Abstract
The use of Chinese-origin rhesus macaques (Macaca mulatta) for infectious disease immunity research is increasing despite the relative lack of major histocompatibility complex (MHC) class I immunogenetics information available for this population. We determined transcript-based MHC class I haplotypes for 385 Chinese rhesus macaques from five different experimental cohorts, providing a concise representation of the full complement of MHC class I major alleles expressed by each animal. In total, 123 Mamu-A and Mamu-B haplotypes were defined in the full Chinese rhesus macaque cohort. We then performed an analysis of haplotype frequencies across the experimental cohorts of Chinese rhesus macaques, as well as a comparison against a group of 96 Indian rhesus macaques. Notably, 35 of the 51 Mamu-A and Mamu-B haplotypes observed in Indian rhesus macaques were also detected in the Chinese population, with 85% of the 385 Chinese-origin rhesus macaques expressing at least one of these class I haplotypes. This unexpected conservation of Indian rhesus macaque MHC class I haplotypes in the Chinese rhesus macaque population suggests that immunologic insights originally gleaned from studies using Indian rhesus macaques may be more applicable to Chinese rhesus macaques than previously appreciated and may provide an opportunity for studies of CD8+ T-cell responses between populations. It may also be possible to extend these studies across multiple species of macaques, as we found evidence of shared ancestral haplotypes between Chinese rhesus and Mauritian cynomolgus macaques.
Keywords: Macaca mulatta, MHC class I, RNA transcript-based haplotypes, immunogenetics
Macaque monkeys are used extensively to model and understand human diseases. Historically, rhesus macaques (Macaca mulatta; Mamu) of Indian origin have been preferred for such research. However, exportation of rhesus macaques from India was halted in 1978 (Southwick and Siddiqi 1994). As it became more difficult to obtain Indian rhesus macaques, researchers turned to other sources, including Chinese-origin rhesus macaques. Chinese rhesus macaques have been used to study infectious diseases including simian immunodeficiency virus/human immunodeficiency virus, avian influenza, malaria, plague, and severe acute respiratory syndrome, as well as studies of xenotransplantation, stem cell therapies, reproduction, and neurobiology (Shen et al. 2007; Chen et al. 2009, 2011, 2012; Choi et al. 2011; Liu et al. 2011; Tian et al. 2011; Wei et al. 2011; Hutnick et al. 2012; Li et al. 2012b; Ling et al. 2013; Mumbauer et al. 2013). Yet comparatively little is known about the immunogenetics of these animals. The genome of a single Chinese rhesus macaque was sequenced in 2011, but whole-genome sequencing technology struggles to accurately characterize highly polymorphic, duplicated regions such as the major histocompatibility complex (MHC) loci and killer immunoglobulin receptor loci (Fang et al. 2011).
The MHC is an exceptionally polymorphic region of the genome responsible for presentation of peptides to T cells (Bontrop 2006). Because of its pivotal role in immune response, understanding MHC genetics is essential for infectious disease research. Our group and others have shown that the repertoire of MHC class I alleles expressed by Chinese rhesus macaques is largely distinct from that observed in Indian rhesus macaques (Otting et al. 2007, 2008; Karl et al. 2008; Otting et al. ; Wu et al. 2008; Ma et al. 2009; Wiseman et al. 2009; Naruse et al. 2010; Doxiadis et al. 2011). Consequently, we inferred MHC class I−restricted CD8+ T-cell responses in these two populations would be unlikely to target many of the same epitopes.
Here we reconsider the relationship between MHC class I genetics of Chinese- and Indian-origin rhesus macaques by focusing on MHC class I transcript−defined haplotypes inherited together, rather than simply analyzing individual allelic variants. Most studies that currently control for the complex rhesus macaque MHC class I region only account for the presence of one or a few widely studied alleles (Mamu-A1*001, etc.), with no regard for the majority of alleles expressed by the animal. Analysis on a haplotype level as presented here provides a more global view of the MHC class I alleles transcribed by each animal, creating the potential for a greater degree of allele matching between animals within a study.
To define haplotypes with high resolution, we deep sequenced complementary DNA (cDNA) amplicons spanning most of exons 2−4 of Mamu-A and Mamu-B genes, the most polymorphic area of MHC class I corresponding to the peptide binding region. A total of 385 Chinese-origin rhesus macaques from five different experimental cohorts were examined, as well as 96 Indian-origin rhesus macaques from two distinct sources. Unexpectedly, we discovered that 35 of 51 of the Mamu-A and Mamu-B haplotypes observed in Indian rhesus macaques also were expressed by a significant majority of Chinese rhesus macaques. Conservation of these haplotypes suggests that functional CD8+ T-cell immunity to pathogens may be more similar between Chinese- and Indian-origin rhesus macaques than previously appreciated.
Materials and Methods
Animals
Whole blood and peripheral blood mononuclear cell samples from 385 Chinese rhesus macaques were obtained over the span of 5 yr from five different sources, including Battelle Biomedical Research Center, Etubics Corporation, the National Institute of Allergy and Infectious Diseases/National Institutes of Health, and Harlan Laboratories, Inc. Cohorts are detailed in Table 1. The core set of 51 Chinese rhesus macaque samples included 45 samples from cohort ChRh #1 and six samples from cohort ChRh #4. The expanded set of 334 Chinese rhesus macaque samples included an additional 142 animals from these two cohorts, as well as 192 animals from the three other sources. It is unknown from which provinces in China the animals or their ancestors were initially obtained, or any degree of relation between the animals sampled.
Table 1. Summary of Chinese- and Indian-origin rhesus macaque cohorts.
| Cohort ID | Total No. Animals | Total No. Chromosomes | Core Set No. Animals | Expanded Set No. Animals |
|---|---|---|---|---|
| ChRh #1 | 147 | 294 | 45 | 102a |
| ChRh #2 | 133 | 266 | − | 133 |
| ChRh #3 | 48 | 96 | − | 48 |
| ChRh #4 | 46 | 92 | 6 | 40a |
| ChRh #5 | 11 | 22 | − | 11 |
| InRh #1 | 49 | 98 | 49 | − |
| InRh #2 | 47 | 94 | 47 | − |
Evaluated using the 248-bp primers; all other animals were amplified with the 638-bp primers.
Whole blood samples from 96 Indian rhesus macaques were obtained from the Wisconsin National Primate Research Center (WNPRC) and New England Primate Research Center (NEPRC) breeding colonies. The WNPRC cohort was selected to be unrelated based on pedigree records whereas the NEPRC cohort was taken from active adult breeding stock. Macaques at the WNPRC were cared for according to protocols approved by the University of Wisconsin-Madison Graduate School Animal Care and Use Committee, whereas macaques housed at the NEPRC were maintained in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the Harvard Medical School Animal Care and Use Committee.
RNA isolation, cDNA synthesis, and polymerase chain reaction (PCR) amplification (normalization and pooling)
RNA was isolated from whole blood or peripheral blood mononuclear cell samples using the Roche MagNA Pure instrument and RNA High Performance kit (Roche, Indianapolis, IN), following manufacturer’s protocols (Supporting Information, File S1). Synthesis of cDNA and PCR amplification were performed as previously described, generating either a 248-bp or 638-bp genotyping amplicon (the shorter amplicon dates back to studies performed before Roche/454 sequencing technology advanced to ~500-bp read lengths) (Wiseman et al. 2009; Fernandez et al. 2011). The amplicons for each sample were tagged during primary PCR with a multiplex identifier sequence, a unique 10-bp tag for each sample (File S1). Amplification was confirmed by running PCR products on a Flash Gel (Lonza Group Ltd, Basel, Switzerland), and bead-based purification was performed using AMPure XP SPRI beads (Agencourt Bioscience Corporation, Beverly, MA) to remove primer dimers. Quantification of purified products was performed using the Quant-iT dsDNA HS Assay kit and a Qubit fluorometer (Invitrogen, Carlsbad, CA). Products were then normalized to 0.3 ng/μL and pooled in equal volumes.
Sequencing
Sequencing for all amplicons was performed using Roche/454 second-generation methods following manufacturer’s protocols for emulsion PCR and pyrosequencing (Roche, Indianapolis, IN). The core set of 51 Chinese rhesus macaques and 96 Indian rhesus macaques were sequenced in a total of five runs on a GS Junior pyrosequencing instrument. The expanded set of 334 Chinese rhesus macaques were sequenced on a combination of GS Junior and GS FLX platforms; both platforms utilize the same basic chemistry, differing only in scale of the run (a single GS Junior run is ~1/8 the scale of a full GS FLX run).
Data analysis
For the core set of 51 Chinese-origin and 96 Indian-origin rhesus macaques, sequences were analyzed by trimming all sequences from the 3′ end to fixed 430-bp lengths, binning sequences into separate animal groups by parsing for the unique multiplex identifier tags, then creating uni-directional contigs of sequence reads assembled at 100% stringency using the program Geneious Pro (BioLegend, San Diego, CA). FASTA format sequences of all contigs for each sample were then exported, and bidirectional reads were assembled at 100% stringency using CodonCode Aligner (CodonCode, Dedham, MA). Bidirectional contigs were interrogated against a curated database of known rhesus macaque MHC class I alleles using the Basic Local Alignment Search Tool (i.e., BLAST), and novel alleles were submitted to GenBank (KC205288-KC205396). Putative haplotypes were inferred by looking for combinations of shared alleles between animals (File S1). Analyses of the Mamu-A and Mamu-B regions were performed independently, since these gene clusters are separated by ~1.2 million base pairs and recombination is relatively common at the population level (Daza-Vamenta et al. 2004).
Once putative haplotypes were defined, a simpler workflow was used to assign haplotype designations to the additional 334 Chinese rhesus macaque samples from the expanded set. All individual sequence reads obtained for each sample were directly compared against our rhesus macaque MHC class I allele database using BLAST-like analysis tool (i.e., BLAT) in a local instance of Galaxy (Penn State, State College, PA; Emory, Atlanta, GA), following the data analysis workflow detailed in Figure S1. Haplotypes were assigned by identifying groups of shared major alleles from the previously defined putative haplotypes.
Results
Haplotype definition based on Mamu-A and Mamu-B transcripts
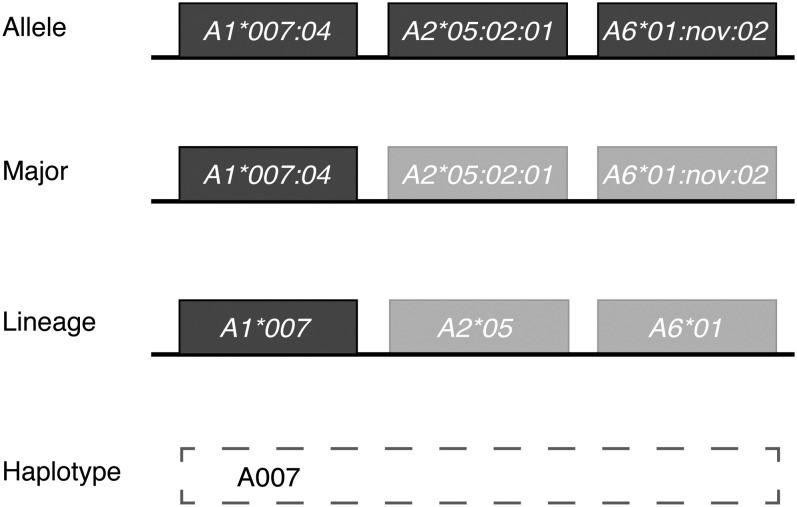
The unparalleled complexity of the nonhuman primate MHC class I region makes it difficult to describe simply the full diversity of class I alleles expressed by an animal. Macaques may encode as many as 30 distinct MHC class I transcripts expressed at widely differing steady state levels in peripheral blood. However, combinations of distinct alleles are inherited together on a chromosome as haplotypes, making it possible to assign a simplified name (i.e., haplotype A007) to each specific combination of alleles. Here, unique haplotypes were defined on the basis of transcriptionally abundant “major” Mamu-A and Mamu-B alleles; an example of this simplification for the A007 haplotype is illustrated in Figure 1. Our operational criterion for defining a “major” allele is that it must exceed a 4% transcript abundance relative to all class I transcripts identified per animal. We previously showed that CD8+ T-cell responses are principally restricted by highly transcribed major MHC class I alleles expressed in macaques, so we believe functionally defining haplotypes based on configurations of major alleles is appropriate for assessing conservation of MHC alleles involved in antigen presentation to CD8+ T cells (Budde et al. 2011).
Figure 1.
Simplification of MHC class I allele calls from full allele down to haplotype designation. Schematic representation of reducing the A007 haplotype observed in Chinese-origin rhesus macaques, from full allele name to major alleles to lineage designation to final haplotype call.
To further streamline haplotype definitions, only lineage level allele names were considered (commonly referred to as two-digit resolution in human HLA genotyping, e.g., all Mamu-A1*007 allelic variants were grouped with the designation Mamu-A1*007; Figure 1). Alleles are named by the nonhuman primate Immuno Polymorphism Database-MHC group based on similarity to other known macaque MHC class I alleles, such that alleles sharing a common lineage level designation are identical or nearly so within the peptide binding regions.
A total of 123 unique haplotypic configurations were defined in the full cohort of 385 Chinese rhesus macaques, consisting of 53 distinct Mamu-A haplotypes and 70 Mamu-B haplotypes (Table S1). Names were assigned to each haplotype based on the presence of what we call a “diagnostic major” allele. Mamu-A region haplotypes typically contained a single major allele, usually from the Mamu-A1 locus, so that allele was considered the “diagnostic major.” A few of the Mamu-A haplotypes and most of the Mamu-B haplotypes expressed two or more major alleles; for these haplotypes, the most transcriptionally abundant allele expressed on the haplotype was typically considered the “diagnostic major” allele. Occasionally the “diagnostic major” allele was selected based on known biological significance. For instance, even though the Mamu-B*017 allele was not typically the most abundant transcript, it was considered the “diagnostic major” due to its known association with spontaneous control of SIV (Yant et al. 2006). Different versions of a base haplotype, denoted with a lowercase letter following the haplotype name (for instance, B001a and B001b), were designated to indicate differences in the complement of major transcripts accompanying a shared diagnostic allele. By this method of haplotype definition, four simple haplotype names represent the full complement of MHC class I major alleles expressed by each macaque (Table S2).
Frequencies of haplotypes across different Chinese rhesus macaque cohorts
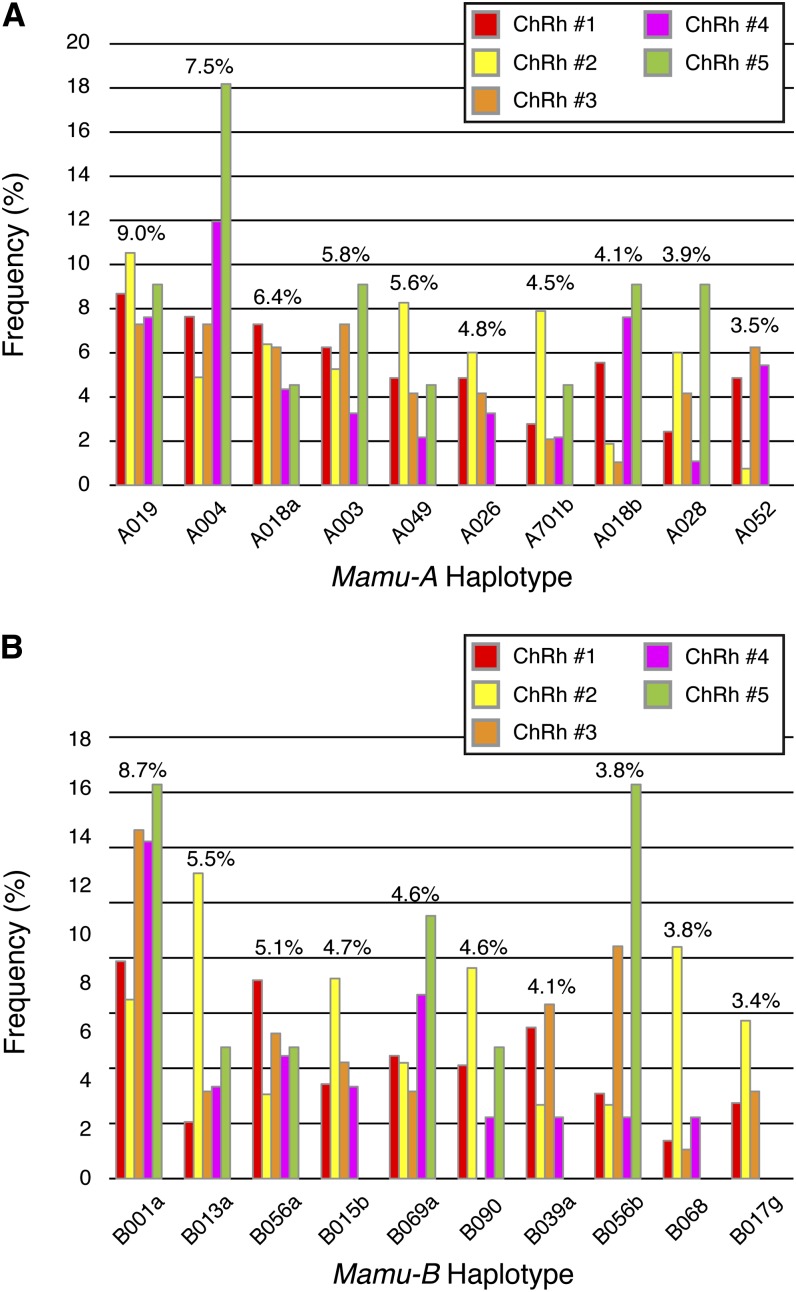
Because the geographical source of macaques within China has been indicated as a factor in frequency of specific MHC class I alleles, we wanted to explore the differences in chromosomal haplotype frequencies between our five experimental cohorts (Li et al. 2012a; Liu et al. 2013). After identifying the 20 most common Chinese rhesus macaque Mamu-A and Mamu-B haplotypes across the data set as a whole, we examined the differences in the frequencies for those haplotypes between the five cohorts (Figure 2). Frequencies were calculated based on the total number of chromosomes examined (770 Chinese rhesus macaque chromosomes), to account for each animal expressing pairs of Mamu-A and Mamu-B haplotypes. Some haplotype frequencies, such as A019, A018a, and B056a, were relatively consistent across the cohorts; however, some frequencies varied greatly from cohort to cohort. One example is the A004 haplotype, which was observed in ~18% of the total chromosomes examined for cohort ChRh #5, but only ~5% for cohort ChRh #2. Overall, frequency differences between cohorts were more pronounced for the Mamu-B haplotypes, and some cohorts did not contain one or more of the 20 most common Mamu-A and Mamu-B haplotypes. This finding is not unexpected for cohort ChRh #5, because it only contained 11 macaques. The remaining cohorts each contained at least 46 macaques, minimizing the effect of sample size on observed frequencies in these cohorts. These results emphasize the point that not all research cohorts of Chinese rhesus macaques are equal from the standpoint of MHC class I diversity, as the complement of major MHC class I alleles expressed by Chinese rhesus macaques can vary greatly between different sample groups. However, despite frequency differences, common haplotypes can still be identified across all five cohorts.
Figure 2.
Frequency distribution by cohort for the 20 overall most frequent haplotypes in Chinese rhesus macaques. (A) Frequencies of the 10 overall most abundant Chinese rhesus macaque Mamu-A haplotypes, separated by cohort, within the full cohort of 385 Chinese rhesus macaques. Average frequency across Chinese rhesus macaque cohorts is listed above the columns. Frequencies were calculated based on total number of chromosomes examined (770 Chinese chromosomes) to account for each animal having two haplotypes for each region. (B) Frequencies of the 10 overall most abundant Mamu-B haplotypes, separated by cohort. Average frequency across all five Chinese rhesus macaque cohorts is listed above the columns.
Haplotype comparison between Chinese and Indian rhesus macaques
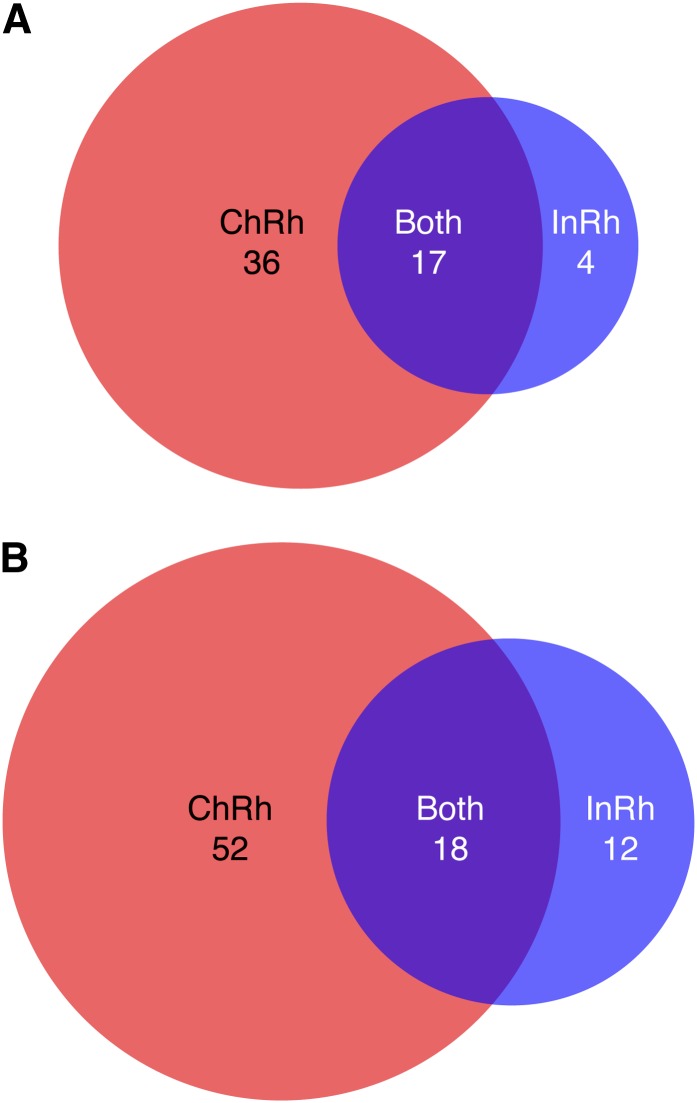
We next performed MHC class I haplotype analysis on a group of 96 Indian rhesus macaques, allowing for haplotype comparison between the two geographically isolated populations. In total, 51 haplotypes were identified in the Indian rhesus macaque cohort, including 21 Mamu-A haplotypes and 30 Mamu-B haplotypes. Interestingly, only 16 haplotypes (four Mamu-A and 12 Mamu-B haplotypes) were unique to Indian rhesus macaques in this comparison (Figure 3). These findings indicate that although the allelic variation between Chinese- and Indian-origin rhesus macaques is largely distinct (Table S3), the two populations frequently inherit shared combinations of closely related allelic variants. Overall, 35 of the 51 MHC class I haplotypes present within Indian rhesus macaques have closely related homologs in the Chinese population.
Figure 3.
Haplotype distribution between Chinese and Indian rhesus macaques by region. (A) Distribution of Mamu-A haplotypes by population within the full cohort of 385 Chinese rhesus macaques and 96 Indian rhesus macaques. (B) Distribution of Mamu-B haplotypes by population within the full cohort.
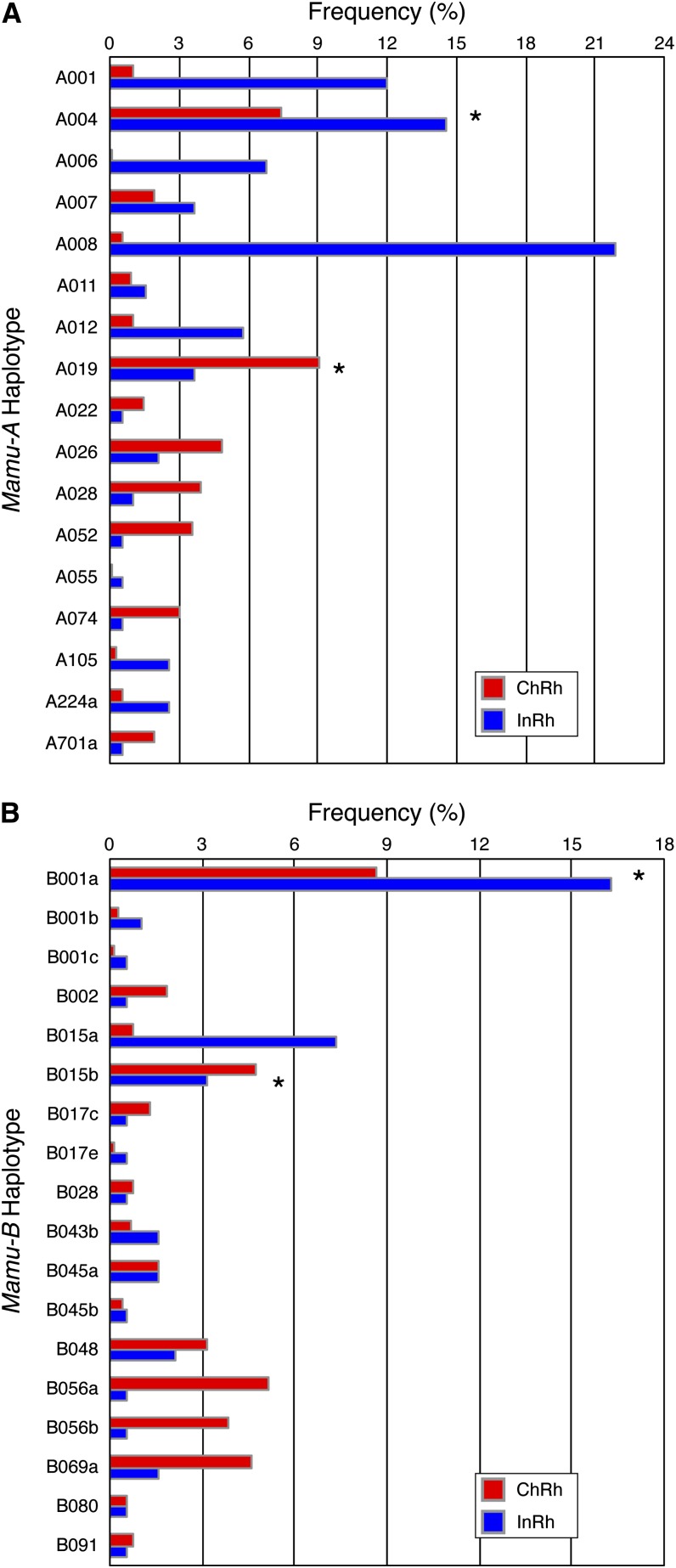
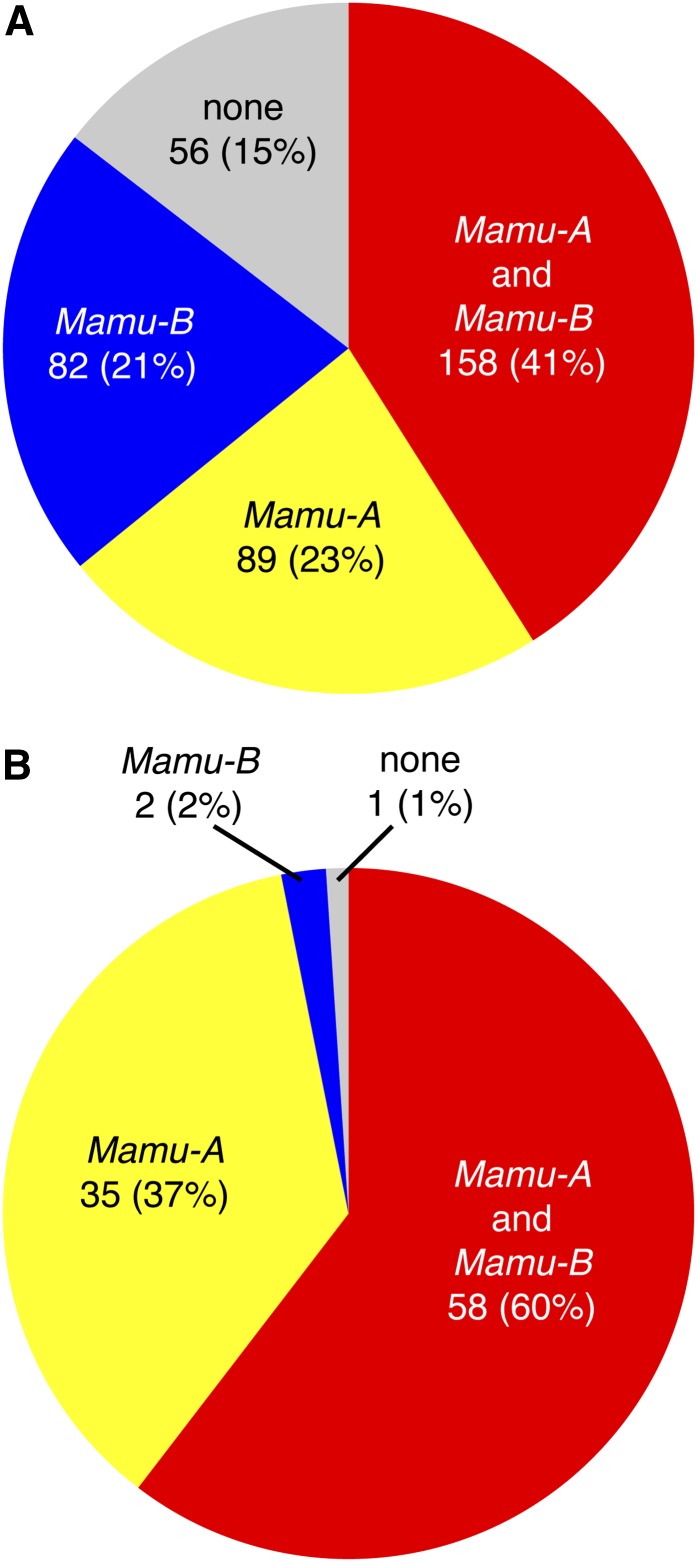
The frequencies of these 35 shared haplotypes in each population are shown in Figure 4; frequencies were again based on total number of chromosomes examined. Although several of these shared haplotypes were not among the most abundant in this study, there were four haplotypes (A004, A019, B001a, and B015b) observed at a chromosomal frequency of at least 3% in each population. Remarkably, 85% of the Chinese rhesus macaques in this study express at least one of the 35 shared Mamu-A or Mamu-B haplotypes (64% carry at least one of the 17 shared Mamu-A haplotypes, and 62% carry one or more of the 18 shared Mamu-B haplotypes). Likewise, 99% of the Indian rhesus macaques in this cohort express at least one haplotype shared with Chinese-origin animals (Figure 5). Therefore, there is a high likelihood that these shared haplotypes were present in prior studies using Indian-origin rhesus macaques that researchers might reproduce or expand upon today using Chinese-origin rhesus macaques. MHC class I genotyping would need to be performed to confirm presence of shared haplotypes between populations for any specific studies.
Figure 4.
Frequency distribution by population for 35 haplotypes shared between Chinese- and Indian-origin rhesus macaques. (A) Average frequencies by population for the 17 shared Mamu-A haplotypes observed in the full cohort of 385 Chinese-origin and 96 Indian-origin rhesus macaques. Frequencies were calculated based on total number of chromosomes examined (770 Chinese and 192 Indian chromosomes) to account for each animal having two haplotypes for each region. Asterisks indicate haplotypes with an average frequency greater than 3% for both populations. (B) Average frequencies by population for the 18 shared Mamu-B haplotypes. Asterisks indicate haplotypes with an average frequency greater than 3% for both populations.
Figure 5.
Abundance of shared MHC class I haplotypes in Chinese- and Indian-origin rhesus macaques. (A) Percentage of animals carrying one or more shared MHC class I haplotypes for the full 385 Chinese rhesus macaque cohort, separated by region (expressing both a shared Mamu-A and a shared Mamu-B haplotype, Mamu-A alone, or Mamu-B alone). Percentages were calculated based on total number of animals examined. (B) Percentage of animals carrying one or more shared MHC class I haplotypes for the 96 Indian rhesus macaque cohort, separated by region.
Rhesus macaque haplotypes shared with Mauritian cynomolgus macaques
We also compared our 139 Chinese- and Indian-origin rhesus macaque haplotypes against the well-characterized panel of Mauritian cynomolgus macaque (Macaca fascicularis; Mafa) haplotypes to explore possible ancestral haplotype sharing between the two species (Wiseman et al. 2007; Budde et al. 2010). Mauritian cynomolgus macaques exhibit extremely limited MHC class I diversity, as the population arose from a small group of founder cynomolgus macaques deposited on the island in the 1500s (Lawler et al. 1995). The MHC class I diversity of Mauritian cynomolgus macaques is effectively described by five Mafa-A and seven Mafa-B haplotypes (one Mafa-A haplotype is commonly associated with three distinct Mafa-B haplotypes) (Wiseman et al. 2007; Budde et al. 2010). Three of our Mamu-A haplotypes (A033, A060, and A063) and two of our Mamu-B haplotypes (B045a and B150) were homologous to Mauritian cynomolgus macaque haplotypes (Table S1). Four of these haplotypes were observed exclusively in Chinese rhesus macaques; the fifth haplotype (B045a) was detected in both Chinese-origin and Indian-origin rhesus macaques.
Discussion
It has previously been shown that the specific MHC class I alleles expressed by Chinese- and Indian-origin rhesus macaques are largely distinct, and those distinctions were thought to equate to an unfathomable MHC class I diversity between the two geographically divided populations (Otting et al. 2007, 2008; Karl et al. 2008; Wu et al. 2008; Ma et al. 2009; Wiseman et al. 2009; Naruse et al. 2010; Doxiadis et al. 2011). However, our model of defining MHC class I haplotypes of coinherited highly expressed major alleles provides a novel approach for assessing putatively functional similarities between macaque populations. Most notably, more than two thirds of the haplotypes observed in Indian rhesus macaques were shared with Chinese rhesus macaques. This indicates that ancestral haplotypes were retained in both populations after the split from their most recent common ancestor ~162,000 years ago, despite subsequent shrinking and expansion of the Indian and Chinese rhesus macaque populations, respectively (Hernandez et al. 2007). Although Chinese rhesus macaques overall display a greater repertoire of MHC class I haplotypes private to the population than Indian rhesus macaques, 85% of the Chinese rhesus macaques in this study express at least one of the shared ancestral Mamu-A or Mamu-B haplotypes described here (Figure 5). These homologous haplotypes may provide a valuable resource for cross-population studies of functional CD8+ T-cell immune response to pathogens.
Does conservation of MHC class I haplotypes correspond to conservation of CD8+ T-cell immune responses? Though this remains to be tested at the haplotype level, a previous study demonstrated that it was possible to create Mamu-B*017:03 (a Chinese rhesus macaque-specific allelic variant of the Mamu-B*017 lineage) monomers and tetramers with the SIV Nef165-173 peptide IW9 (Ouyang et al. 2009). This amino acid sequence was characterized as a CTL epitope restricted by Mamu-B*017:01 in Indian rhesus macaques, an allele that has been associated with control of SIVmac239 viral replication (Mothe et al. 2002; Yant et al. 2006). Mamu-B*017:03 differs from Mamu-B*017:01 by four amino acids, three of which occur in the peptide binding domain encoded by exons 2-3. These amino acid substitutions did not appear to alter the ability of Mamu-B*017:03 to bind SIV Nef165-173 peptide IW9 (Ouyang et al. 2009). If the highly similar allelic variants expressed on homologous haplotypes are also able to bind the same peptides, it is very likely those haplotypes would mount similar CD8+ T-cell responses in both Chinese- and Indian-origin rhesus macaques. To extend these observations, class I alleles of specific interest sharing a lineage designation would need to be analyzed for predicted binding preferences as well as the functional ability to present specific peptides.
There is also early evidence that sharing of homologous haplotypes may be a pan-macaque phenomenon. Comparison of the rhesus macaque haplotypes identified in this study to an established panel of Mauritian cynomolgus macaque haplotypes revealed a total of five putative orthologous haplotypes, including three of the five Mafa-A haplotypes. Interestingly, the cynomolgus macaque haplotypes with putative rhesus macaque orthologs account for more than 62% and 20% of the Mafa-A and Mafa-B diversity, respectively, in Mauritian cynomolgus macaques (Budde et al. 2010). It may therefore be possible to also explore whether rhesus and cynomolgus macaques with conserved ancestral MHC class I haplotypes mount similar CD8+ T-cell immune responses. Additional haplotype similarities are also likely to exist between rhesus macaques and cynomolgus macaques of other geographic populations, or between rhesus macaques and other macaque species, but a current lack of defined haplotypes for these other populations make comparisons difficult.
Overall, this study provides the first large-scale description of MHC class I transcript-based haplotypes in rhesus macaques. It begins to explore some of the variability of haplotypes observed between independent experimental research cohorts of Chinese rhesus macaques, and provides a valuable chromosomal haplotype frequency survey for groups looking to study high-frequency Chinese rhesus macaque alleles. Importantly, it highlights the unexpected sharing of Indian rhesus macaque haplotypes with rhesus macaques from China, which may facilitate comparisons of MHC class I−mediated response to pathogens across populations.
Supplementary Material
Acknowledgments
We thank Battelle Biomedical Research Center, Etubics Corporation, the National Institute of Allergy and Infectious Diseases/National Institutes of Health, and Harlan Laboratories, Inc., for providing us with Chinese rhesus macaque samples, and the New England Primate Research Center for providing an independent source of Indian rhesus macaque samples. We also thank the staff of the W.M. Keck Center for Comparative and Functional Genomics at the University of Illinois at Urbana-Champaign for performing Roche/454 GS FLX sequencing. This work was supported by the National Institute of Allergy and Infectious Diseases (HHSN266200400088C, HHSN272201100013C), the National Center for Research Resources (P51 RR000167), and the Office of Research Infrastructure Programs (P51 OD011106) of the National Institutes of Health. This research was conducted at a facility constructed with support from the Research Facilities Improvement Program (RR15459-01, RR020141-01).
Footnotes
Communicating editor: D.-J. De Koning
Literature Cited
- Bontrop R. E., 2006. Comparative genetics of MHC polymorphisms in different primate species: duplications and deletions. Hum. Immunol. 67: 388–397 [DOI] [PubMed] [Google Scholar]
- Budde M. L., Wiseman R. W., Karl J. A., Hanczaruk B., Simen B. B., et al. , 2010. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics 62: 773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde M. L., Lhost J. J., Burwitz B. J., Becker E. A., Burns C. M., et al. , 2011. Transcriptionally abundant major histocompatibility complex class I alleles are fundamental to non-human primate SIV-specific CD8+ T cell responses. J. Virol. 85: 3250–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Deng W., Jia C., Dai X., Zhu H., et al. , 2009. Pathological lesions and viral localization of influenza A (H5N1) virus in experimentally infected Chinese rhesus macaques: implications for pathogenesis and viral transmission. Arch. Virol. 154: 227–233 [DOI] [PubMed] [Google Scholar]
- Chen S., Lai C., Wu X., Lu Y., Han D., et al. , 2011. Variability of bio-clinical parameters in Chinese-origin Rhesus macaques infected with simian immunodeficiency virus: a nonhuman primate AIDS model. PLoS ONE 6: e23177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Niu Y., Yang S., He X., Ji S., et al. , 2012. The available time window for embryo transfer in the rhesus monkey (Macaca mulatta). Am. J. Primatol. 74: 165–173 [DOI] [PubMed] [Google Scholar]
- Choi H. J., Kim M. K., Lee H. J., Ko J. H., Jeong S. H., et al. , 2011. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest. Ophthalmol. Vis. Sci. 52: 6643–6650 [DOI] [PubMed] [Google Scholar]
- Daza-Vamenta R., Glusman G., Rowen L., Guthrie B., Geraghty D. E., 2004. Genetic divergence of the rhesus macaque major histocompatibility complex. Genome Res. 14: 1501–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxiadis G. G., de Groot N., Otting N., Blokhuis J. H., Bontrop R. E., 2011. Genomic plasticity of the MHC class I A region in rhesus macaques: extensive haplotype diversity at the population level as revealed by microsatellites. Immunogenetics 63: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Zhang Y., Zhang R., Yang L., Li M., et al. , 2011. Genome sequence and global sequence variation map with 5.5 million SNPs in Chinese rhesus macaque. Genome Biol. 12: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C. S., Reece J. C., Saepuloh U., De Rose R., Ishkandriati D., et al. , 2011. Screening and confirmatory testing of MHC class I alleles in pig-tailed macaques. Immunogenetics 63: 511–521 [DOI] [PubMed] [Google Scholar]
- Hernandez R. D., Hubisz M. J., Wheeler D. A., Smith D. G., Ferguson B., et al. , 2007. Demographic histories and patterns of linkage disequilibrium in Chinese and Indian rhesus macaques. Science 316: 240–243 [DOI] [PubMed] [Google Scholar]
- Hutnick N. A., Myles D. J., Hirao L., Scott V. L., Ferraro B., et al. , 2012. An optimized SIV DNA vaccine can serve as a boost for Ad5 and provide partial protection from a high-dose SIVmac251 challenge. Vaccine 30: 3202–3208 [DOI] [PubMed] [Google Scholar]
- Karl J. A., Wiseman R. W., Campbell K. J., Blasky A. J., Hughes A. L., et al. , 2008. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics 60: 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S. H., Sussman R. W., Taylor L. L., 1995. Mitochondrial DNA of the Mauritian macaques (Macaca fascicularis): an example of the founder effect. Am. J. Phys. Anthropol. 96: 133–141 [DOI] [PubMed] [Google Scholar]
- Li A., Wang X., Liu Y., Zhao Y., Liu B., et al. , 2012a Preliminary observations of MHC class I A region polymorphism in three populations of Chinese-origin rhesus macaques. Immunogenetics 64: 887–894 [DOI] [PubMed] [Google Scholar]
- Li Q., Ruan Z., Zhang H., Peng N., Zhao S., et al. , 2012b Characterization of peripheral blood T lymphocyte subsets in Chinese rhesus macaques with repeated or long-term infection with Plasmodium cynomolgi. Parasitol. Res. 110: 961–969 [DOI] [PubMed] [Google Scholar]
- Ling B., Rogers L., Johnson A., Piatak M., Lifson J., et al. , 2013. Effect of combination antiretroviral therapy on Chinese rhesus macaques of SIV infection. AIDS Res. Hum. Retroviruses (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wei Q., Alvarez X., Wang H., Du Y., et al. , 2011. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 85: 4025–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li A., Wang X., Sui L., Li M., et al. , 2013. Mamu-B genes and their allelic repertoires in different populations of Chinese-origin rhesus macaques. Immunogenetics 65: 273–280 [DOI] [PubMed] [Google Scholar]
- Ma X., Tang L. H., Qu L. B., Ma J., Chen L., 2009. Identification of 17 novel major histocompatibility complex-A alleles in a population of Chinese-origin rhesus macaques. Tissue Antigens 73: 184–187 [DOI] [PubMed] [Google Scholar]
- Mothe B. R., Sidney J., Dzuris J. L., Liebl M. E., Fuenger S., et al. , 2002. Characterization of the peptide-binding specificity of Mamu-B*17 and identification of Mamu-B*17-restricted epitopes derived from simian immunodeficiency virus proteins. J. Immunol. 169: 210–219 [DOI] [PubMed] [Google Scholar]
- Mumbauer A., Gettie A., Blanchard J., Cheng-Mayer C., 2013. Efficient mucosal transmissibility but limited pathogenicity of R5 SHIVSF162P3N in Chinese origin rhesus macaques. J. Acquir. Immune Defic. Syndr. 62: 496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse T. K., Chen Z., Yanagida R., Yamashita T., Saito Y., et al. , 2010. Diversity of MHC class I genes in Burmese-origin rhesus macaques. Immunogenetics 62: 601–611 [DOI] [PubMed] [Google Scholar]
- Otting N., de Vos-Rouweler A. J., Heijmans C. M., de Groot N. G., Doxiadis G. G., et al. , 2007. MHC class I A region diversity and polymorphism in macaque species. Immunogenetics 59: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N., Heijmans C. M., van der Wiel M., de Groot N. G., Doxiadis G. G., et al. , 2008. A snapshot of the Mamu-B genes and their allelic repertoire in rhesus macaques of Chinese origin. Immunogenetics 60: 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang D., Wang X., He X., Xu L., Shi H., et al. , 2009. Construction of soluble Mamu-b*1703, a class I major histocompatibility complex of Chinese rhesus macaques, monomer and tetramer loaded with a simian immunodeficiency virus peptide. Cell. Mol. Immunol. 6: 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z. M., Xu W. F., Li C. Y., 2007. Cue-invariant detection of centre-surround discontinuity by V1 neurons in awake macaque monkey. J. Physiol. 583: 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick C. H., Siddiqi M. F., 1994. Population status of nonhuman primates in Asia, with emphasis on rhesus macaques in India. Am. J. Primatol. 34: 51–59 [DOI] [PubMed] [Google Scholar]
- Tian G., Qiu Y., Qi Z., Wu X., Zhang Q., et al. , 2011. Histopathological observation of immunized rhesus macaques with plague vaccines after subcutaneous infection of Yersinia pestis. PLoS ONE 6: e19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Sun Z., He X., Tan T., Lu B., et al. , 2011. Derivation of rhesus monkey parthenogenetic embryonic stem cells and its microRNA signature. PLoS ONE 6: e25052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman R. W., Wojcechowskyj J. A., Greene J. M., Blasky A. J., Gopon T., et al. , 2007. Simian immunodeficiency virus SIVmac239 infection of major histocompatibility complex-identical cynomolgus macaques from Mauritius. J. Virol. 81: 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman R. W., Karl J. A., Bimber B. N., O’Leary C. E., Lank S. M., et al. , 2009. Major histocompatibility complex genotyping with massively parallel pyrosequencing. Nat. Med. 15: 1322–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Bassinger S., Montoya G. D., Chavez L., Jones C. E., et al. , 2008. Allelic diversity within the high frequency Mamu-A2*05/Mane-A2*05 (Mane-A*06)/Mafa-A2*05 family of macaque MHC-A loci. Tissue Antigens 72: 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant L. J., Friedrich T. C., Johnson R. C., May G. E., Maness N. J., et al. , 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80: 5074–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.