Abstract
Context
Depression and dementia are common in older adults and often co-occur, but it is unclear whether depression is an etiologic risk factor for dementia.
Objective, Design, Setting and Participants
To clarify the timing and etiology of the association, we examined depressive symptoms assessed in mid-life (1964–1973) and late-life (1994–2000) and the risks of dementia, Alzheimer’s disease (AD) and vascular dementia (VaD) (2003–2009) in a retrospective cohort study of 13,535 long-term Kaiser Permanente members. Depressive symptoms were categorized as none, mid-life only, late-life only or both. Cox proportional hazards models (age as time-scale) adjusted for demographics and medical comorbidities were used to examine depressive symptom category and risk of dementia, AD or VaD.
Main Outcome Measure
Any medical record diagnosis of dementia; Neurology clinic diagnosis of AD or VaD.
Results
Subjects had a mean (standard deviation) age of 81 (5) years in 2003; 58% were women and 25% were non-white. Depressive symptoms were present in 14.1% of subjects in mid-life only, 9.2% late-life only, and 4.2% both. Over 6 years, 23.1% were diagnosed with dementia (5.5% AD, 2.3% VaD). The adjusted hazard of dementia was increased by approximately 20% for mid-life depressive symptoms only (Hazard Ratio [95% confidence interval]: 1.19 [1.07, 1.32]), 70% for late-life symptoms only (1.72 [1.54, 1.92]), and 80% for both (1.77 [1.52, 2.06]). When we examined AD and VaD separately, subjects with late-life depressive symptoms only had a two-fold increase in AD risk (2.06 [1.67, 2.55]) whereas subjects with both mid-life and late-life symptoms had more than a three-fold increase in VaD risk (3.51 [2.44, 5.05]).
Conclusions
Depressive symptoms in mid-life or late-life are associated with an increased risk of developing dementia. Depression that begins in late-life may be part of the AD prodrome, while recurrent depression may be etiologically associated with increased risk of VaD.
BACKGROUND
There are currently 5.3 million individuals in the U.S. with Alzheimer’s disease (AD), and the associated healthcare costs in 2010 were 172 billion dollars.1 Prevalence and costs of AD and other dementias are projected to rise dramatically over the next 40 years unless a prevention or cure can be found.2 Therefore, it is critical to gain a greater understanding of the key risk factors and etiologic underpinnings of dementia from a population-based perspective.
Depression commonly occurs in individuals with cognitive impairment and dementia.3 Although some studies have found that depression coincides with4–6 or follows7,8 the onset of dementia in older adults, most studies and several meta-analyses have concluded that depression precedes dementia and is associated with approximately a two-fold increase in the risk of developing cognitive impairment or dementia.9–16 It remains controversial, however, whether depression reflects an etiologic risk factor, is part of the dementia prodrome or shares genetic or other neuropathologic features with dementia.10,17
Vascular disease has been hypothesized as one of the potential mechanisms underlying the association between depression and dementia.18 There is evidence of a reciprocal relationship between vascular disease and depression, in which each condition is associated with an increased risk of developing the other.19–21 There also is growing evidence that vascular disease contributes to the clinical manifestation of dementia symptoms.22–24 Several cross-sectional studies have found that depressive symptoms are more common in vascular dementia (VaD) than AD,25–28 which provides some support for the vascular-depression-dementia hypothesis. However, to our knowledge, prior studies have not determined whether depression is more likely to lead to VaD longitudinally, which would provide greater support for an etiologic association attributable to vascular disease.
Another limitation of most prior studies in this area is they have had relatively short follow-up periods. Given that dementia has a long preclinical period, these studies cannot clearly differentiate between etiologic risk factors and symptoms of preclinical disease. Thus, life-course studies with extended follow-up periods are critical for clarifying the timing and nature of the association between depression and dementia.
We examined the association between depressive symptoms and dementia over 45 years in a longitudinal study of more than 13,000 long-term members of the Kaiser Permanente Medical Care Program of Northern California. The primary goals of our study were to 1) clarify the timing of the association by examining the effects of depressive symptoms in mid-life and late-life and 2) clarify the role of vascular disease by examining associations with AD and VaD separately.
METHODS
Study population
The study population consisted of members of the Kaiser Permanente Medical Care Program of Northern California who participated in a voluntary health examination called the Multiphasic Health Checkup (MHC) in San Francisco and Oakland during 1964–73 when they were 40–55 years old. Kaiser is a nonprofit, integrated health maintenance organization that provides comprehensive inpatient and outpatient care to more than one fourth of the population in the geographic areas served. The MHC was administered at the San Francisco and Oakland medical clinics to collect a data on health habits and medical conditions of Kaiser members. The original MHC cohort included 30,392 individuals. Our analyses excluded individuals who had a dementia diagnosis (N=4,627), died (N=6,818) or ended their Kaiser membership (N=2,662) prior to January 1, 2003, as well as those who were missing data on race (N=26) or education (N=2,724) for a final analytic cohort of 13,535 individuals (Figure 1).
Figure 1.
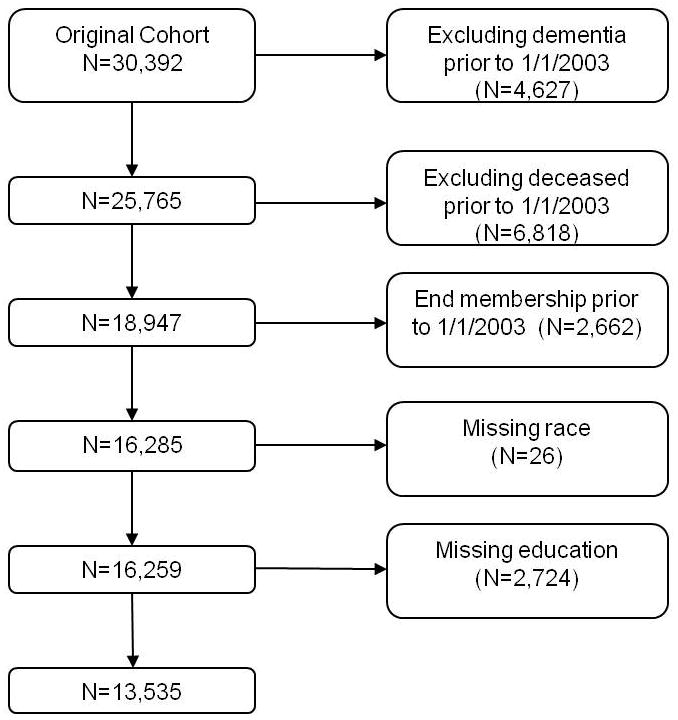
Flow chart of study participants.
Study procedures were approved by the Kaiser Division of Research Internal Ethics Committee, the Committee on Human Research at the University of California, San Francisco (UCSF) and the Research & Development Committee at the San Francisco Veterans Affairs Medical Center (SFVAMC). Analyses were performed by experienced Kaiser data analysts. To maximize patient confidentiality, non-Kaiser investigators were provided with data summaries only.
Mid-life depressive symptoms
As part of the mid-life health survey, Kaiser members were asked: “Do you often feel unhappy or depressed?” Study participants who answered ‘yes’ to this question were classified as having depressive symptoms in mid-life. We also searched hospitalization records and classified individuals who were hospitalized for depression from 1971–1979 as having mid-life depressive symptoms. Depression was determined using the following International Classification of Diseases – 9th edition (ICD-9) diagnostic codes: 296.2 (major depressive disorder), 296.3 (recurrent major depressive disorder), 298.0 (depressive type psychosis), 300.4 (dysthymic disorder) and 311.0 (depressive disorder not elsewhere classified). Eight percent of those classified as having mid-life depressive symptoms had been hospitalized for their depression; of these 57% also answered ‘yes’ to the survey question on depressive symptoms.
Late-life depressive symptoms
Late-life depression was determined by searching Kaiser’s comprehensive electronic medical record database system, which includes diagnoses from all inpatient and outpatient encounters at Kaiser medical centers and clinics, for depression diagnoses from 1/1/1994 – 12/31/2000 using the same ICD-9 codes described above. Additional episodes of late-life depression were identified by searching hospitalization records from 1990–1999.
Dementia diagnoses
Dementia diagnoses were determined from Kaiser’s electronic medical record system from 1/1/03 – 7/31/09. We used a three-year lag between depression diagnoses and dementia ascertainment to better ensure that our dementia cases reflected incident diagnoses. Specific diagnoses included AD (331.0), VaD (290.40), presenile/senile dementia (290.0, 290.10), dementia with depressive features (290.13, 290.21, 290.43) or behavioral disturbances (294.10, 294.11)
All diagnoses were used for analyses of all-cause dementia. Analyses of AD and VaD were restricted to diagnoses made in Neurology clinics to maximize diagnostic specificity. In these analyses, individuals who developed other types of dementia were excluded. We have previously used similar procedures to identify cases of dementia, AD and VaD among Kaiser members.29–32
Other Measures
The MHC questionnaire included a detailed interview on demographics, health behaviors, health status, and medical as well as family history. Race/ethnicity was self-reported as Asian, Black, Caucasian or Other and was included in analyses as a potential confounder. In addition to the MHC questionnaire, several clinical measurements were also collected at the midlife exam, including height, weight, systolic and diastolic blood pressure. Height and weight were combined to calculate body mass index (BMI, kg/m2). Participants were considered to have mid-life hypertension if they had one of the following: self-report of physician diagnosed hypertension, use of antihypertensive medication, systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Mid-life diabetes status was defined by self report of physician diagnosed diabetes, use of insulin or oral hypoglycemic agents, a fasting glucose (last food eaten in ≥8 hours) of ≥140 mg/dl or a non-fasting (last food eaten in ≤4 hours) glucose of ≥200 mg/dl.
Stroke, hypertension and cardiovascular disease diagnoses also were recorded from outpatient records and hospital discharge diagnoses from 1978 through the end of the study using the following ICD-9 codes: ischemic stroke (433–438), hemorrhagic stroke (430–432), and cardiovascular disease (410, 411, 413, 414, 428, 440, 443, V717).
Analyses
Subjects were classified into one of four depression groups: no depressive symptoms, mid-life only, late-life only, or both. Those with no depressive symptoms were used as the comparison group in all analyses. The unadjusted percentage of individuals who developed dementia, AD and VaD was compared across the depression groups using Chi-square tests. Survival analysis and Cox proportional hazards models using age as the time scale were used to compare the time to dementia, AD and VaD in each of the four depression groups. Person-years were calculated from the onset of follow-up (1/1/03) to the date of dementia diagnosis, with individuals censored at death, end of Kaiser membership or end of the study period (7/31/09). Information on mortality was obtained through the California Automated Mortality Linkage System. Final multivariable models were adjusted for demographic factors (sex, race, education) and number of medical comorbidities.
RESULTS
Our final sample included 13,535 long-term Kaiser members without any type of dementia at the onset of follow-up (1/1/03). The mean (standard deviation) age of study participants at the beginning of follow-up was 81 (5) years and the mean age at dementia diagnosis or censoring was 86 (4) years; 58% were women and 25% were non-white (Table 1). Overall, 72.5% of subjects had no depressive symptoms at mid-life or late-life, 14.1% had mid-life symptoms only, 9.2% had late-life symptoms only, and 4.2% had both. Subjects with depressive symptoms at any point over the life course were more likely to be women and to have a history of diabetes or hypertension. Asian subjects were more likely to have no depressive symptoms while black subjects were more likely to have depressive symptoms in mid-life only and white subjects were more likely to have depressive symptoms in late-life only. In addition, individuals with depressive symptoms at mid-life only or both mid-life and late-life were less likely to have a college education, and individuals with symptoms in late-life only or both mid-life and late-life were more likely to have a history of stroke or heart disease. Hyperlipidemia and body mass index did not differ between the depression groups.
Table 1.
Baseline Characteristics of 13,535 Study Participants by Depressive Symptom Category*
| None (N=9,808) | Mid-Life Only (N=1,913) | Late-Life Only (N=1,239) | Mid-Life and Late-Life (N=575) | p-value | |
|---|---|---|---|---|---|
| Age in 2003 (years), mean (SD) | 81.1 (4.5) | 80.8 (4.3) | 81.5 (4.6) | 80.9 (4.6) | <.001 |
| Age at event or censored (years), mean (SD) | 85.9 (4.4) | 85.5 (4.3) | 85.8 (4.6) | 85.2 (4.7) | <.001 |
|
| |||||
| Female gender | 5,192 (52.9) | 1,358 (71.0) | 844 (68.1) | 439 (76.4) | <.001 |
| Race/ethnicity | |||||
| Asian | 662 (6.8) | 68 (3.6) | 48 (3.9) | 17 (3.0) | <.001 |
| Black | 1,338 (13.6) | 323 (16.9) | 127 (10.3) | 84 (14.6) | <.001 |
| White | 7,379 (75.2) | 1,411 (73.8) | 1,023 (82.6) | 440 (76.5) | <.001 |
| Other | 429 (4.4) | 111 (5.8) | 41 (3.3) | 34 (5.9) | <.001 |
| Education | |||||
| Grade school | 1,304 (13.3) | 364 (19.0) | 169 (13.6) | 107 (18.6) | <.001 |
| High school | 3,319 (33.8) | 755 (39.5) | 429 (34.6) | 231 (40.2) | <.001 |
| College | 5,185 (52.9) | 794 (41.5) | 641 (51.7) | 237 (41.2) | <.001 |
| Medical conditions | |||||
| Stroke | 2,689 (27.4) | 537 (28.1) | 449 (36.2) | 197 (34.3) | <.001 |
| Midlife diabetes | 1,876 (19.1) | 416 (21.8) | 274 (22.1) | 140 (24.4) | <.001 |
| Hypertension | 8,231 (83.9) | 1,653 (86.4) | 1,078 (87.0) | 506 (88.0) | <.001 |
| Hyperlipidemia | 5,892 (60.1) | 1,172 (61.3) | 759 (61.3) | 356 (61.9) | 0.58 |
| Cardiovascular disease | 5,148 (52.5) | 1,001 (52.3) | 754 (60.9) | 354 (61.6) | <.001 |
| Midlife body mass index, mean (SD) | 24.9 (3.6) | 25.0 (4.1) | 24.9 (3.8) | 25.0 (4.0) | 0.79 |
Values are number (%) unless indicated otherwise. P-values based on Chi-square for categories variables and analysis of variance for continuous variables.
During the 6-year follow-up period, 20.7% of subjects with no depressive symptoms developed dementia compared to 23.5% of those with mid-life symptoms only, 31.4% of those with late-life symptoms only and 31.5% of those with both mid-life and late-life symptoms (Table 2). After adjustment for demographics and number of medical comorbidities, the hazard of dementia was significantly increased by approximately 20% for those with depressive symptoms in mid-life only (HR, 1.19; 95% CI: 1.07, 1.32), 70% for those with late-life symptoms only (HR, 1.72; 95% CI: 1.54, 1.92), and 80% for those with both (HR, 1.77; 95% CI: 1.52, 2.06).
Table 2.
Depressive Symptoms and Risk of All-Cause Dementia
| All-Cause Dementia | |||
|---|---|---|---|
|
| |||
| Depressive Symptoms | No (%) | Unadjusted HR* (95% CI) | Adjusted HR* (95% CI) |
| None | 2,026 (20.7) | Ref. | Ref. |
| Mid-life only | 450 (23.5) | 1.22 (1.10, 1.35) | 1.19 (1.07, 1.32) |
| Late-life only | 389 (31.4) | 1.69 (1.51, 1.88) | 1.72 (1.54, 1.92) |
| Mid-life and late-life | 181 (31.5) | 1.80 (1.54, 2.09) | 1.77 (1.52, 2.06) |
HR, Hazard Ratio; CI, Confidence Interval.
Adjusted for education, gender, race, and number of comorbidities.
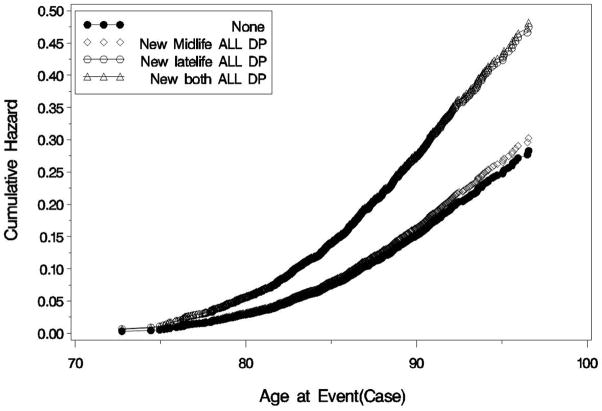
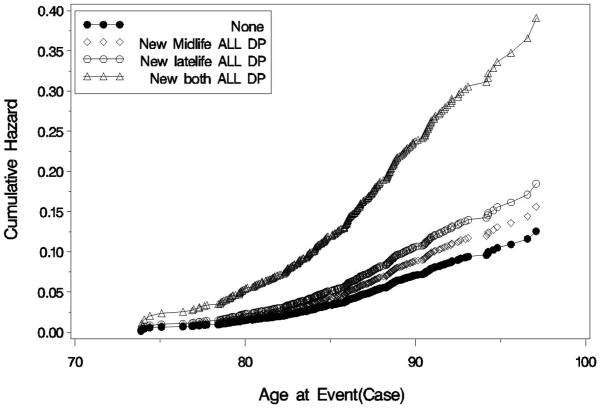
When we repeated analyses looking at diagnoses of AD and VaD from Neurology clinics, 5.5% had received a diagnosis of AD and 2.3% had received a diagnosis of VaD (Table 3). In analyses adjusted for demographics and number of medical comorbidities, subjects with mid-life depressive symptoms only did not have a significantly increased risk of either AD (HR, 1.06; 95% CI: 0.85, 1.33) or VaD (HR, 1.24; 95% CI: 0.90, 1.72). In contrast, subjects with late-life depressive symptoms only had a two-fold increase in the risk of AD (HR, 2.06; 95% CI: 1.67, 2.55) and a nearly 50% increase in the risk of VaD (HR, 1.47; 95% CI: 1.01, 2.14). Subjects with both mid-life and late-life depressive symptoms also had a two-fold increase in the risk of AD (HR, 1.99; 95% CI: 1.47, 2.69) as well as more than a three-fold increase in the risk of VaD (HR, 3.51; 95% CI: 2.44, 5.05). The cumulative hazard of AD and VaD by depressive symptom category is shown in Figures 2a and 2b, respectively.
Table 3.
Depressive Symptoms and Risk of Alzheimer’s Disease (AD) and Vascular Dementia (VaD)
| AD
|
VaD
|
|||||
|---|---|---|---|---|---|---|
| Depressive Symptoms | No (%) | Unadjusted HR (95% CI) | Adjusted HR* (95% CI) | No (%) | Unadjusted HR (95% CI) | Adjusted HR* (95% CI) |
| None | 500 (5.1) | Ref. | Ref. | 201 (2.1) | Ref. | Ref. |
| Mid-life only | 97 (5.1) | 1.1 (0.9, 1.41) | 1.1 (0.9, 1.3) | 46 (2.4) | 1.3 (0.9, 1.7) | 1.2 (0.9, 1.7) |
| Late-life only | 105 (8.5) | 1.9 (1.6, 2.4) | 2.1 (1.7, 2.6) | 32 (2.6) | 1.5 (1.1, 2.2) | 1.5 (1.0, 2.1) |
| Mid-life and late-life | 47 (8.2) | 2.0 (1.5, 2.7) | 2.0 (1.5, 2.7) | 35 (6.1) | 3.7 (2.6, 5.3) | 3.5 (2.4, 5.1) |
HR, Hazard Ratio; CI, Confidence Interval.
Adjusted for education, gender, race, and number of comorbidities.
Figure 2.
The unadjusted cumulative hazard of Alzheimer’s disease (AD) and vascular dementia (VaD) is shown for those with no depressive symptoms (filled circles), mid-life symptoms only (open diamonds), late-life symptoms only (open circles) and both mid-life and late-life symptoms (open triangles).
Figure 2a. Cumulative Hazard of Alzheimer’s Disease by Depressive Symptom Category Compared to those with no depressive symptoms, the unadjusted cumulative hazard of AD was approximately doubled in those with late-life depressive symptoms only or both mid-life and late-life symptoms but was not significantly different in those with mid-life symptoms only.
Figure 2b. Cumulative Hazard of Vascular Dementia by Depressive Symptom Category
Compared to those with no depressive symptoms, the unadjusted cumulative hazard of VaD was increased by 50% in those with late-life depressive symptoms only and was nearly quadrupled in those with both mid-life and late-life depressive symptoms but was not significantly different in those with mid-life symptoms only.
COMMENT
In this study of more than 13,000 long-term Kaiser members, depressive symptoms in either mid-life or late-life were associated with an increased risk of developing dementia. In addition, the risk of AD was approximately doubled in individuals with depressive symptoms in late-life (either alone or in combination with mid-life symptoms) while the risk of VaD was more than tripled in those with both mid-life and late-life depressive symptoms. These findings have important public health implications because they raise hope that adequate treatment of depression in mid-life may reduce dementia risk in late-life.
Our findings are consistent with a large body of literature suggesting that depression in late-life is associated with an increased risk of developing cognitive impairment, dementia and AD.9–16 They also are consistent with a small number of prior studies suggesting that depression earlier in life also is associated with an increased risk of dementia. An early case-control study found that depression was associated with increased dementia risk even if the first episode occurred 25 years prior to onset,33 and a more recent study found an association between the number of depressive episodes and risk of dementia over 25 years.15 However, these studies did not differentiate between single versus recurrent depressive episodes and, therefore, did not explicitly examine dementia risk in the subset of subjects who only had depressive symptoms in mid-life.
There has been an ongoing debate in the field as to whether the association between depression and dementia reflects an etiologic relationship or whether depression is a prodromal symptom of dementia.10,17 Our results suggest that the answer may differ depending on the dementia subtype. Depression that presents for the first time in late life may reflect the earliest symptoms of dementia, particularly AD, in some individuals. Future studies should examine whether it may be helpful clinically to monitor these individuals for symptoms of cognitive deterioration suggestive of dementia. It is possible that earlier recognition of dementia could facilitate better management of healthcare through earlier treatment with memory-enhancing agents, when they are most likely to be effective, as well as greater involvement of caregivers, simplification of medication regimens and earlier discussions regarding goals of care.
On the other hand, recurrence of depression in late life may reflect a long-term process of subclinical cerebrovascular changes that may predispose toward development of VaD. This hypothesis is consistent with the vascular-depression-dementia hypothesis18 and is supported by prior studies in which white matter hyperintensities on cerebral MRIs—which are considered to be markers of underlying cerebrovascular disease—are associated with greater risk of both depression and dementia in late life.34–37 Although our study provides support for the vascular-depression-dementia hypothesis, it remains possible that other hypothesized mechanisms also play a role in the association between depression and dementia. In particular, the hypothamalic-pituitary-adrenal (HPA) axis has been proposed as an alternative mechanism in which chronic or recurrent depression leads to hypercortisolemia which, in turn, results in hippocampal damage and increased vulnerability to dementia.9,10
Strengths of this study include the large sample size, which enabled us to study VaD as well as AD; the integrated health-care delivery setting, which minimizes the impact of access to healthcare and enabled us to adjust for other medical comorbidities over the life-span; and the availability of data from both mid-life and late-life, which enabled examination of depression over the life course. Limitations of the study include the mid-life depressive symptom measure, which was based primarily on a single self-reported question and likely resulted in lack of specificity. In addition, use of electronic medical record data for diagnoses of late-life depression and dementia likely resulted in low sensitivity and under-recognition of these conditions. We also were not able to confirm diagnoses of AD and VaD using operational criteria or through postmortem or neuroimaging studies. Given that vascular disease is an independent risk factor for AD, that pure VaD is relatively rare, and that the majority of individuals with dementia at the population level have mixed AD/VaD pathology, it is likely that some of what was diagnosed as VaD in this study may have reflected a more mixed etiology. To the extent that these misclassifications were non-differential (i.e., misclassification of depressive symptom status was similar in those with and without dementia diagnoses and misclassification of dementia status was similar in those with and without depressive symptoms), our findings would be biased toward the null; thus, the true magnitude of these associations may be stronger than observed in this study. We also were unable to analyze data related to use of anti-depressants or other psychotropic medications, which should be examined in future studies. In addition, our measures of vascular risk factors were relatively limited, so we were unable to directly examine the role of vascular disease as the etiologic mediator of the association between depression and VaD. Finally, since we excluded dementia cases diagnosed prior to 1/1/03, it is important to note that our findings are restricted to late-life dementia.
CONCLUSIONS
Our findings suggest that chronic depression over the lifecourse may be etiologically associated with increased risk of dementia, particularly VaD, while depression that occurs for the first time in late life is likely to reflect a prodromal stage of dementia, particularly AD. Future studies are needed to determine whether adequate treatment of depression in mid-life or late-life may help to maintain cognitive function and delay dementia onset. Given the anticipated increase in dementia prevalence over the next 40 years, even a small reduction in dementia risk would have a tremendous public health impact.38
Acknowledgments
Funding/support: NARSAD (Dr Barnes); National Institutes of Health (Dr. Yaffe: K24 AG031155; R01 MH086498); Kaiser Permanente Community Benefits (Dr. Whitmer).
Funding support and role of sponsor
Funding was provided by NARSAD (Dr Barnes), the National Institutes of Health (Dr. Yaffe: K24 AG031155; R01 MH086498) and Kaiser Permanente Community Benefits (Dr. Whitmer). Sponsors did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
Presented at the International Conference on Alzheimer’s Disease, Honolulu, HI, July 12, 2010.
Conflicts of interest and financial disclosures
We have no conflicts of interest.
Data access and responsibility
Dr. Whitmer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions
Study concept and design: Barnes, Whitmer.
Acquisition of data: Whitmer.
Analysis and interpretation of data: Barnes, Yaffe, Byers, McCormick, Schaefer, Whitmer.
Drafting of the manuscript: Barnes.
Critical revision of the manuscript for important intellectual content: Yaffe, Byers, McCormick, Schaefer, Whitmer.
Statistical analysis: Whitmer.
Obtained funding: Barnes, Whitmer.
Administrative, technical or material support. Whitmer.
Supervision. Whitmer.
Additional contributors: Jufen Zhou, MS, performed all of the data analyses.
Contributor Information
Deborah E. Barnes, Department of Psychiatry, University of California, San Francisco, and Mental Health, San Francisco Veterans Affairs Medical Center.
Kristine Yaffe, Departments of Psychiatry, Neurology and Epidemiology & Biostatistics, University of California, San Francisco, and Mental Health, San Francisco Veterans Affairs Medical Center.
Amy L. Byers, Department of Psychiatry, University of California, San Francisco, and Mental Health, San Francisco Veterans Affairs Medical Center.
Mark McCormick, Kaiser Division of Research, Oakland, CA.
Catherine Schaefer, Kaiser Division of Research, Oakland, CA.
Rachel A. Whitmer, Kaiser Division of Research, Oakland, CA.
References
- 1.Alzheimer’s Association. [Accessed November 5, 2010];Alzheimer’s Disease Facts & Figures. http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf.
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007 Jul;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Steffens DC, Fisher GG, Langa KM, Potter GG, Plassman BL. Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. Int Psychogeriatr. 2009 Oct;21(5):879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heun R, Kockler M, Ptok U. Depression in Alzheimer’s disease: is there a temporal relationship between the onset of depression and the onset of dementia? Eur Psychiatry. 2002 Sep;17(5):254–258. doi: 10.1016/s0924-9338(02)00678-8. [DOI] [PubMed] [Google Scholar]
- 5.Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry. 2006 Feb;63(2):153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 6.Dufouil C, Fuhrer R, Dartigues JF, Alperovitch A. Longitudinal analysis of the association between depressive symptomatology and cognitive deterioration. Am J Epidemiol. 1996 Oct 1;144(7):634–641. doi: 10.1093/oxfordjournals.aje.a008974. [DOI] [PubMed] [Google Scholar]
- 7.Vinkers DJ, Gussekloo J, Stek ML, Westendorp RG, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. Bmj. 2004 Oct 16;329(7471):881. doi: 10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia: a community-based prospective study. Arch Gen Psychiatry. 1999 Mar;56(3):261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- 9.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001 Dec;35(6):776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 10.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006 May;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999 May;56(5):425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 12.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006 Mar;63(3):273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008 Apr;65(4):439–445. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Hoganson GM, Rajan KB, Barnes LL, Mendes de Leon CF, Evans DA. Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology. Jul 6;75(1):21–26. doi: 10.1212/WNL.0b013e3181e620c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. Jul 6;75(1):27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. Jul 6;75(1):35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, Silberberg D, Trevisan M. NIH State-of-the-Science Conference Statement: Preventing Alzheimer’s Disease and Cognitive Decline. NIH Consens State Sci Statements. 2010 Apr 28;27(4) [PubMed] [Google Scholar]
- 18.Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003 Aug;51(8):1178–1180. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997 Oct;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006 Dec 15;60(12):1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006 Oct;18(5):433–441. doi: 10.1080/09540260600935447. [DOI] [PubMed] [Google Scholar]
- 22.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002 Feb;1(1):61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 23.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005 Jan 25;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005 Aug 23;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballard C, Neill D, O’Brien J, McKeith IG, Ince P, Perry R. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J Affect Disord. 2000 Aug;59(2):97–106. doi: 10.1016/s0165-0327(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 26.Bowirrat A, Oscar-Berman M, Logroscino G. Association of depression with Alzheimer’s disease and vascular dementia in an elderly Arab population of Wadi-Ara, Israel. Int J Geriatr Psychiatry. 2006 Mar;21(3):246–251. doi: 10.1002/gps.1455. [DOI] [PubMed] [Google Scholar]
- 27.Newman SC. The prevalence of depression in Alzheimer’s disease and vascular dementia in a population sample. J Affect Disord. 1999 Jan-Mar;52(1–3):169–176. doi: 10.1016/s0165-0327(98)00070-6. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Lee SB, Lee TJ, Lee DY, Jhoo JH, Youn JC, Choo IH, Choi EA, Jeong JW, Choe JY, Woo JI, Kim KW. Depression in vascular dementia is quantitatively and qualitatively different from depression in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(2):67–73. doi: 10.1159/000097039. [DOI] [PubMed] [Google Scholar]
- 29.Rusanen M, Kivipelto M, Quesenberry CP, Jr, Zhou J, Whitmer RA. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med. Oct 25; doi: 10.1001/archinternmed.2010.393. [DOI] [PubMed] [Google Scholar]
- 30.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007 Apr;4(2):103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 32.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009 Apr 15;301(15):1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003 May;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 34.Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression--a longitudinal evaluation. Biol Psychiatry. 1997 Sep 1;42(5):367–374. doi: 10.1016/S0006-3223(96)00363-0. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann LL, Masurier M Le, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79(6):619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 36.Steffens DC, MacFall JR, Payne ME, Welsh-Bohmer KA, Krishnan KR. Grey-matter lesions and dementia. Lancet. 2000 Nov 11;356(9242):1686–1687. doi: 10.1016/S0140-6736(05)70393-7. [DOI] [PubMed] [Google Scholar]
- 37.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010 Jul 26;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011 Sep;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]