Abstract
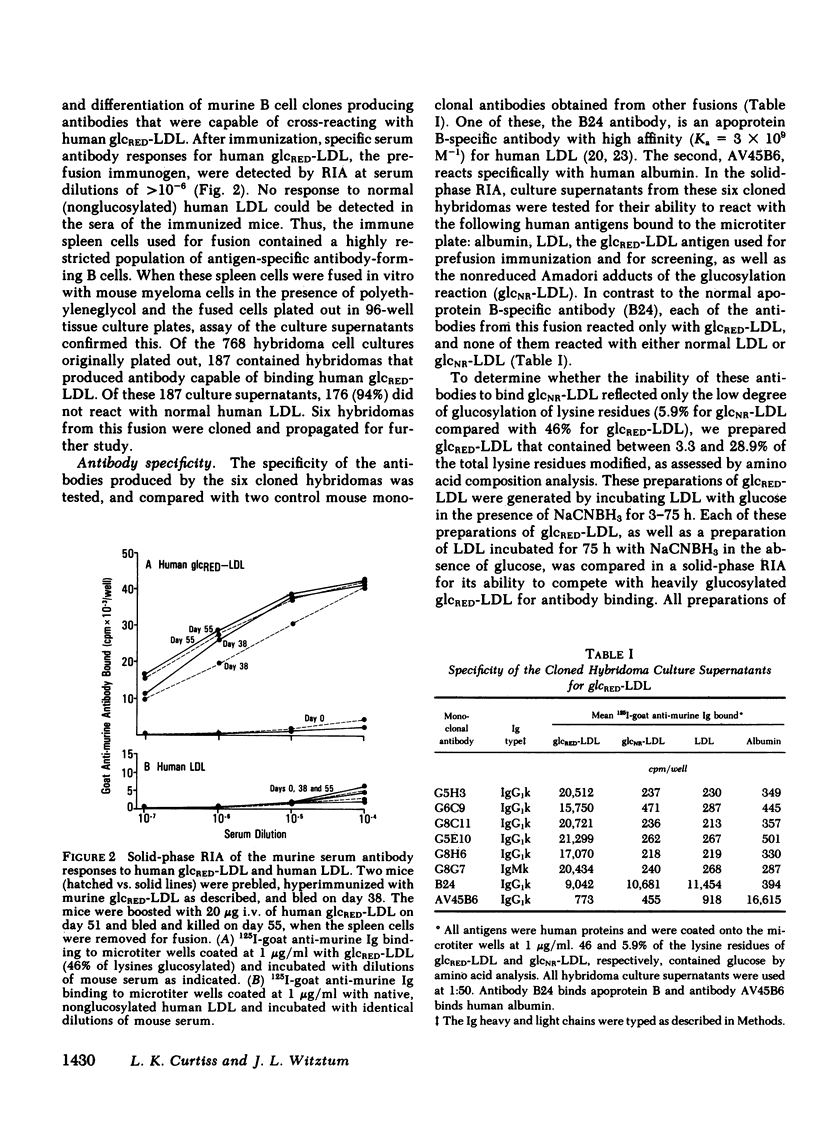
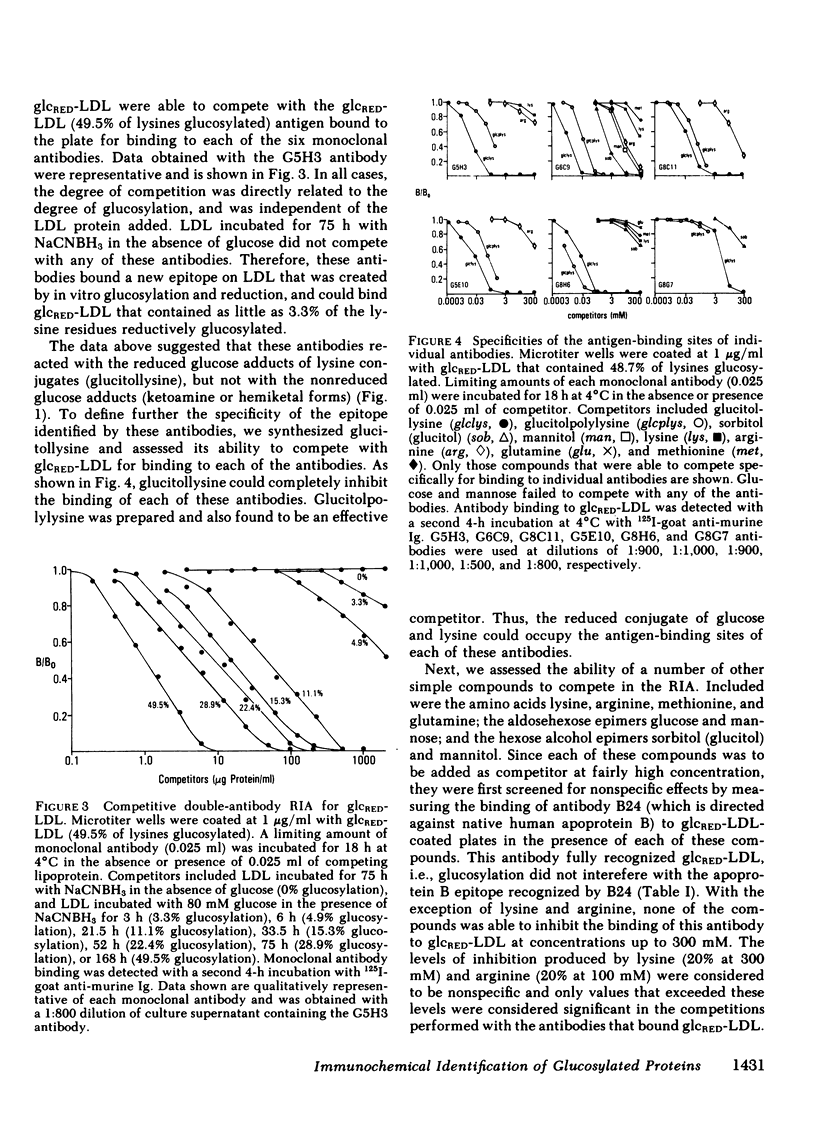
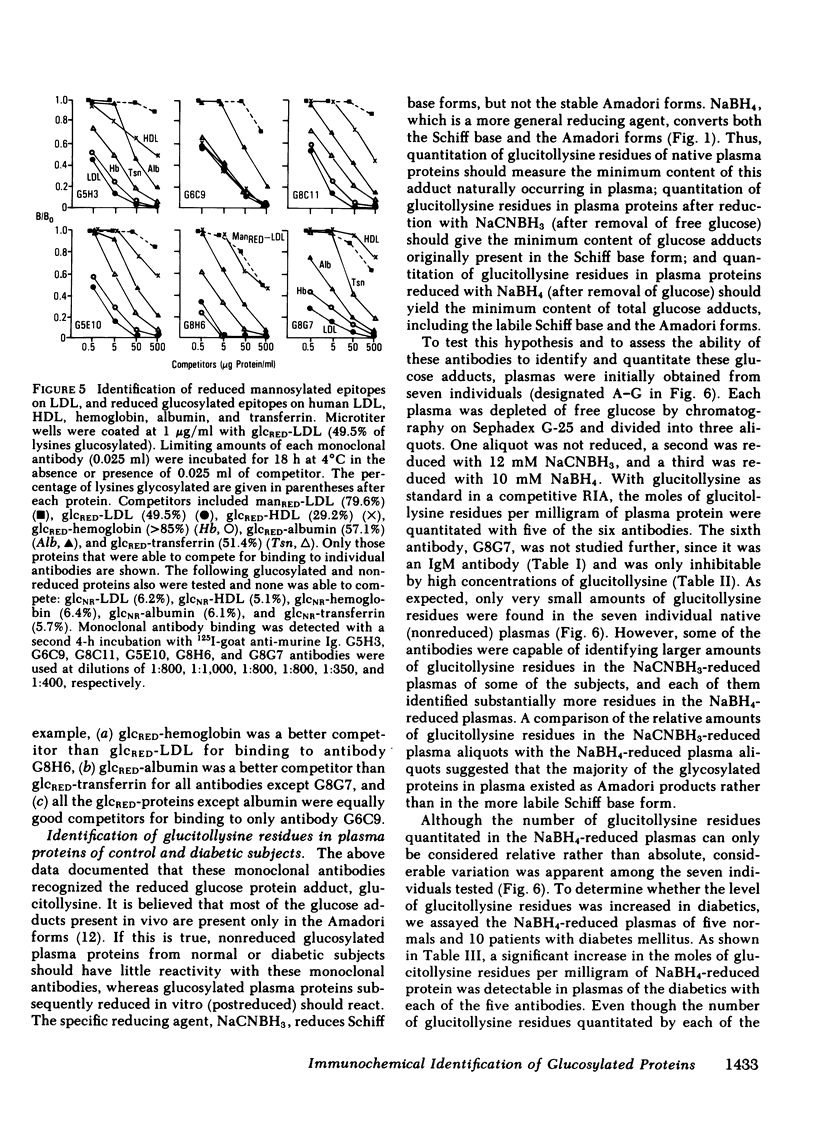
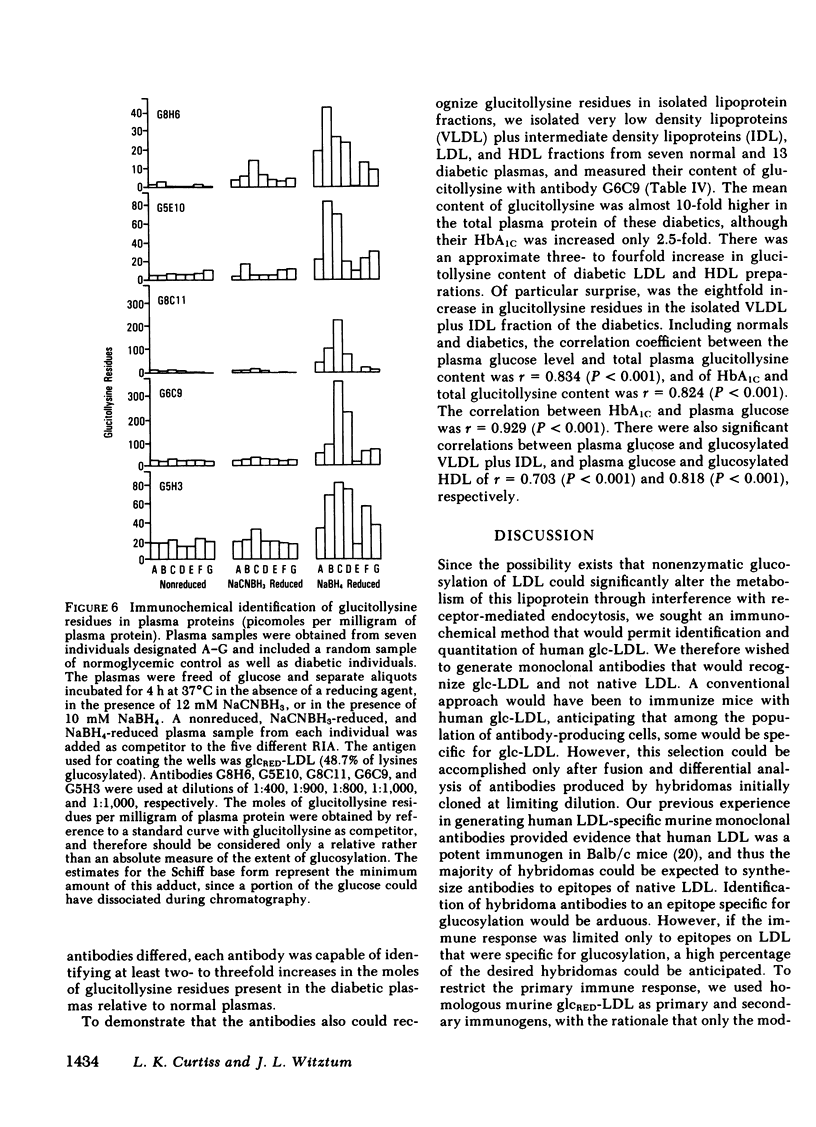
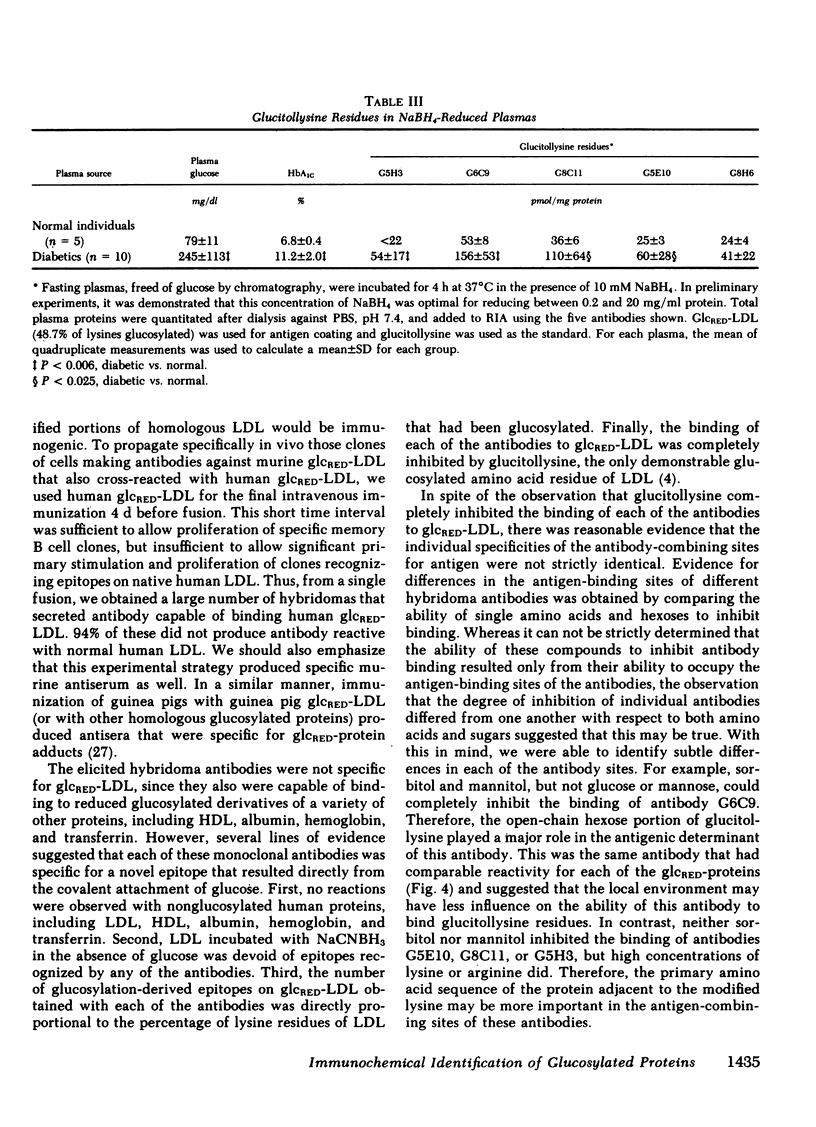
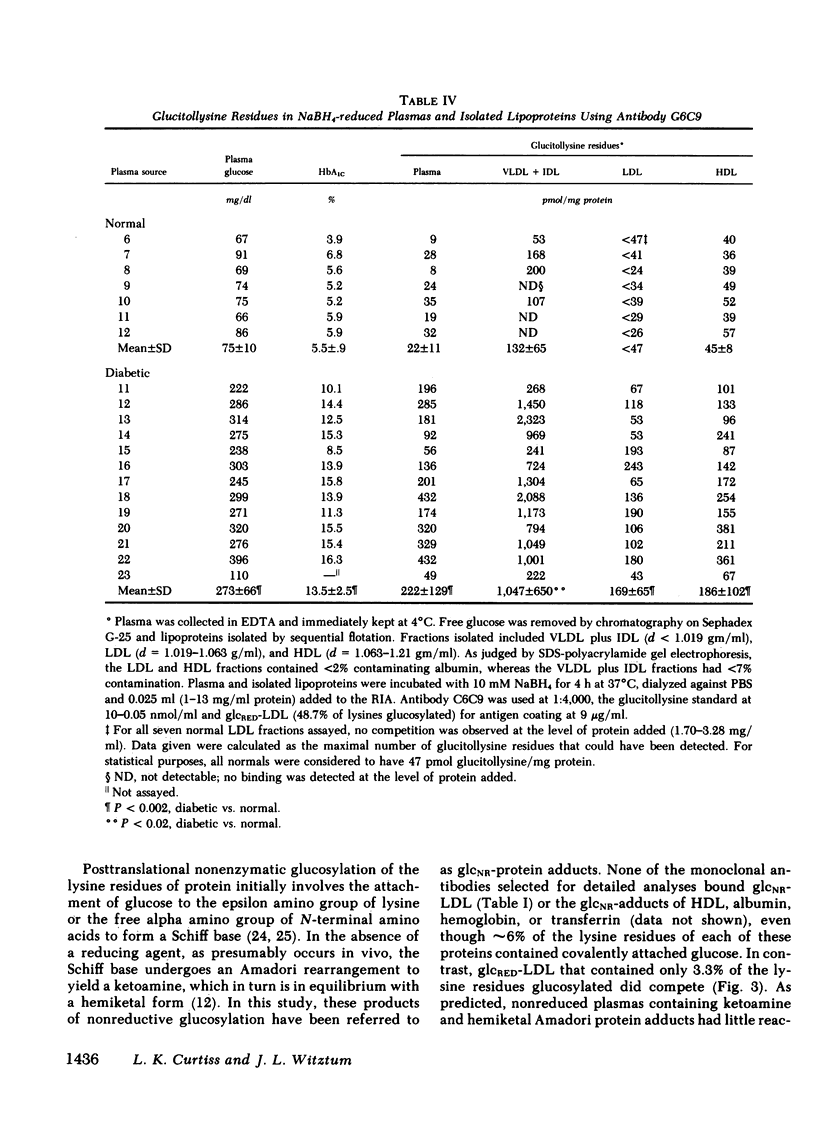
Modifications of plasma lipoprotein structure and function resulting from in vivo post-translational nonenzymatic glycosylation may play a role in the premature atherosclerosis of patients with diabetes mellitus. This report describes the generation and characterization of six unique murine monoclonal antibodies that bind glucosylated human plasma lipoproteins, but do not react with normal plasma lipoproteins. This was accomplished by immunizing mice with homologous glucosylated low density lipoprotein. In competitive inhibition radioimmunoassays, the dominant epitope recognized by these antibodies on glucosylated low density lipoprotein was identified as glucitollysine, the reduced hexose alcohol form of glucose conjugated to the epsilon amino group of lysine. Each of these antibodies was capable of identifying glucitollysine epitopes on all reduced glucosylated proteins studied, including high density lipoprotein, albumin, hemoglobin, and transferrin. These antibodies were also capable of identifying and quantitating glucitollysine residues on the total plasma proteins and isolated lipoproteins of normal and diabetic individuals after reduction of the proteins with NaBH4. Preliminary data suggest that diabetic total plasma proteins and isolated lipoproteins contain at least threefold more immunochemically detectable glucitollysine residues than nondiabetic plasma proteins and lipoproteins. The technique described in this report should allow production of region-specific antibodies to any immunogenic modification of a protein.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissé E., Berger W., Flückiger R. Quantitation of glycosylated hemoglobin. Elimination of labile glycohemoglobin during sample hemolysis at pH 5. Diabetes. 1982 Jul;31(7):630–633. doi: 10.2337/diab.31.7.630. [DOI] [PubMed] [Google Scholar]
- Bodansky H. J., Wolf E., Cudworth A. G., Dean B. M., Nineham L. J., Bottazzo G. F., Matthews J. A., Kurtz A. B., Kohner E. M. Genetic and immunologic factors in microvascular disease in type I insulin-dependent diabetes. Diabetes. 1982 Jan;31(1):70–74. doi: 10.2337/diab.31.1.70. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Measurement of glycosylated amino acids and peptides from urine of diabetic patients using affinity chromatography. Diabetes. 1980 Dec;29(12):1044–1047. doi: 10.2337/diab.29.12.1044. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. Evaluation of glycosylated hemoglobin diabetic patients. Diabetes. 1981 Jul;30(7):613–617. doi: 10.2337/diab.30.7.613. [DOI] [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Immunochemical heterogeneity of human plasma apolipoprotein B. I. Apolipoprotein B binding of mouse hybridoma antibodies. J Biol Chem. 1982 Dec 25;257(24):15213–15221. [PubMed] [Google Scholar]
- Ditzel J. Oxygen transport impairment in diabetes. Diabetes. 1976;25(2 Suppl):832–838. [PubMed] [Google Scholar]
- Gonen B., Baenziger J., Schonfeld G., Jacobson D., Farrar P. Nonenzymatic glycosylation of low density lipoproteins in vitro. Effects on cell-interactive properties. Diabetes. 1981 Oct;30(10):875–878. doi: 10.2337/diab.30.10.875. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Bunn H. F. Kinetic analysis of the nonenzymatic glycosylation of hemoglobin. J Biol Chem. 1981 May 25;256(10):5204–5208. [PubMed] [Google Scholar]
- Javid J., Pettis P. K., Koenig R. J., Cerami A. Immunologic characterization and quantification of haemoglobin A1c. Br J Haematol. 1978 Mar;38(3):329–337. doi: 10.1111/j.1365-2141.1978.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Kesaniemi Y. A., Witztum J. L., Steinbrecher U. P. Receptor-mediated catabolism of low density lipoprotein in man. Quantitation using glucosylated low density lipoprotein. J Clin Invest. 1983 Apr;71(4):950–959. doi: 10.1172/JCI110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Kurup I. V. Nonenzymatic glycosylation of human plasma low density lipoprotein. Evidence for in vitro and in vivo glucosylation. Metabolism. 1982 Apr;31(4):348–353. doi: 10.1016/0026-0495(82)90109-3. [DOI] [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McFarland K. F., Catalano E. W., Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatic glucosylation of serum proteins in diabetes mellitus. Diabetes. 1979 Nov;28(11):1011–1014. doi: 10.2337/diab.28.11.1011. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Doberne L., Greenfield M. S. Comparison of insulin secretion and in vivo insulin action in nonobese and moderately obese individuals with non-insulin-dependent diabetes mellitus. Diabetes. 1982 May;31(5 Pt 1):382–384. doi: 10.2337/diab.31.5.382. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Cottam G. L. Glycosylation of LDL decreases its ability to interact with high-affinity receptors of human fibroblasts in vitro and decreases its clearance from rabbit plasma in vivo. Biochim Biophys Acta. 1982 Nov 12;713(2):199–207. doi: 10.1016/0005-2760(82)90237-5. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Cottam G. L. Glycosylation of human LDL and its metabolism in human skin fibroblasts. Biochem Biophys Res Commun. 1982 Feb 11;104(3):977–983. doi: 10.1016/0006-291x(82)91345-6. [DOI] [PubMed] [Google Scholar]
- Schwartz B. A., Gray G. R. Proteins containing reductively aminated disaccharides. Synthesis and chemical characterization. Arch Biochem Biophys. 1977 Jun;181(2):542–549. doi: 10.1016/0003-9861(77)90261-2. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Witztum J. L., Kesaniemi Y. A., Elam R. L. Comparison of glucosylated low density lipoprotein with methylated or cyclohexanedione-treated low density lipoprotein in the measurement of receptor-independent low density lipoprotein catabolism. J Clin Invest. 1983 Apr;71(4):960–964. doi: 10.1172/JCI110850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens V. J., Rouzer C. A., Monnier V. M., Cerami A. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2918–2922. doi: 10.1073/pnas.75.6.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao B. P., Curtiss L. K., Edgington T. S. Immunochemical heterogeneity of human plasma apolipoprotein B. II. Expression of apolipoprotein B epitopes on native lipoproteins. J Biol Chem. 1982 Dec 25;257(24):15222–15228. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Witztum J. L., Fisher M., Pietro T., Steinbrecher U. P., Elam R. L. Nonenzymatic glucosylation of high-density lipoprotein accelerates its catabolism in guinea pigs. Diabetes. 1982 Nov;31(11):1029–1032. doi: 10.2337/diacare.31.11.1029. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Steinbrecher U. P., Fisher M., Kesaniemi A. Nonenzymatic glucosylation of homologous low density lipoprotein and albumin renders them immunogenic in the guinea pig. Proc Natl Acad Sci U S A. 1983 May;80(9):2757–2761. doi: 10.1073/pnas.80.9.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Church D. B., Turtle J. R. The measurement of glycosylated hemoglobin in man and animals by aminophenylboronic acid affinity chromatography. Diabetes. 1982 Aug;31(8 Pt 1):701–705. doi: 10.2337/diab.31.8.701. [DOI] [PubMed] [Google Scholar]



