Abstract
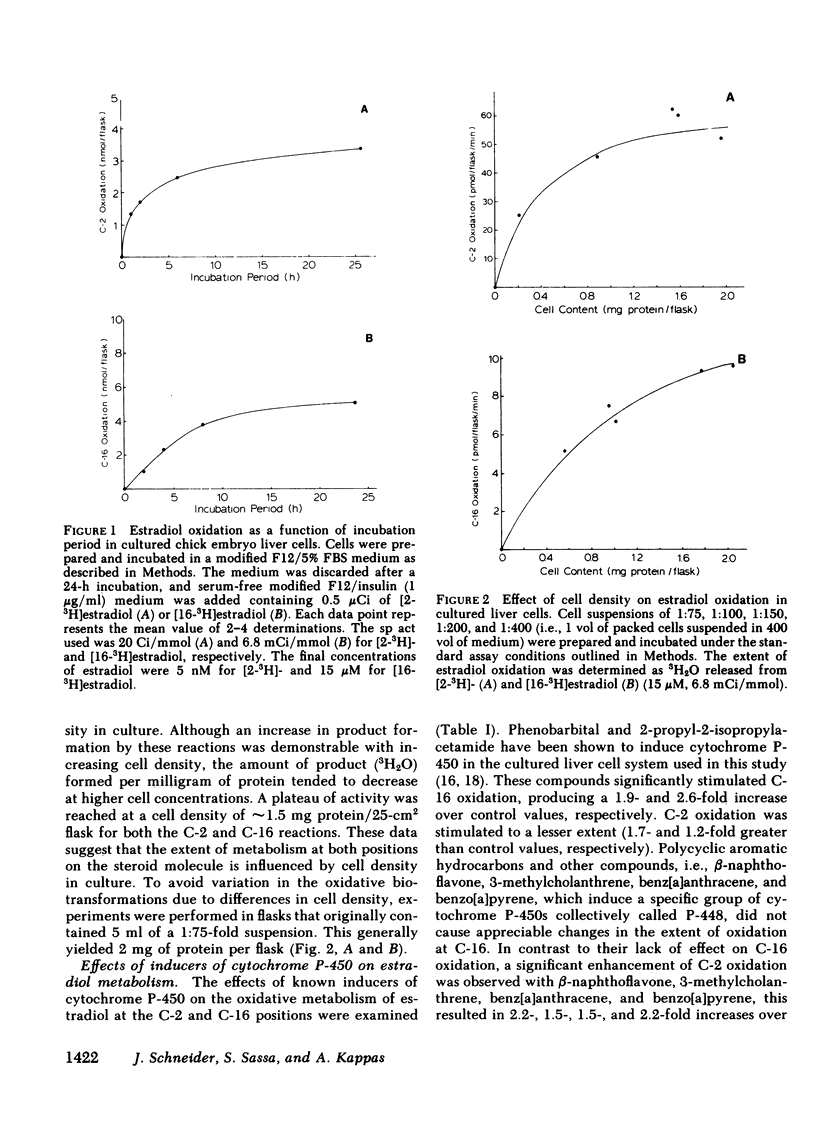
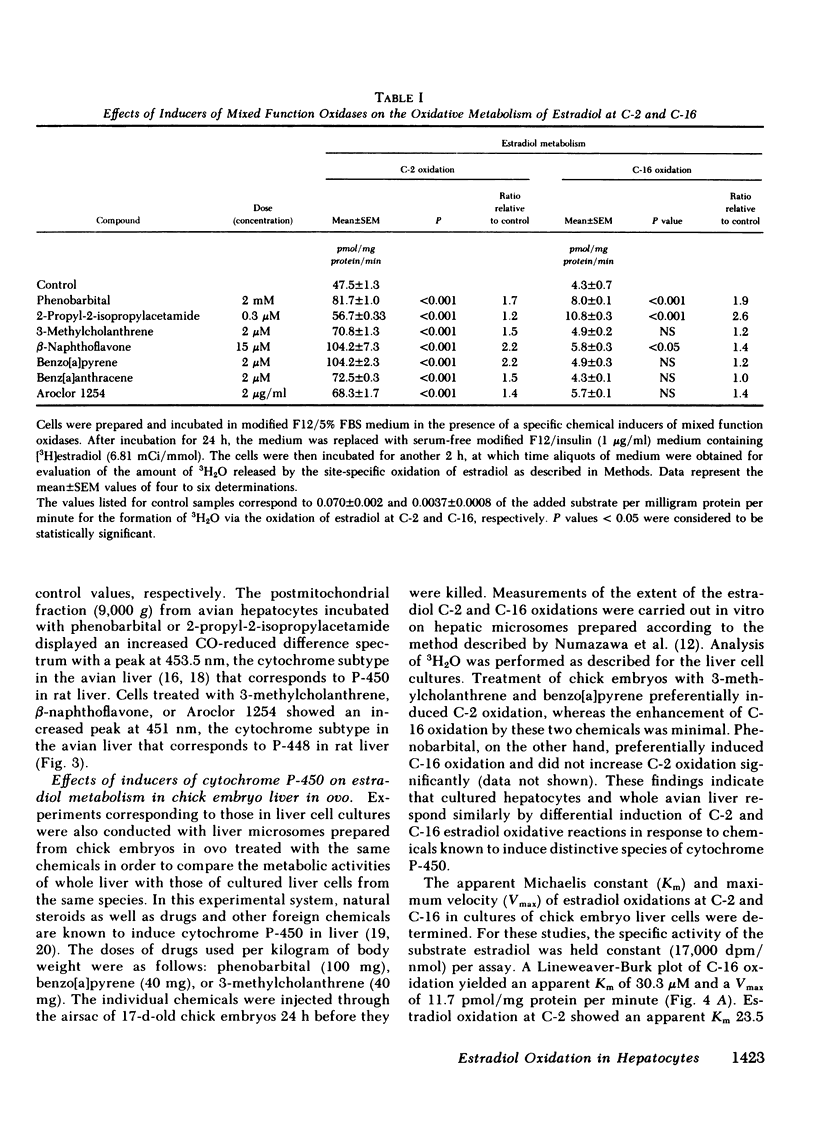
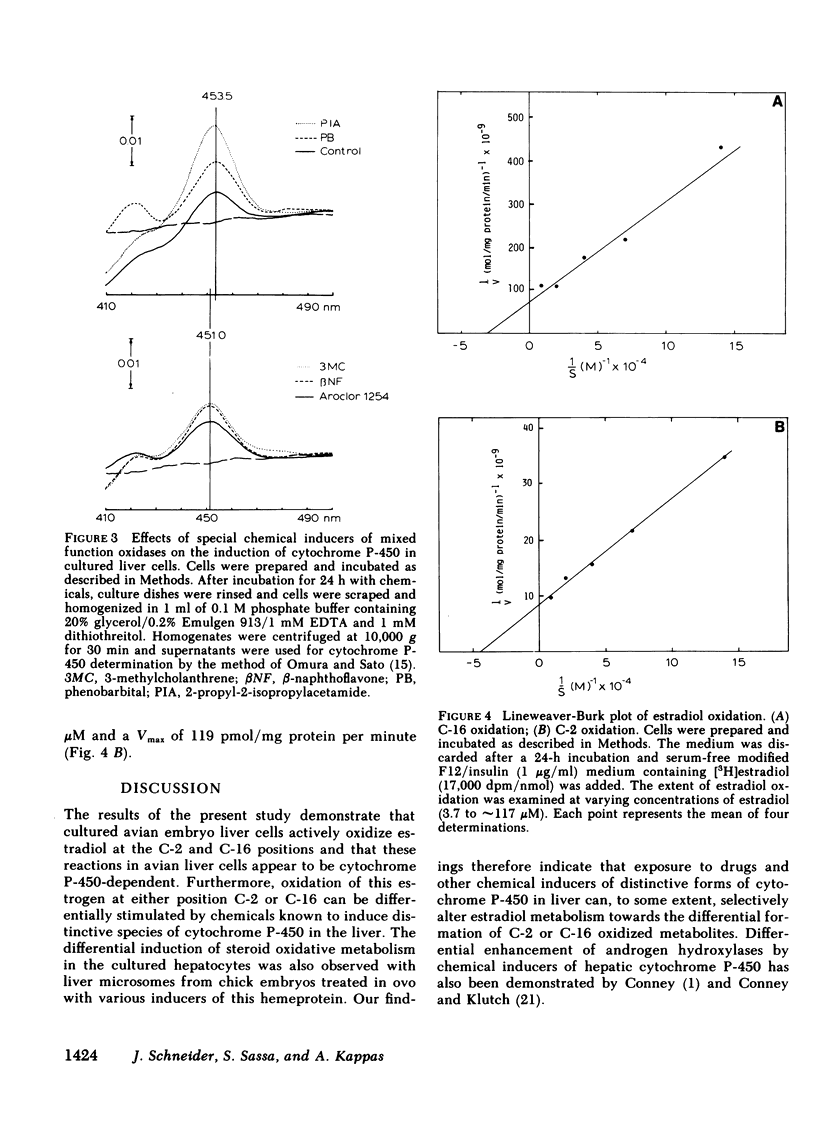
The oxidative metabolism of estradiol (the natural estrogen 2,3,5(10)-estratriene-3,17 beta-diol) at positions C-2 and C-16 was examined in primary cultures of chick embryo liver cells using estradiol which was labeled with 3H specifically at either the C-2 or C-16 position as the substrate. Oxidation of estradiol by the cultured liver cells was assessed by the release of 3H which accumulated as 3H2O in the culture medium; both C-2 and C-16 oxidative reactions were detectable in the liver cell cultures by this technique. When incubated with a concentration of estradiol substrate close to the Michaelis constant (Km), approximately 45.8 pmol [2-3H]estradiol and 5.0 pmol [16-3H]estradiol/mg protein per minute underwent oxidative metabolism in untreated cells. Total amounts of oxidized product formation after 2 h of incubation were 28 and 5 pmol/mg protein for C-2 and C-16 oxidation, respectively. Treatment of cultures with phenobarbital or 2-propyl-2-isopropylacetamide significantly increased oxidation at C-16 (1.9-fold and 2.6-fold greater than control values, respectively), whereas no significant change in C-16 oxidation was observed after treatment of the cultures with 3-methylcholanthrene, benzo[a]pyrene, or benz[a]anthracene. The latter chemicals, however, were found to increase the extent of oxidation at C-2 significantly (i.e., 1.5-2.2-fold increases over control values). The increase in C-2 oxidation after treatment of cultures with phenobarbital or 2-propyl-2-isopropylacetamide was significantly less than that observed for oxidation at C-16. The apparent Km values for these oxidations in control cultures were 23.5 and 30.3 microM for C-2 and C-16 oxidation, respectively; corresponding maximum velocity (Vmax) values were 119 and 11.7 pmol/mg protein per minute, respectively. These data indicate that the C-2 and C-16 oxidations of estradiol take place in cultured avian hepatocytes and that the extent of metabolism at these positions on the hormone molecule can be altered by chemicals, such as drugs and polycyclic aromatic hydrocarbons, which induce distinctive species of cytochrome P-450 in the liver.
Full text
PDF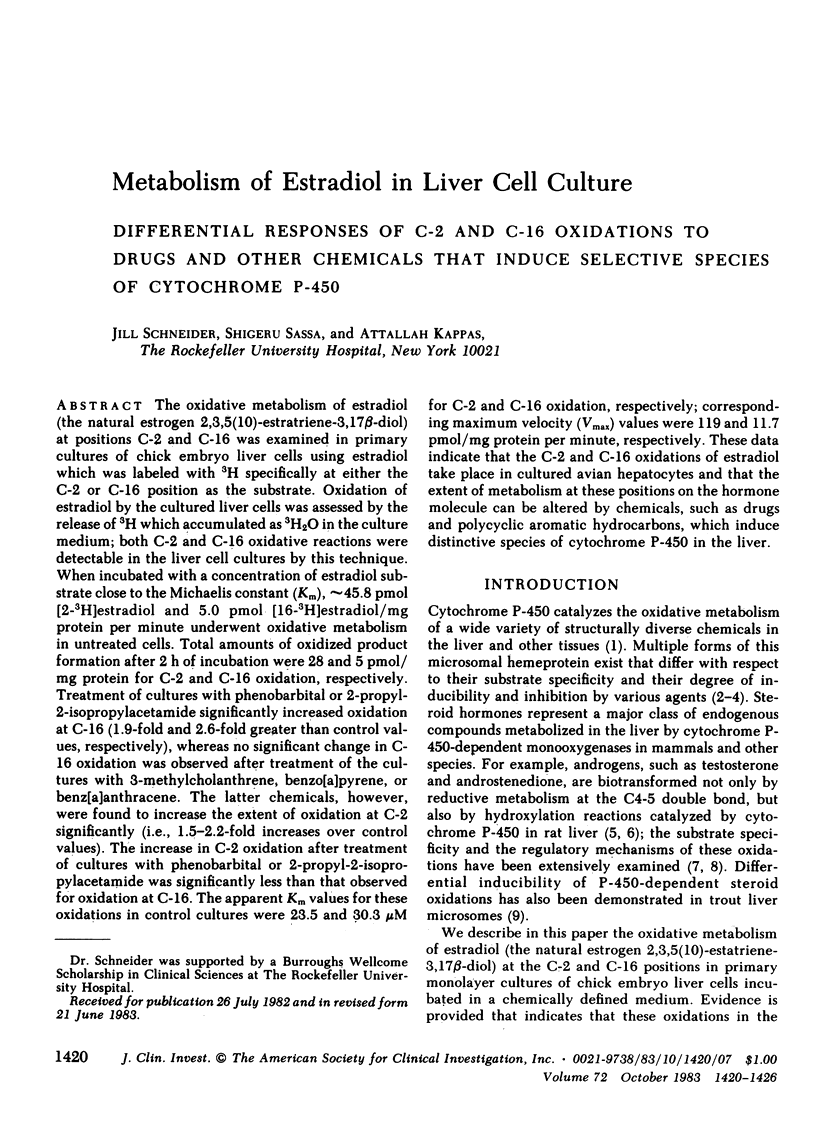



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Sinclair J. F., Sinclair P., Meyers U. A. Drug-mediated induction of cytochrome(s) P-450 and drug metabolism in cultured hepatocytes maintained in chemically defined medium. J Biol Chem. 1979 Mar 25;254(6):2148–2153. [PubMed] [Google Scholar]
- Anderson K. E., Conney A. H., Kappas A. Nutritional influences on chemical biotransformations in humans. Nutr Rev. 1982 Jun;40(6):161–171. doi: 10.1111/j.1753-4887.1982.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Anderson K. E., Freddara U., Kappas A. Induction of hepatic cytochrome P-450 by natural steroids: relationship to the induction of delta-aminolevulinate synthase and porphyrin accumulation in the avian embryo. Arch Biochem Biophys. 1982 Sep;217(2):597–608. doi: 10.1016/0003-9861(82)90542-2. [DOI] [PubMed] [Google Scholar]
- Berg A., Gustafsson J. A. Regulation of hydroxylation of 5 alpha-androstane-3 beta, 17 beta-diol in liver microsomes from male and female rats. J Biol Chem. 1973 Sep 25;248(18):6559–6567. [PubMed] [Google Scholar]
- Brueggemeier R. W. Kinetics of rat liver microsomal estrogen 2-hydroxylase. Evidence for sex differences at initial velocity conditions. J Biol Chem. 1981 Oct 25;256(20):10239–10242. [PubMed] [Google Scholar]
- CONNEY A. H., KLUTCH A. Increased activity of androgen hydroxylases in liver microsomes of rats pretreated with phenobarbital and other drugs. J Biol Chem. 1963 May;238:1611–1617. [PubMed] [Google Scholar]
- CONNEY A. H., KLUTCH A. Increased activity of androgen hydroxylases in liver microsomes of rats pretreated with phenobarbital and other drugs. J Biol Chem. 1963 May;238:1611–1617. [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- De Verneuil H., Sassa S., Kappas A. Effects of polychlorinated biphenyl compounds, 2,3,7,8-tetrachlorodibenzo-p-dioxin, phenobarbital and iron on hepatic uroporphyrinogen decarboxylase. Implications for the pathogenesis of porphyria. Biochem J. 1983 Jul 15;214(1):145–151. doi: 10.1042/bj2140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P. W., Goodridge A. G. Coordinate regulation of acetyl coenzyme A carboxylase and fatty acid synthetase in liver cells of the developing chick in vivo and in culture. Arch Biochem Biophys. 1978 Sep;190(1):332–344. doi: 10.1016/0003-9861(78)90283-7. [DOI] [PubMed] [Google Scholar]
- Fishman J. Aromatic hydroxylation of estrogens. Annu Rev Physiol. 1983;45:61–72. doi: 10.1146/annurev.ph.45.030183.000425. [DOI] [PubMed] [Google Scholar]
- Fishman J., Bradlow H. L., Schneider J., Anderson K. E., Kappas A. Radiometric analysis of biological oxidations in man: sex differences in estradiol metabolism. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4957–4960. doi: 10.1073/pnas.77.8.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J., Martucci C. Differential biological activity of estradiol metabolites. Pediatrics. 1978 Dec;62(6 Pt 2):1128–1133. [PubMed] [Google Scholar]
- Giger U., Meyer U. A. Induction of delta-aminolevulinate synthase and cytochrome P-450 hemoproteins in hepatocyte culture. Effect of glucose and hormones. J Biol Chem. 1981 Nov 10;256(21):11182–11190. [PubMed] [Google Scholar]
- Goodridge A. G., Adelman T. G. Regulation of malic enzyme synthesis by insulin triiodothyronine, and glucagon in liver cells in culture. J Biol Chem. 1976 May 25;251(10):3027–3032. [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid control of porphyrin and heme biosynthesis: a new biological function of steroid hormone metabolites. Proc Natl Acad Sci U S A. 1967 May;57(5):1463–1467. doi: 10.1073/pnas.57.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieninger G., Granick S. Synthesis and secretion of plasma proteins by embryonic chick hepatocytes: changing patterns during the first three days of culture. J Exp Med. 1978 Jun 1;147(6):1806–1823. doi: 10.1084/jem.147.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson T., Rafter J., Gustafsson J. A. Effects of some common inducers on the hepatic microsomal metabolism of androstenedione in rainbow trout with special reference to cytochrome P-450-dependent enzymes. Biochem Pharmacol. 1980 Feb 15;29(4):583–587. doi: 10.1016/0006-2952(80)90380-9. [DOI] [PubMed] [Google Scholar]
- Haugen D. A., van der Hoeven T. A., Coon M. J. Purified liver microsomal cytochrome P-450. Separation and characterization of multiple forms. J Biol Chem. 1975 May 10;250(9):3567–3570. [PubMed] [Google Scholar]
- Heinrichs W. L., Colás A. The selective stimulation, inhibition, and physicochemical alteration of the 7- and 16-alpha-hydroxylases of 3-beta-hydroxyandrost-5-en-17-one and drug-metabolizing enzymes in hepatic microsomal fractions. Biochemistry. 1968 Jun;7(6):2273–2280. doi: 10.1021/bi00846a033. [DOI] [PubMed] [Google Scholar]
- Huang M. T., West S. B., Lu A. Y. Separation, purification, and properties of multiple forms of cytochrome P-450 from the liver microsomes of phenobarbital-treated mice. J Biol Chem. 1976 Aug 10;251(15):4659–4665. [PubMed] [Google Scholar]
- KAPPAS A., PALMER R. H. Selected aspects of steroid pharmacology. Pharmacol Rev. 1963 Mar;15:123–167. [PubMed] [Google Scholar]
- Kappas A. Biologic actions of some natural steroids on the liver. N Engl J Med. 1968 Feb 15;278(7):378–384. doi: 10.1056/NEJM196802152780707. [DOI] [PubMed] [Google Scholar]
- Kupfer D., Miranda G. K., Bulger W. H. A facile assay for 2-hydroxylation of estradiol by liver microsomes. Anal Biochem. 1981 Sep 1;116(1):27–34. doi: 10.1016/0003-2697(81)90317-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., Kuntzman R., West S., Jacobson M., Conney A. H. Reconstituted liver microsomal enzyme system that hydroxylates drugs, other foreign compounds, and endogenous substrates. II. Role of the cytochrome P-450 and P-448 fractions in drug and steroid hydroxylations. J Biol Chem. 1972 Mar 25;247(6):1727–1734. [PubMed] [Google Scholar]
- Martucci C., Fishman J. Direction of estradiol metabolism as a control of its hormonal action--uterotrophic activity of estradiol metabolites. Endocrinology. 1977 Dec;101(6):1709–1715. doi: 10.1210/endo-101-6-1709. [DOI] [PubMed] [Google Scholar]
- Numazawa M., Kiyono Y., Nambara T. A simple radiometric assay for estradiol 2-hydroxylase activity. Anal Biochem. 1980 May 15;104(2):290–295. doi: 10.1016/0003-2697(80)90077-9. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Rifkind A. B., Gillette P. N., Song C. S., Kappas A. Drug stimulation of -aminolevulinic acid synthetase and cytochrome P-450 in vivo in chick embryo liver. J Pharmacol Exp Ther. 1973 May;185(2):214–225. [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Levin W. Purification of characterization of a minor form of hepatic microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls. Arch Biochem Biophys. 1982 Jun;216(1):272–288. doi: 10.1016/0003-9861(82)90212-0. [DOI] [PubMed] [Google Scholar]
- Sassa S., Bradlow H. L., Kappas A. Steroid induction of delta-aminolevulinic acid synthase and porphyrins in liver. Structure-activity studies and the permissive effects of hormones on the induction process. J Biol Chem. 1979 Oct 25;254(20):10011–10020. [PubMed] [Google Scholar]
- Sassa S., Golde D. W., Li C. H. Growth hormone stimulates drug-induced porphyrin formation in liver cells maintained in a serum-free medium. J Biol Chem. 1981 Sep 10;256(17):8882–8884. [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Sinclair J. F., Sinclair P. R., Bonkowsky H. L. Hormonal requirements for the induction of cytochrome P-450 in hepatocytes cultured in a serum-free medium. Biochem Biophys Res Commun. 1979 Feb 14;86(3):710–717. doi: 10.1016/0006-291x(79)91771-6. [DOI] [PubMed] [Google Scholar]
- Werringloer J., Estabrook R. W. Heterogeneity of liver microsomal cytochrome P-450: the spectral characterization of reactants with reduced cytochrome P-450. Arch Biochem Biophys. 1975 Mar;167(1):270–286. doi: 10.1016/0003-9861(75)90463-4. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Recent studies on the mechanism of action of testosterone. N Engl J Med. 1972 Dec 21;287(25):1284–1291. doi: 10.1056/NEJM197212212872508. [DOI] [PubMed] [Google Scholar]



