Abstract
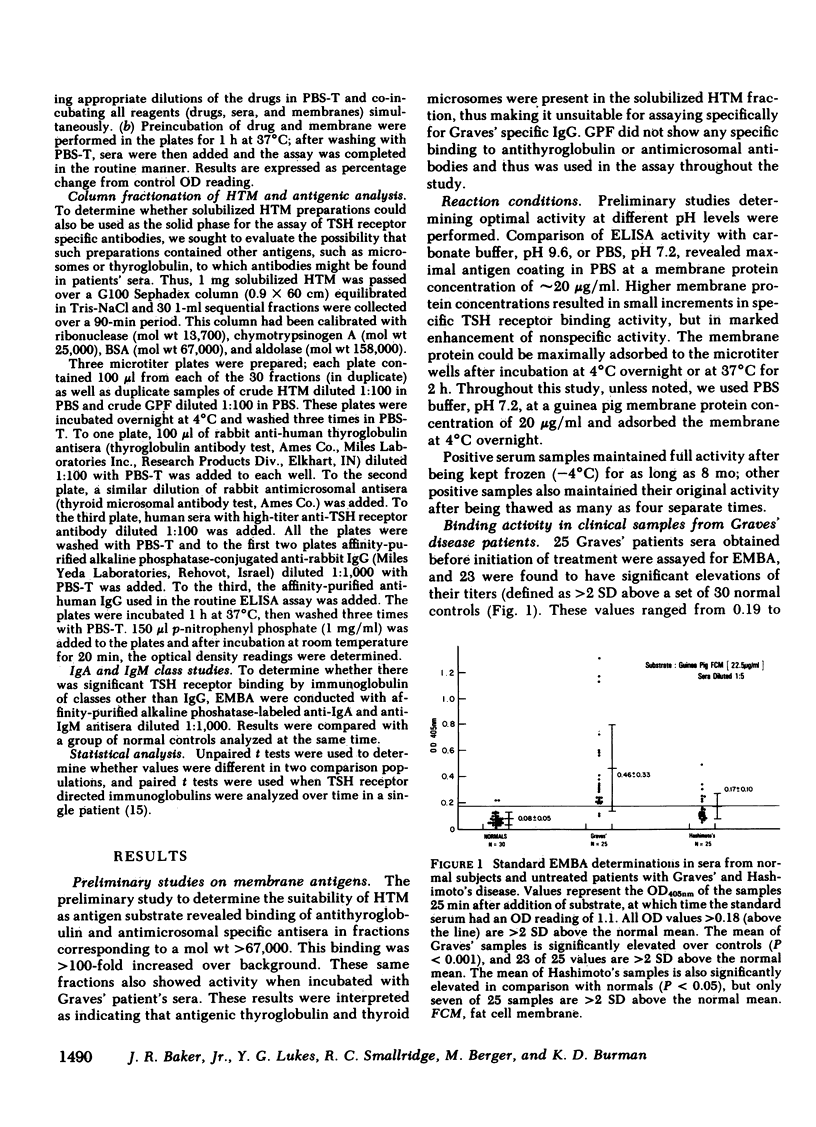
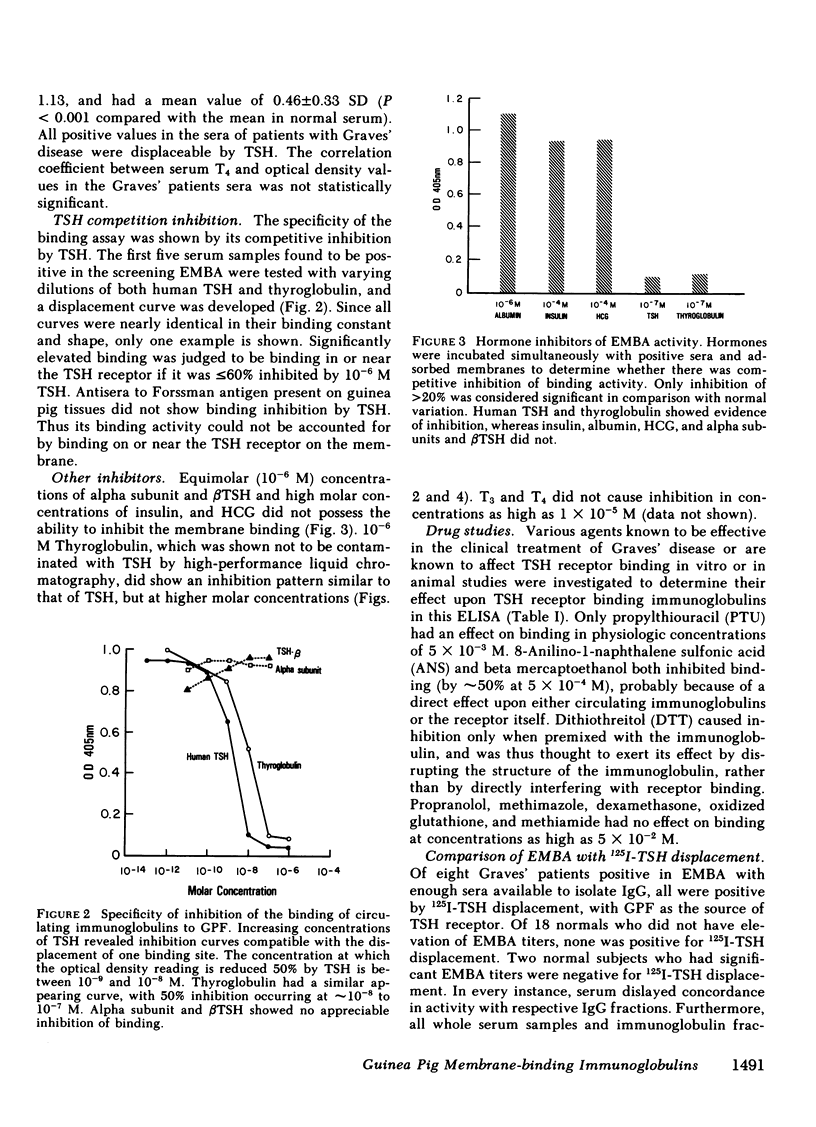
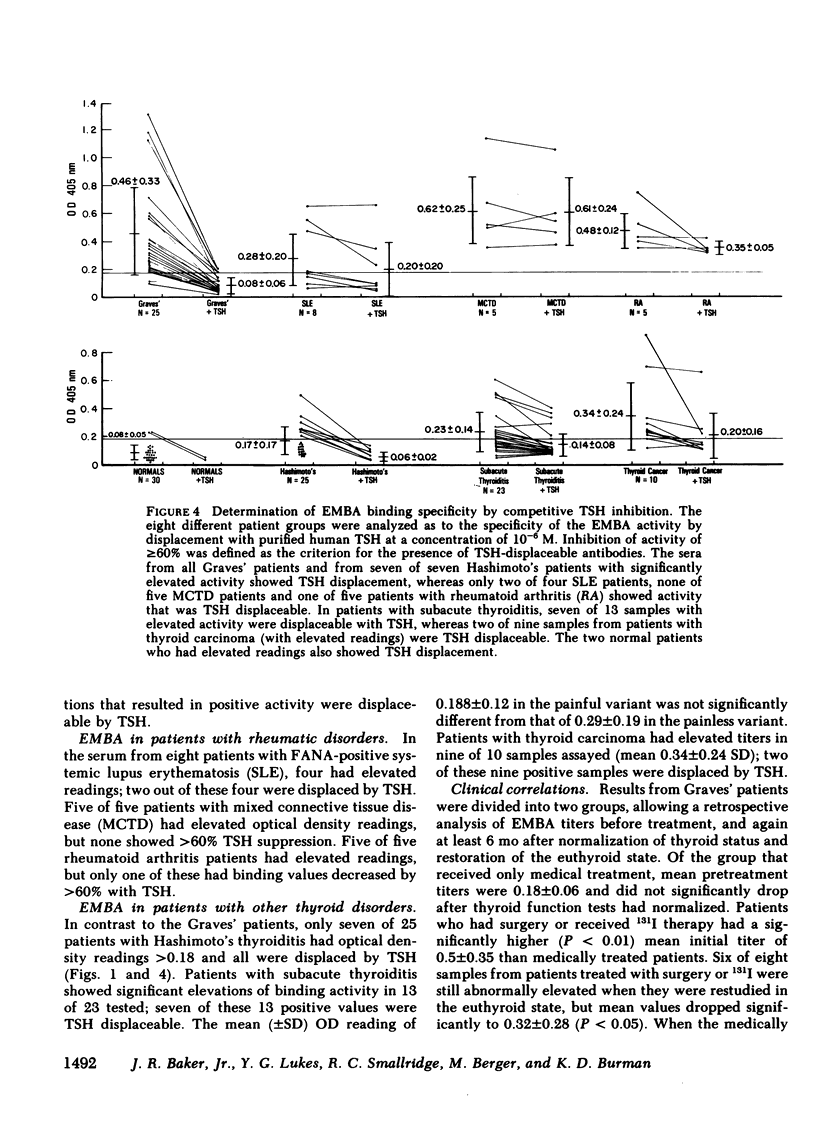
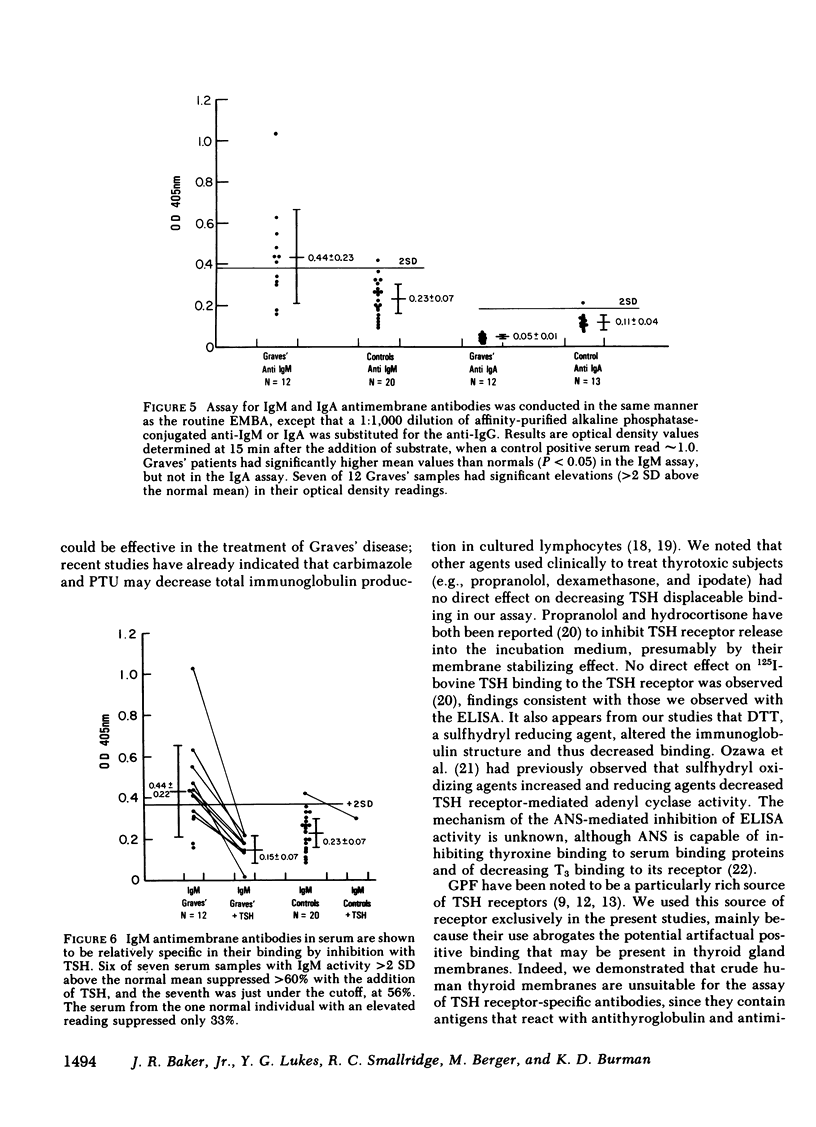
To obviate several problems inherent in indirect thyroid-stimulating hormone (TSH) receptor antibody assays, we developed an enzyme-linked immunosorbent assay (ELISA) that measures antibodies binding to guinea pig fat cell membrane, which contain high concentrations of TSH receptors. Solubilized guinea pig fat cell membranes were adsorbed to plastic microtiter plates and served as the solid-phase antigen. Test sera and affinity-purified alkaline phosphatase-conjugated anti-human IgG were co-incubated with membranes, after which p-nitrophenyl phosphate was added. Results were read when a positive control reached a standard color change (OD405nm). Specificity of this assay was demonstrated by the inability of albumin, insulin, TSH subunits, propranolol, or dexamethasone to block binding 30. normal subjects had a mean OD value of 0.080 +/- 0.050 (SD). 23 of 25 untreated Graves' patients had OD values at least 2 SD above the normal mean (Grave's mean +/- SD; 0.46 +/- 0.33, P less than 0.001) and in each case 10(-6) M TSH inhibited the binding by at least 60%, suggesting that the immunoglobulins were directed at the TSH receptor. Seven of 25 serum samples from patients with Hashimoto's disease, seven of 23 serum samples from patients with transient hyperthyroidism (subacute thyroiditis or painless thyrotoxic thyroiditis), and two of 10 samples from patients with thyroid carcinoma had significant elevations in the titers of membrane-directed immunoglobulins. Graves' patients who were treated with ablative therapy at least 6 mo earlier and who were euthyroid when restudied continued to have abnormally elevated membrane-directed immunoglobulins in six of eight samples studied. Further studies involved the substitution of affinity-purified alkaline phosphatase anti-IgM antisera for the anti-IgG antisera routinely used. Seven of 12 serum samples from patients with Graves' disease had significant elevations in binding which in every instance was inhibited by greater than 60% by 10(-6) M TSH. In sum, the present results indicate that (a) we have developed a sensitive, specific, reproducible, convenient ELISA for the measurement both of the total amount of circulating membrane-directed antibodies and of TSH-displaceable membrane-directed immunoglobulins. (b) This ELISA detected significant elevations in TSH-displaceable guinea pig membrane binding in 23 of 25 untreated Graves' patients as well as in approximately 30% of patients with Hashimoto's thyroiditis and subacute thyroiditis. (c) Elevated membrane directed antibodies may continue to be present many months or years after restoration of the euthyroid state. (d) Circulating membrane binding IgM immunoglobulins have been detected in patients with Graves' disease. Further studies using this ELISA should prove useful in a variety of investigative and clinical studies.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall G. N., Chopra I. J., Solomon D. H., Kruger S. R. Serum protein inhibition of thyrotropin binding to human thyroid tissue. J Clin Endocrinol Metab. 1978 Nov;47(5):967–973. doi: 10.1210/jcem-47-5-967. [DOI] [PubMed] [Google Scholar]
- Bech K., Nistrup Madsen S. Influence of treatment with radioiodine and propylthiouracil on thyroid stimulating immunoglobulins in Graves' disease. Clin Endocrinol (Oxf) 1980 Nov;13(5):417–424. doi: 10.1111/j.1365-2265.1980.tb03406.x. [DOI] [PubMed] [Google Scholar]
- Borges M., Ingbar J. C., Endo K., Amir S., Uchimura H., Nagataki S., Ingbar S. H. A new method for assessing the thyrotropin binding inhibitory activity in the immunoglobulins and whole serum of patients with Graves' disease. J Clin Endocrinol Metab. 1982 Mar;54(3):552–558. doi: 10.1210/jcem-54-3-552. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Kertiles L. P., Reichlin S. Partial purification and characterization of thyrotropin binding inhibitory immunoglobulins from normal human plasma. J Clin Endocrinol Metab. 1983 Jan;56(1):156–163. doi: 10.1210/jcem-56-1-156. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Lukes Y. G., Latham K. R., Wartofsky L. Ipodate and 8-anilino-1-naphthalene sulfonic acid block receptor binding of T3 in rat liver. Horm Metab Res. 1980 Dec;12(12):685–687. doi: 10.1055/s-2007-999232. [DOI] [PubMed] [Google Scholar]
- Cooper D. S., Saxe V. C., Meskell M., Maloof F., Ridgway E. C. Acute effects of propylthiouracil (PTU) on thyroidal iodide organification and peripheral iodothyronine deiodination: correlation with serum PTU levels measured by radioimmunoassay. J Clin Endocrinol Metab. 1982 Jan;54(1):101–107. doi: 10.1210/jcem-54-1-101. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Yeo P. P., Evered D. C., Clark F., Smith B. R., Hall R. Value of thyroid-stimulating-antibody determinations in predicting short-term thyrotoxic relapse in Graves' disease. Lancet. 1977 Jun 4;1(8023):1181–1182. doi: 10.1016/s0140-6736(77)92719-2. [DOI] [PubMed] [Google Scholar]
- Endo K., Amir S. M., Ingbar S. H. Development and evaluation of a method for the partial purification of immunoglobulins specific for Graves' disease. J Clin Endocrinol Metab. 1981 Jun;52(6):1113–1123. doi: 10.1210/jcem-52-6-1113. [DOI] [PubMed] [Google Scholar]
- Endo K., Borges M., Amir S., Ingbar S. H. Preparation of 125I-labeled receptor-purified Graves' immunoglobulins: properties of their binding to human thyroid membranes. J Clin Endocrinol Metab. 1982 Sep;55(3):566–576. doi: 10.1210/jcem-55-3-566. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Immunoregulation in autoimmunity. J Allergy Clin Immunol. 1980 Jul;66(1):5–17. doi: 10.1016/0091-6749(80)90132-3. [DOI] [PubMed] [Google Scholar]
- Fenzi G., Hashizume K., Roudebush C. P., DeGroot L. J. Changes in thyroid-stimulating immunoglobulins during antithyroid therapy. J Clin Endocrinol Metab. 1979 Apr;48(4):572–576. doi: 10.1210/jcem-48-4-572. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Amir S. M., Petersen A. W., Ingbar S. H. Preparation of biologically active 125I-TSH. Endocrinology. 1974 Nov;95(5):1228–1233. doi: 10.1210/endo-95-5-1228. [DOI] [PubMed] [Google Scholar]
- Grabar P. Hypothesis. Auto-antibodies and immunological theories: an analytical review. Clin Immunol Immunopathol. 1975 Nov;4(4):453–466. doi: 10.1016/0090-1229(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Hashizume K., DeGroot L. J. Release of thyrotropin receptor from thyroid plasma membranes: effect of hydrocortisone, propranolol, and adenosine 3',5'-monophosphate. Endocrinology. 1980 May;106(5):1463–1468. doi: 10.1210/endo-106-5-1463. [DOI] [PubMed] [Google Scholar]
- Hashizume K., Fenzi G., DeGroot L. J. Thyroglobulin inhibition of thyrotropin binding to thyroid plasma membrane. J Clin Endocrinol Metab. 1978 Apr;46(4):679–685. doi: 10.1210/jcem-46-4-679. [DOI] [PubMed] [Google Scholar]
- How J., Topliss D. J., Strakosch C., Lewis M., Row V. V., Volpé R. T lymphocyte sensitization and suppressor T lymphocyte defect in patients long after treatment for Graves' disease. Clin Endocrinol (Oxf) 1983 Jan;18(1):61–71. doi: 10.1111/j.1365-2265.1983.tb03187.x. [DOI] [PubMed] [Google Scholar]
- Kleinmann R. E., Braverman L. E., Vagenakis A. G., Butcher R. W., Clark R. B. A new method for measurement of human thyroid-stimulating immunoglobulins. J Lab Clin Med. 1980 Apr;95(4):581–589. [PubMed] [Google Scholar]
- Lind I., Harboe M., Folling I. Protein A reactivity of two distinct groups of human monoclonal IgM. Scand J Immunol. 1975;4(8):843–848. doi: 10.1111/j.1365-3083.1975.tb03726.x. [DOI] [PubMed] [Google Scholar]
- McGregor A. M., Petersen M. M., McLachlan S. M., Rooke P., Smith B. R., Hall R. Carbimazole and the autoimmune response in Graves' disease. N Engl J Med. 1980 Aug 7;303(6):302–307. doi: 10.1056/NEJM198008073030603. [DOI] [PubMed] [Google Scholar]
- McGregor A. M., Smith B. R., Hall R., Petersen M. M., Miller M., Dewar P. J. Prediction of relapse in hyperthyroid Graves' disease. Lancet. 1980 May 24;1(8178):1101–1103. doi: 10.1016/s0140-6736(80)91551-2. [DOI] [PubMed] [Google Scholar]
- O'Donnell J., Trokoudes K., Silverberg J., Row V., Volpé R. Thyrotropin displacement activity of serum immunoglobulins from patients with Graves' disease. J Clin Endocrinol Metab. 1978 May;46(5):770–777. doi: 10.1210/jcem-46-5-770. [DOI] [PubMed] [Google Scholar]
- Ozawa Y., Chopra I. J., Solomon D. H., Smith F. The role of sulfhydryl groups in thyrotropin binding and adenylate cyclase activities of thyroid plasma membranes. Endocrinology. 1979 Nov;105(5):1221–1225. doi: 10.1210/endo-105-5-1221. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Schleusener H., Kotulla P., Finke R., Sörje H., Meinhold H., Adlkofer F., Wenzel K. W. Relationship between thyroid status and Graves' disease-specific immunoglobulins. J Clin Endocrinol Metab. 1978 Aug;47(2):379–384. doi: 10.1210/jcem-47-2-379. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Binding of thyroid stimulators to thyroid membranes. FEBS Lett. 1974 Jun 15;42(3):301–304. doi: 10.1016/0014-5793(74)80751-9. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- Strakosch C. R., Wenzel B. E., Row V. V., Volpé R. Immunology of autoimmune thyroid diseases. N Engl J Med. 1982 Dec 9;307(24):1499–1507. doi: 10.1056/NEJM198212093072407. [DOI] [PubMed] [Google Scholar]
- Sugenoya A., Kidd A., Row V. V., Volpé R. Correlation between thyrotropin-displacing activity and human thyroid-stimulating activity by immunoglobulins from patients with Graves' disease and other thyroid disorders. J Clin Endocrinol Metab. 1979 Mar;48(3):398–402. doi: 10.1210/jcem-48-3-398. [DOI] [PubMed] [Google Scholar]
- Talal N. Autoimmunity and the immunologic network. Arthritis Rheum. 1978 Sep-Oct;21(7):853–861. doi: 10.1002/art.1780210719. [DOI] [PubMed] [Google Scholar]
- Tao T. W., Kriss J. P. Membrane-binding antibodies in patients with Graves' disease and other autoimmune diseases. J Clin Endocrinol Metab. 1982 Nov;55(5):935–940. doi: 10.1210/jcem-55-5-935. [DOI] [PubMed] [Google Scholar]
- Tate R. L., Holmes J. M., Kohn L. D., Winand R. J. Characteristics of a solubilized thyrotropin receptor from bovine thyroid plasma membranes. J Biol Chem. 1975 Aug 25;250(16):6527–6533. [PubMed] [Google Scholar]
- Teng C. S., Yeung R. T. Changes in thyroid-stimulating antibody activity in Graves' disease treated with antithyroid drug and its relationship to relapse: a prospective study. J Clin Endocrinol Metab. 1980 Jan;50(1):144–147. doi: 10.1210/jcem-50-1-144. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Yeung R. T., Khoo R. K., Alagaratnam T. T. A prospective study of the changes in thyrotropin binding inhibitory immunoglobulins in Graves' disease treated by subtotal thyroidectomy or radioactive iodine. J Clin Endocrinol Metab. 1980 Jun;50(6):1005–1010. doi: 10.1210/jcem-50-6-1005. [DOI] [PubMed] [Google Scholar]
- Weiss I., Davies T. F. Inhibition of immunoglobulin-secreting cells by antithyroid drugs. J Clin Endocrinol Metab. 1981 Dec;53(6):1223–1228. doi: 10.1210/jcem-53-6-1223. [DOI] [PubMed] [Google Scholar]
- Wood L. C., Ingbar S. H. Hypothyroidism as a late sequela in patient with Graves' disease treated with antithyroid agents. J Clin Invest. 1979 Nov;64(5):1429–1436. doi: 10.1172/JCI109601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M., Banovac K. Clinical significance of assay of thyroid-stimulating antibody in Graves' disease. Ann Intern Med. 1980 Jul;93(1):28–32. doi: 10.7326/0003-4819-93-1-28. [DOI] [PubMed] [Google Scholar]
- de Bruin T. W., Van der Heide D., Querido A. Thyrotrophin binding inhibition by anti-thyrotrophin receptor antibodies in Graves' disease which is not reflected by 1.6 M ammonium sulphate precipitates. Clin Endocrinol (Oxf) 1982 Jul 1;17(1):77–84. doi: 10.1111/j.1365-2265.1982.tb02636.x. [DOI] [PubMed] [Google Scholar]



