, it cannot be ruled out that additional retinal cell types may also be contributing to the production of CNTF, VEGF, or PEDF.
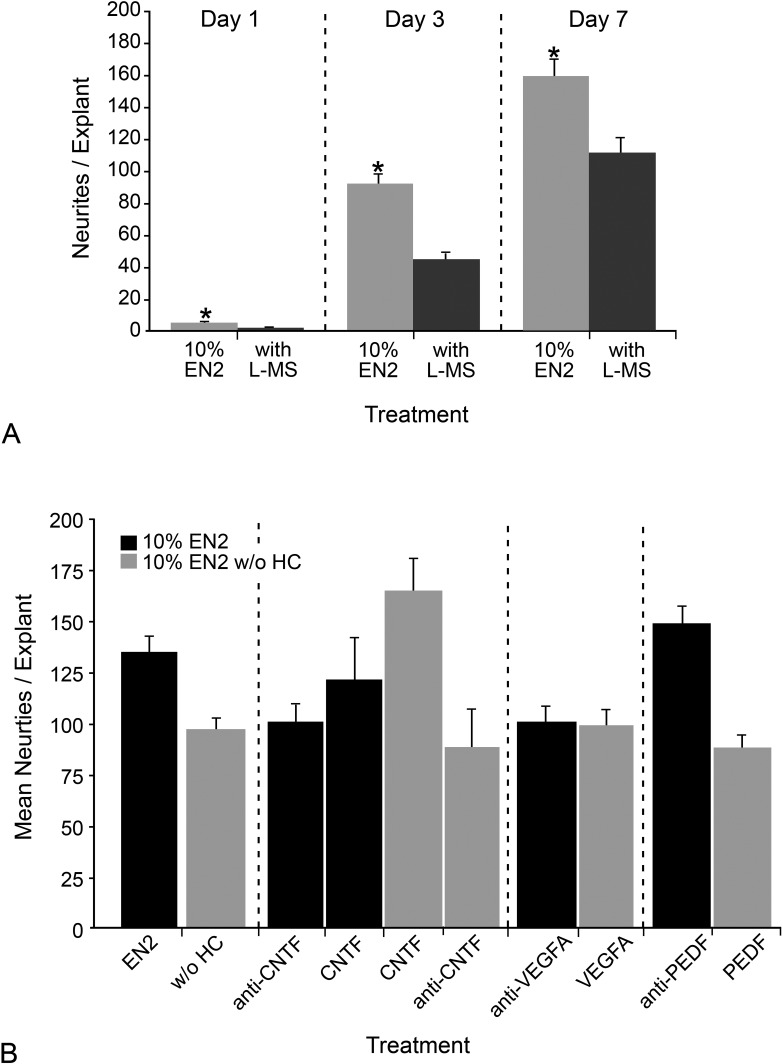
To test if the increase in Glul transcript level was relevant to the mechanism of HC action in stimulating more neurite outgrowth in explants, we used the GLUL inhibitor L-methionine sulfoximine in EN266–70 and monitored neurite outgrowth (Fig. 7A). At both days 4 and 7 in culture, neurite outgrowth was significantly diminished in explants treated with EN2 with the inhibitor compared with explants in just EN2 (P = 8.00 × 10−9 and P = 6.19 × 10−4). This indicated that one contribution of HC to a regenerative environment in the explant system may have been to prevent toxic buildup of glutamate by increasing GLUL and subsequent glutamate recycling.
Figure 7.
GLUL and CNTF were critical to increased neurite outgrowth stimulated by EN2. (A) Histograph showing the mean neurites per explant (±SEM) in explants from PN7 CB6F1 mice treated with EN2 or EN2 with 1 mg/mL L-methionine sulfoximine (L-MS) at days 1, 3, and 7 in culture. By day 3 and day 7, inhibition of GLUL resulted in a significant (*P < 0.001) decrease in neurite outgrowth. (B) Histograph of the mean neurites per explant (±SEM) in explants treated with EN2; EN2 with 0.2 μg/mL neutralizing antibody to either CNTF, VEGFA, or PEDF; EN2 without HC; or EN2 without HC and with 1 μg/mL of exogenous CNTF, VEGFA, or PEDF. In explants treated with EN2 with neutralizing antibody to CNTF, there was a significant 28.41% (P = 0.032) decrease in neurites relative to EN2-treated explants, while in explants treated with EN2 without HC and with CNTF, there was a significant 65.36% (P = 0.00088) increase in neurites relative to EN2 without HC–treated explants. In explants treated with EN2 with neutralizing antibody to VEGFA, there was a 15.03% decrease in neurites, while in explants treated with EN2 without HC and with VEGFA, there was an 11.41% increase in neurites. Antibodies to PEDF in EN2, or the addition of exogenous PEDF to EN2 without HC, had no effect on neurite outgrowth. Table 4 contains additional details on P values for different treatments relative to treatment with EN2 or EN2 without HC.
To test if the increases in Cntf, Pedf, or VegfA transcripts played any part in increasing neurite outgrowth, a neutralizing antibody to either CNTF, VEGFA, or PEDF was added to explants grown in EN2 (Fig. 7B). The reverse experiment was also performed: exogenous CNTF, VEGFA, or PEDF was added to EN2 without HC. In addition, for CNTF, the CNTF neuralizing antibody was added to EN2 without HC, and CNTF protein was added to EN2. A complete summary of P values for this experiment is given in Table 4.
Table 4.
P Values for Growth Factor Treatments Compared with EN2 or EN2 without HC
|
Treatment |
P
(vs. 10% EN2) |
P
(vs. 10% EN2 without HC) |
| 10% EN2 without HC | 0.0014 | |
| 10% EN2 + anti-CNTF | 0.0043 | 0.42 |
| 10% EN2 + CNTF | 0.28 | 0.14 |
| 10% EN2 without HC+ CNTF | 0.051 | 0.0002 |
| 10% EN2 without HC+ anti-CNTF | 0.018 | 0.33 |
| 10% EN2+ anti-VEGFA | 0.0018 | 0.37 |
| 10% EN2 without HC+ VEGFA | 0.0010 | 0.43 |
| 10% EN2 + anti-PEDF | 0.15 | 1.89 × 10−5 |
| 10% EN2 without HC+ PEDF | 1.19 × 10−5 | 0.16 |
All values were calculated by unpaired Student's t-test.
Addition of anti-CNTF to EN2 resulted in a significant decrease in neurite outgrowth from 134.47 ± 8.13 neurites in EN2 to 98.82 ± 10.23 neurites in EN2 with anti-CNTF (P = 0.0002). Addition of CNTF to EN2 without HC resulted in a significant increase in neurite outgrowth from 96.51 ± 6.17 neurites in EN2 without HC to 164.67 ± 16.12 neurites in EN2 without HC + CNTF (P = 0.00088). This increase was not significant when compared with EN2 (P = 0.051). Explants treated with EN2 + CNTF had 120.85 ± 21.17 neurites, which was not significantly different from explants treated with EN2 alone (P = 0.28). Explants treated with EN2 without HC + anti-CNTF had 87.53 ± 19.40 neurites, which was not significantly different from explants treated with EN2 without HC alone (P = 0.33). These data demonstrate that the HC-stimulated increase in Cntf expression was important to the mechanism by which EN2 caused more neurite outgrowth from retinal explants.
When anti-VEGFA was added to EN2, there was a significant decrease in neurites from 134.47 ± 8.13 neurites in EN2 to 99.98 ± 8.27 neurites in EN2 with anti-VEFA (P = 0.0018). Addition of VEGFA to EN2 without HC resulted in no change in neurites, with 96.51 ± 6.17 neurites in EN2 without HC to 98.36 ± 8.07 neurites in EN2 without HC + VEGFA (P = 0.43). It appears that the increased VegfA expression was important to HC-stimulated neurite outgrowth, but there was some type of further interaction between HC and VEGFA that could not be recapitulated by addition of VEGFA to EN2 without HC.
Explants treated with EN2 + anti-PEDF had 147.88 ± 9.70 neurites, which was not significantly different from explants treated with EN2 alone (P = 0.15), and explants treated with EN2 without HC with PEDF had 86.77 ± 7.50 neurites, which was not significantly different from explants treated with EN2 without HC alone (P = 0.16). Increased PEDF did not appear to be critical to HC's mode of action.
Discussion
Recent studies have shown that complex trophic environments increase the regenerative potential of RGCs compared with single growth factors.17,71–73 To better define what factors are beneficial in combination, we set out to characterize a minimally complex mixture of different factors that was able to enhance RGC neurite outgrowth. Initially, we used commercial media supplements G5 and N2, which contained different combinations of components, including growth factors, hormones, co-factors, and other small proteins and molecules. We found that of the commercially available supplements, G5 was able to promote neurite outgrowth from retinal explants. EN2, a supplement we generated by adding G5 components to N2, was better than either supplement alone for stimulating increased numbers of neurites that were also greater in length and appeared to be more complexly branched. Overall, these experiments suggest that the combination of molecules in EN2 promoted the greatest growth-stimulatory effect on RGCs competent to sprout and grow axons.
A significant part of the EN2 neurite outgrowth effect was mediated by HC. Removing HC from EN2 resulted in a significant decrease in neurite outgrowth. A major consideration in assessing the effect of HC was to determine if HC acted directly on RGCs or if the effect was mediated through other cell types present in the retinal explant. Lindsey and Weinreb48 show that purified RGCs in culture survived best in a complex media containing HC, progesterone, and insulin and that removal of any of these components either singly or in pairs reduced RGC survival. Isolated RGCs survived similarly in EN2 with or without HC, and neither EN2 nor EN2 without HC was effective in preventing RGC death in a crush model. Thus, there is no evidence then that EN2 increases the pool of RGCs available to generate neurites. It is important to note that HC added by itself to N2 did not stimulate neurite outgrowth from explants and that in isolated RGCs, EN2 did not stimulate significant neurite outgrowth, while EN2 without HC did. The latter piece of data could suggest that HC is actually suppressing neurite outgrowth from isolated RGCs, but we do not believe this was the case since G5, which did contain HC, was able to increase neurite outgrowth from isolated RGCs and was able to prevent RGC cell death after ON crush. Although the differences in neurite outgrowth by EN2 on isolated RGCs versus explants may be influenced by species differences (rats versus mice), we think that this is unlikely given that RGC survival was similar in response to the supplements between the cell culture and ON crush paradigms. Rather we think that the effect of HC requires the synergistic interaction with other components contributed by both G5 and N2 and that the effects of HC appear to be mediated by retinal cell types other than RGCs.
The inhibitor studies using antagonists of the NR3C1 and NR3C2 high-affinity receptors may provide some insight into the cells that are most responsive to HC. Studies of the retina of chicks and salamanders have shown that the NR3C1 receptor is more highly expressed in Müller cells and astrocytes than other cell types in the retina including RGCs, although receptor distribution in the mouse has not been extensively characterized.64,74,75 The NR3C2 receptor is predominately expressed in the retinal pigmented epithelium and cone photoreceptors of rodents, while in humans, NR3C2 is predominately expressed in the nonpigmented epithelium of the ciliary body.76–78 Most NR3C2 in the retina is unoccupied by glucocorticoid ligands because of the presence of the enzyme 11β-hydroxysteroid dehydrogenase 2, which converts cortisol (HC) to its inactive form cortisone in epithelial tissues.62,78 Our data showing that the NR3C1 antagonist mifepristone, but not the NR3C2 antagonist spironolactone, significantly decreases neurite outgrowth from retinal explants indicates that the likely action of HC neurite induction is mediated through the NR3C1 receptor and thus via the retinal macroglia. This is further bolstered by our data showing that expression of Gfap and Glul is increased in response to HC.
Our speculation that macroglia contribute to the ability of RGCs to sprout and grow axons is not novel, and many studies have implicated these cells as key sites of mediation of factors that can influence axon regeneration.21,46,47,79–83 The nature of the interaction with macroglia is still not known, however, as these cells have had both negative and positive trophic effects attributed to them. For example, astrocytes in co-cultures of neurons typically present a nonpermissive growth environment that has been linked to the expression of the intermediate filament GFAP.84 Interestingly, injuries to the retina, including glaucoma,85 experimentally-induced glaucoma,86 ON transection,43 and ON crush,87 are characterized by the local activation of Müller cells and astrocytes, which is exemplified by the increased expression of GFAP and decreased expression of the glutamate-scavenging enzyme GLUL in Müller cells.41,72,88 While reactive macroglia are largely associated with a growth inhibitory environment, these cells are also known to produce potent growth factors, such as FGF2,26,89 CNTF,37,81 and BDNF,47 all of which can have neuroprotective and/or axonal growth stimulatory effects on RGCs.
In our explant system, we see a clear role for HC in modifying the gene expression profile of the macroglia, especially the Müller cells, to produce a more permissive growth environment. Explants treated with EN2 had increases in expression of Gfap, Glul, Glt1, Cntf, Pedf, and VegfA compared with explants treated with EN2 without HC, as well as intense co-immunoreactivity for GFAP and GLUL, CNTF, VEGF, and PEDF. Inhibiting the action of GLUL, CNTF, or VEGFA significantly decreased the number of neurites from explants. Supplementing EN2 without HC with CNTF, but not VEGFA, stimulated neurite outgrowth on par with that from EN2. Based on these data, increases in GLUL and CNTF are key factors in the HC role of aiding neurite stimulation from EN2-treated explants, while it remains unclear what may be happening with PEDF and VEGFA. Since inhibiting VEGFA was detrimental to neurite outgrowth, but supplementing exogenous VEGFA could not recapitulate the HC effect, it may be that VEGFA can promote expression of neurotrophic molecules only in synergy with HC.
CNTF is one of the best-studied growth factors for RGC survival and axon regeneration.9,11,13,25,27,35–37,80,90–92 It is known both to be produced by Müller cells and to act upon Müller cells.81,88,93,94 The studies cited highlight how CNTF is a key factor in maintaining proper relationships between the Müller cells and RGCs. Thus, it is not entirely surprising to see CNTF being such a key component of EN2's mode of action.
One of the most important functions of Müller cells is to regulate glutamate homeostasis within the retina.41,42,95,96 In the developing retina, GLUL expression is induced in the Müller glia by cortisol.64 Glutamate excitotoxicity is a thought to be a key feature of many retinal diseases and is very detrimental to axon regeneration.55,97–102 Both Glul and Glt1 transcripts are increased in EN2-treated explants relative to EN2 without HC–treated explants. One interpretation of the HC effect on explants is that increased glutamate turnover prevents glutamate excitotoxicity in the explant system, creating an overall better environment for regeneration. It is unclear whether the increase in Glul transcript in EN2-treated explants is greater than endogenous expression of Glul in age-matched retinas. Thus, a second interpretation would be that EN2 preserves glutamate recycling rather than increasing it. Regardless, HC does support glutamate recycling, and this is likely an important part of EN2's increased stimulation of neurites.
While CNTF and the glutamate recycling molecules have modes of action that are reasonably clear in terms of how they might support neurite outgrowth, there is no clear interpretation of how the increase in Gfap may be beneficial to neurite outgrowth. Interestingly, GFAP has multiple glucocorticoid receptor elements for NR3C1 to bind in its promoter.103,104 Rozovsky et al.105 found that corticosterone (the murine glucocorticoid equivalent of HC) decreased GFAP expression in astrocytes co-cultured with neurons. Heiduschka and Thanos49 observed that an intravitreal injection of aurintricarboxylic acid (a apoptosis inhibitor) and cortisol promoted an increase in GFAP that was associated with increased RGC survival and regeneration in retinas of rats following ON transection. Interestingly, Gfap expression can be modulated by not only glucocorticoids, like HC, but also by FGF2 and CNTF.103 Experiments are currently under way to directly investigate the role of HC in regulating GFAP expression and the direct role of GFAP on neurite outgrowth in our culture system.
The mechanism by which EN2 stimulated neurite outgrowth is complex, since some components, such as EGF, FGF2, and insulin, can affect RGC neuritogenesis directly,17,18,26,29 and others, such as HC, affect surrounding cells in a way that creates a more growth permissive environment. The complexity of this mechanism is a strength of this strategy for regeneration because many of the pathways activated can amplify and interact with each other, resulting in an effect that is greater than the sum of its parts. We have demonstrated that HC contributes to neurite outgrowth by modulating macroglial gene expression. The Müller cells are strongly implicated as the target of the HC effect and contribute to a regenerative environment for RGC axon regeneration by producing the potent growth factor CNTF as well as maintaining glutamate recycling.
Supplementary Material
Acknowledgments
We thank Jenny Peterson and Paul Bertics for technical advice, Jon Detloff for counting cells of Nissl-stained retinas, Albee Messing for the GFAP antibody, and Cassandra Schlamp for technical advice and comments on the manuscript.
Footnotes
This work was supported by grants including NIH 5T32GM08349 Biotechnology Training Grant, R01EY12223 (RWN), and Vision CORE grant P30EY016665 from the National Eye Institute, the University of Wisconsin Eye Research Institute's Alice R. McPherson Endowment for the Visual Sciences, and the Lew Wasserman Research Award (RWN) from Research to Prevent Blindness, Inc.
Disclosure: K.A. Toops, None; C. Berlinicke, None; D.J. Zack, None; R.W. Nickells, None
References
- 1. Nickells RW. The molecular biology of retinal ganglion cell death: caveats and controversies. Brain Res Bull. 2004;62:439–446. [DOI] [PubMed] [Google Scholar]
- 2. Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–287. [PubMed] [Google Scholar]
- 3. Nickells RW. Ganglion cell death in glaucoma: from mice to men. Vet Ophthalmol. 2007;10(suppl 1);88–94. [DOI] [PubMed] [Google Scholar]
- 4. Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways—a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662. [DOI] [PubMed] [Google Scholar]
- 5. Libby RT, Li Y, Savinova OV, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baptiste DC, Powell KJ, Jollimore CA, et al. Effects of minocycline and tetracycline on retinal ganglion cell survival after axotomy. Neuroscience. 2005;134:575–582. [DOI] [PubMed] [Google Scholar]
- 7. Logan A, Ahmed Z, Baird A, Gonzalez AM, Berry M. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain. 2006;129:490–502. [DOI] [PubMed] [Google Scholar]
- 8. Blanco RE, Soto I, Duprey-Diaz M, Blagburn JM. Up-regulation of brain-derived neurotrophic factor by application of fibroblast growth factor-2 to the cut optic nerve is important for long-term survival of retinal ganglion cells. J Neurosci Res. 2008;86:3382–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leaver SG, Cui Q, Plant GW, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13:1328–1341. [DOI] [PubMed] [Google Scholar]
- 10. Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch Ophthalmol. 2006;124:520–526. [DOI] [PubMed] [Google Scholar]
- 11. Lingor P, Tonges L, Pieper N, et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. [DOI] [PubMed] [Google Scholar]
- 12. Nork TM, Poulsen GL, Nickells RW, et al. Protection of ganglion cells in experimental glaucoma by retinal laser photocoagulation. Arch Ophthalmol. 2000;118:1242–1250. [DOI] [PubMed] [Google Scholar]
- 13. van Adel BA, Arnold JM, Phipps J, Doering LC, Ball AK. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005;63:215–234. [DOI] [PubMed] [Google Scholar]
- 14. Yan Q, Wang J, Matheson CR, Urich JL. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF). J Neurobiol. 1999;38:382–390. [DOI] [PubMed] [Google Scholar]
- 15. Johnson EC, Guo Y, Cepurna WO, Morrison JC. Neurotrophin roles in retinal ganglion cell survival: lessons from rat glaucoma models. Exp Eye Res. 2009;88:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lebrun-Julien F, Di Polo A. Molecular and cell-based approaches for neuroprotection in glaucoma. Optom Vis Sci. 2008;85:417–424. [DOI] [PubMed] [Google Scholar]
- 17. Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. [DOI] [PubMed] [Google Scholar]
- 18. So KF, Yip HK. Regenerative capacity of retinal ganglion cells in mammals. Vision Res. 1998;38:1525–1535. [DOI] [PubMed] [Google Scholar]
- 19. Raju TR, Rao MS, Nagaraja TN, Meti BL, Schulz M. Retinal ganglion cell survival and neurite regeneration in vitro after cell death period are dependent upon target derived trophic factor and retinal glial factor(s). Brain Res. 1994;664:247–251. [DOI] [PubMed] [Google Scholar]
- 20. Cui Q, Yin Y, Benowitz LI. The role of macrophages in optic nerve regeneration. Neuroscience. 2009;158:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Melo Reis RA, Cabral-da-Silva MC, de Mello FG, Taylor JS. Muller glia factors induce survival and neuritogenesis of peripheral and central neurons. Brain Res. 2008;1205:1–11. [DOI] [PubMed] [Google Scholar]
- 22. Berry M, Ahmed Z, Lorber B, Douglas M, Logan A. Regeneration of axons in the visual system. Restor Neurol Neurosci. 2008;26:147–174. [PubMed] [Google Scholar]
- 23. Klocker N, Braunling F, Isenmann S, Bahr M. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport. 1997;8:3439–3442. [DOI] [PubMed] [Google Scholar]
- 24. Klocker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3′-kinase/protein kinase B signaling. J Neurosci. 2000;20:6962–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. [DOI] [PubMed] [Google Scholar]
- 26. Blanco RE, Lopez-Roca A, Soto J, Blagburn JM. Basic fibroblast growth factor applied to the optic nerve after injury increases long-term cell survival in the frog retina. J Comp Neurol. 2000;423:646–658. [PubMed] [Google Scholar]
- 27. Ju WK, Kim KY, Lee MY, et al. Up-regulated CNTF plays a protective role for retrograde degeneration in the axotomized rat retina. Neuroreport. 2000;11:3893–3896. [DOI] [PubMed] [Google Scholar]
- 28. Blanco RE, López-Roca A, Soto J, Blagburn JM. Basic fibroblast growth factor applied to the optic nerve after injury increases long-term cell survival in the frog retina. J Comp Neurol. 2000;423:646–658. [PubMed] [Google Scholar]
- 29. Webber CA, Chen YY, Hehr CL, Johnston J, McFarlane S. Multiple signaling pathways regulate FGF-2-induced retinal ganglion cell neurite extension and growth cone guidance. Mol Cell Neurosci. 2005;30:37–47. [DOI] [PubMed] [Google Scholar]
- 30. Pang IH, Zeng H, Fleenor DL, Clark AF. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci. 2007;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. [DOI] [PubMed] [Google Scholar]
- 33. Cen LP, Luo JM, Zhang CW, et al. Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Invest Ophthalmol Vis Sci. 2007;48:4257–4266. [DOI] [PubMed] [Google Scholar]
- 34. Pernet V, Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129:1014–1026. [DOI] [PubMed] [Google Scholar]
- 35. Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. [DOI] [PubMed] [Google Scholar]
- 36. Park K, Luo JM, Hisheh S, Harvey AR, Cui Q. Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J Neurosci. 2004;24:10806–10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. [DOI] [PubMed] [Google Scholar]
- 38. Kerrison JB, Lewis RN, Otteson DC, Zack DJ. Bone morphogenetic proteins promote neurite outgrowth in retinal ganglion cells. Mol Vis. 2005;11:208–215. [PubMed] [Google Scholar]
- 39. Michler-Stuke A, Bottenstein JE. Proliferation of glial-derived cells in defined media. J Neurosci Res. 1982;7:215–228. [DOI] [PubMed] [Google Scholar]
- 40. Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. [DOI] [PubMed] [Google Scholar]
- 42. Bringmann A, Pannicke T, Biedermann B, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54:143–160. [DOI] [PubMed] [Google Scholar]
- 43. Huxlin KR, Dreher Z, Schulz M, Dreher B. Glial reactivity in the retina of adult rats. Glia. 1995;15:105–118. [DOI] [PubMed] [Google Scholar]
- 44. Lorber B, Berry M, Douglas MR, Nakazawa T, Logan A. Activated retinal glia promote neurite outgrowth of retinal ganglion cells via apolipoprotein E. J Neurosci Res. 2009;87:2645–2652. [DOI] [PubMed] [Google Scholar]
- 45. Bahr M. Adult rat retinal glia in vitro: effects of in vivo crush-activation on glia proliferation and permissiveness for regenerating retinal ganglion cell axons. Exp Neurol. 1991;111:65–73. [DOI] [PubMed] [Google Scholar]
- 46. Garcia DM, Koke JR. Astrocytes as gate-keepers in optic nerve regeneration—a mini-review. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:135–138. [DOI] [PubMed] [Google Scholar]
- 47. Seki M, Tanaka T, Sakai Y, et al. Muller cells as a source of brain-derived neurotrophic factor in the retina: noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Muller cells. Neurochem Res. 2005;30:1163–1170. [DOI] [PubMed] [Google Scholar]
- 48. Lindsey JD, Weinreb RN. Survival and differentiation of purified retinal ganglion cells in a chemically defined microenvironment. Invest Ophthalmol Vis Sci. 1994;35:3640–3648. [PubMed] [Google Scholar]
- 49. Heiduschka P, Thanos S. Cortisol promotes survival and regeneration of axotomised retinal ganglion cells and enhances effects of aurintricarboxylic acid. Graefes Arch Clin Exp Ophthalmol. 2006;244:1512–1521. [DOI] [PubMed] [Google Scholar]
- 50. Marcus RC, Wang LC, Mason CA. Retinal axon divergence in the optic chiasm: midline cells are unaffected by the albino mutation. Development. 1996;122:859–868. [DOI] [PubMed] [Google Scholar]
- 51. Kleene R, Mzoughi M, Joshi G, et al. NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J Neurosci. 2010;30:10784–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miki A, Miki K, Ueno S, et al. Prolonged blockade of VEGF receptors does not damage retinal photoreceptors or ganglion cells. J Cell Physiol. 2010;224:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Semaan SJ, Schlamp CL, Nickells RW. Dominant inheritance of retinal ganglion cell resistance to optic nerve crush in mice. BMC Neurosci. 2007;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schlamp CL, Thliveris AT, Li Y, et al. Insertion of the beta Geo promoter trap into the Fem1c gene of ROSA3 mice. Mol Cell Biol. 2004;24:3794–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40:1004–1008. [PubMed] [Google Scholar]
- 56. Schlamp CL, Johnson EC, Li Y, Morrison JC, Nickells RW. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol Vis. 2001;7:192–201. [PubMed] [Google Scholar]
- 57. Pelzel HR, Schlamp CL, Nickells RW. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci. 2010;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. [DOI] [PubMed] [Google Scholar]
- 60. Drager UC, Olsen JF. Ganglion cell distribution in the retina of the mouse. Invest Ophthalmol Vis Sci. 1981;20:285–293. [PubMed] [Google Scholar]
- 61. Sutanto W, De Kloet ER. Species-specificity of corticosteroid receptors in hamster and rat brains. Endocrinology. 1987;121:1405–1411. [DOI] [PubMed] [Google Scholar]
- 62. Lu NZ, Wardell SE, Burnstein KL, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. [DOI] [PubMed] [Google Scholar]
- 63. Gorovits R, Ben-Dror I, Fox LE, Westphal HM, Vardimon L. Developmental changes in the expression and compartmentalization of the glucocorticoid receptor in embryonic retina. Proc Natl Acad Sci U S A. 1994;91:4786–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grossman R, Fox LE, Gorovits R, Ben-Dror I, Reisfeld S, Vardimon L. Molecular basis for differential expression of glutamine synthetase in retina glia and neurons. Brain Res Mol Brain Res. 1994;21:312–320. [DOI] [PubMed] [Google Scholar]
- 65. Harada T, Harada C, Watanabe M, et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci U S A. 1998;95:4663–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rowe WB, Meister A. Identification of L-methionine-S-sulfoximine as the convulsant isomer of methionine sulfoximine. Proc Natl Acad Sci U S A. 1970;66:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ronzio RA, Rowe WB, Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969;8:1066–1075. [DOI] [PubMed] [Google Scholar]
- 68. Ghoddoussi F, Galloway MP, Jambekar A, Bame M, Needleman R, Brusilow WS. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. J Neurol Sci. 2010;290:41–47. [DOI] [PubMed] [Google Scholar]
- 69. Gorovits R, Avidan N, Avisar N, Shaked I, Vardimon L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc Natl Acad Sci U S A. 1997;94:7024–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shaked I, Ben-Dror I, Vardimon L. Glutamine synthetase enhances the clearance of extracellular glutamate by the neural retina. J Neurochem. 2002;83:574–580. [DOI] [PubMed] [Google Scholar]
- 71. Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lorber B, Berry M, Logan A. Different factors promote axonal regeneration of adult rat retinal ganglion cells after lens injury and intravitreal peripheral nerve grafting. J Neurosci Res. 2008;86:894–903. [DOI] [PubMed] [Google Scholar]
- 73. Cui Q, Yin Y, Benowitz LI. The role of macrophages in optic nerve regeneration. Neuroscience. 2008;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reisfeld S, Vardimon L. Cell to cell contacts control the transcription activity of the glucocorticoid receptor. Mol Endocrinol. 1994;8:1224–1233. [DOI] [PubMed] [Google Scholar]
- 75. Psarra AM, Bochaton-Piallat ML, Gabbiani G, Sekeris CE, Tsacopoulos M. Mitochondrial localization of glucocortocoid receptor in glial (Muller) cells in the salamander retina. Glia. 2003;41:38–49. [DOI] [PubMed] [Google Scholar]
- 76. Mirshahi M, Nicolas C, Mirshahi A, et al. The mineralocorticoid hormone receptor and action in the eye. Biochem Biophys Res Commun. 1996;219:150–156. [DOI] [PubMed] [Google Scholar]
- 77. Golestaneh N, Picaud S, Mirshahi M. The mineralocorticoid receptor in rodent retina: ontogeny and molecular identity. Mol Vis. 2002;8:221–225. [PubMed] [Google Scholar]
- 78. Suzuki T, Sasano H, Kaneko C, Ogawa S, Darnel AD, Krozowski ZS. Immunohistochemical distribution of 11beta-hydroxysteroid dehydrogenase in human eye. Mol Cell Endocrinol. 2001;173:121–125. [DOI] [PubMed] [Google Scholar]
- 79. Xue LP, Lu J, Cao Q, Hu S, Ding P, Ling EA. Muller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience. 2006;139:723–732. [DOI] [PubMed] [Google Scholar]
- 80. Chun MH, Ju WK, Kim KY, et al. Upregulation of ciliary neurotrophic factor in reactive Muller cells in the rat retina following optic nerve transection. Brain Res. 2000;868:358–362. [DOI] [PubMed] [Google Scholar]
- 81. Honjo M, Tanihara H, Kido N, Inatani M, Okazaki K, Honda Y. Expression of ciliary neurotrophic factor activated by retinal Muller cells in eyes with NMDA- and kainic acid-induced neuronal death. Invest Ophthalmol Vis Sci. 2000;41:552–560. [PubMed] [Google Scholar]
- 82. Woldemussie E, Wijono M, Ruiz G. Muller cell response to laser-induced increase in intraocular pressure in rats. Glia. 2004;47:109–119. [DOI] [PubMed] [Google Scholar]
- 83. Gimenez y Ribotta M, Menet V, Privat A. The role of astrocytes in axonal regeneration in the mammalian CNS. Prog Brain Res. 2001;132:587–610. [DOI] [PubMed] [Google Scholar]
- 84. Menet V, Gimenez y, Ribotta M, Chauvet N, et al. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. J Neurosci. 2001;21:6147–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hiscott PS, Grierson I, Trombetta CJ, Rahi AH, Marshall J, McLeod D. Retinal and epiretinal glia—an immunohistochemical study. Br J Ophthalmol. 1984;68:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang X, Tay SS, Ng YK. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Exp Brain Res. 2000;132:476–484. [DOI] [PubMed] [Google Scholar]
- 87. Chen H, Weber AJ. Expression of glial fibrillary acidic protein and glutamine synthetase by Muller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factor. Glia. 2002;38:115–125. [DOI] [PubMed] [Google Scholar]
- 88. Fischer AJ, Omar G, Eubanks J, McGuire CR, Dierks BD, Reh TA. Different aspects of gliosis in retinal Muller glia can be induced by CNTF, insulin, and FGF2 in the absence of damage. Mol Vis. 2004;10:973–986. [PubMed] [Google Scholar]
- 89. Kostyk SK, D'Amore PA, Herman IM, Wagner JA. Optic nerve injury alters basic fibroblast growth factor localization in the retina and optic tract. J Neurosci. 1994;14:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Avwenagha O, Campbell G, Bird MM. The outgrowth response of the axons of developing and regenerating rat retinal ganglion cells in vitro to neurotrophin treatment. J Neurocytol. 2003;32:1055–1075. [DOI] [PubMed] [Google Scholar]
- 91. Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci. 2009;41:233–246. [DOI] [PubMed] [Google Scholar]
- 93. Harada T, Harada C, Kohsaka S, et al. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ju WK, Lee MY, Hofmann HD, Kirsch M, Chun MH. Expression of CNTF in Muller cells of the rat retina after pressure-induced ischemia. Neuroreport. 1999;10:419–422. [DOI] [PubMed] [Google Scholar]
- 95. Taylor S, Srinivasan B, Wordinger RJ, Roque RS. Glutamate stimulates neurotrophin expression in cultured Muller cells. Brain Res Mol Brain Res. 2003;111:189–197. [DOI] [PubMed] [Google Scholar]
- 96. Rauen T, Wiessner M. Fine tuning of glutamate uptake and degradation in glial cells: common transcriptional regulation of GLAST1 and GS. Neurochem Int. 2000;37:179–189. [DOI] [PubMed] [Google Scholar]
- 97. Kruchkova Y, Ben-Dror I, Herschkovitz A, David M, Yayon A, Vardimon L. Basic fibroblast growth factor: a potential inhibitor of glutamine synthetase expression in injured neural tissue. J Neurochem. 2001;77:1641–1649. [DOI] [PubMed] [Google Scholar]
- 98. Maier K, Merkler D, Gerber J, et al. Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation. Neurobiol Dis. 2007;25:514–525. [DOI] [PubMed] [Google Scholar]
- 99. Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci. 2002;43:2236–2243. [PubMed] [Google Scholar]
- 100. Moreno MC, Sande P, Marcos HA, de Zavalia N. Keller Sarmiento MI, Rosenstein RE. Effect of glaucoma on the retinal glutamate/glutamine cycle activity. Faseb J. 2005;19:1161–1162. [DOI] [PubMed] [Google Scholar]
- 101. Shen W, Slaughter MM. A non-excitatory paradigm of glutamate toxicity. J Neurophysiol. 2002;87:1629–1634. [DOI] [PubMed] [Google Scholar]
- 102. Vardimon L. Neuroprotection by glutamine synthetase. Isr Med Assoc J. 2000;2(suppl);46–51. [PubMed] [Google Scholar]
- 103. Gomes FC, Paulin D. Moura Neto V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res. 1999;32:619–631. [DOI] [PubMed] [Google Scholar]
- 104. Laping NJ, Teter B, Nichols NR, Rozovsky I, Finch CE. Glial fibrillary acidic protein: regulation by hormones, cytokines, and growth factors. Brain Pathol. 1994;4:259–275. [DOI] [PubMed] [Google Scholar]
- 105. Rozovsky I, Laping NJ, Krohn K, Teter B, O'Callaghan JP, Finch CE. Transcriptional regulation of glial fibrillary acidic protein by corticosterone in rat astrocytes in vitro is influenced by the duration of time in culture and by astrocyte-neuron interactions. Endocrinology. 1995;136:2066–2073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.