Abstract
Rosacea is a common skin disease with a high impact on quality of life. Characterized by erythema, edema, burning pain, immune infiltration, and facial skin fibrosis, rosacea has all the characteristics of neurogenic inflammation, a condition induced by sensory nerves via antidromically released neuromediators. To investigate the hypothesis of a central role of neural interactions in the pathophysiology, we analyzed molecular and morphological characteristics in the different subtypes of rosacea by immunohistochemistry, double immunofluorescence, morphometry, real-time PCR, and gene array analysis, and compared the findings with those for lupus erythematosus or healthy skin. Our results showed significantly dilated blood and lymphatic vessels. Signs of angiogenesis were only evident in phymatous rosacea. The number of mast cells and fibroblasts was increased in rosacea, already in subtypes in which fibrosis is not clinically apparent, indicating early activation. Sensory nerves were closely associated with blood vessels and mast cells, and were increased in erythematous rosacea. Gene array studies and qRT-PCR confirmed upregulation of genes involved in vasoregulation and neurogenic inflammation. Thus, dysregulation of mediators and receptors implicated in neurovascular and neuroimmune communication may be crucial at early stages of rosacea. Drugs that function on neurovascular and/or neuroimmune communication may be beneficial for the treatment of rosacea.
INTRODUCTION
Rosacea is a common chronic inflammatory skin disease primarily characterized by transient or persistent facial erythema, telangiectasia, papules, pustules and/or edema, and burning pain, possibly resulting in fibrotic, phymatous rosacea (Marks, 1989; Wilkin et al., 2002; Powell, 2005). As the etiology and pathogenesis remains uncertain, treatment mainly targets the symptoms instead of modulating the pathophysiological process (Crawford et al., 2004).
Four different subtypes have been defined based on typical clinical characteristics: erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PhR), and ocular rosacea (Wilkin et al., 2002).
Endogenous key factors of this complex pathogenic interplay—blood vessels, lymphatic vessels, fibroblasts (FBs), and cells of the immune system—have been identified (Marks, 1969; Jansen and Plewig, 1997; Aroni et al., 2004; Crawford et al., 2004; Gomaa et al., 2007). However, the underlying mechanisms of onset and maintenance of molecular and cellular alterations, and the connection between involved cells, are not yet understood (Crawford et al., 2004).
Several triggers that exacerbate rosacea have been identified: UV radiation, temperature changes (heat and cold), chemical irritation, strong emotions, alcoholic beverages, and spicy food (Jansen and Plewig, 1997), and probably microbial agents (Lacey et al., 2007; Whitfeld et al., 2011). Rosacea patients appear susceptible to certain banal stimuli. This modification of cutaneous sensitivity indicates the relevance of the sensory and/or autonomic nervous system in the pathogenesis of the disease (Steinhoff et al., 2011; Guzman-Sanchez et al., 2007). Interestingly, neurogenic inflammation, a condition evoked by released neuropeptides after stimulation of sensory nerve endings (Cevikbas et al., 2007), resembles clinical features of rosacea such as local erythema, edema, hyperemia, and recruitment of leukocytes to the site of inflammation. In addition, stimulation of mast cells (MCs) by neuropeptides and consecutive release of histamine, tryptase, and other mediators mediates inflammation, as well as itchy and/or burning sensations (Steinhoff et al., 2000; Arck et al., 2006; Ikoma et al., 2006).
With respect to sensory and autonomic nerves in this disease, one problem is that the RNA is harbored in ganglia and is therefore not detectable by skin biopsies. Another limiting factor is that the local concentration of released neuromediators is often under the detection limit of protein assays or immunohistochemistry. Therefore, little investigation in this aspect of rosacea has been made until now. For a comprehensive approach, we analyzed the neurovascular and neuroimmune alterations at different clinical stages of rosacea, both on the RNA and protein level by quantitative real-time RT-PCR (qRT-PCR), and by immunohistochemistry using markers for nerves, blood vessel, endothelial cells, lymphatic vessels, FBs, and MCs.
RESULTS
Anatomical association of sensory nerves, MCs, and blood vessels in rosacea
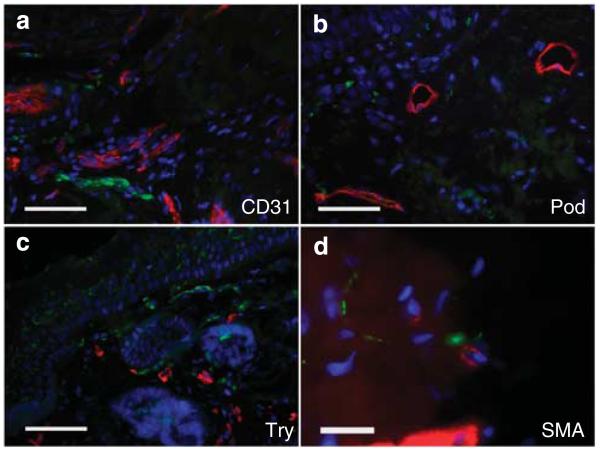
To determine the anatomical association of facial unmyelinated sensory nerves with the vascular and immune system (MCs) in rosacea, we performed double immunofluorescence (IF, Figure 1). A close colocalization was observed between PGP9.5-positive sensory nerves and blood vessels, as well as MCs. Some myofibroblasts were colocalized with free nerve endings. Lymphatic vessels were rarely colocalized with unmyelinated nerves.
Figure 1. Association of different vascular and immune structures with sensory nerves in human facial skin of rosacea patients, as shown by double immunofluorescence staining.
Colocalization of sensory nerves (PGP9.5) was determined in combination with CD31 for blood vessels (a), podoplanin (Pod) for lymphatic vessels (b), tryptase (Try) for mast cells (c), and smooth muscle actin (SMA) for myofibroblasts or blood vessels (d). Our data show a close anatomical association of unmyelinated nerves, especially with blood vessels and mast cells, and less with lymphatic vessels or myofibroblasts (bar = 300 μm; a–c and bar = 100 μm; d).
Marked vasodilatation, not angiogenesis, in rosacea
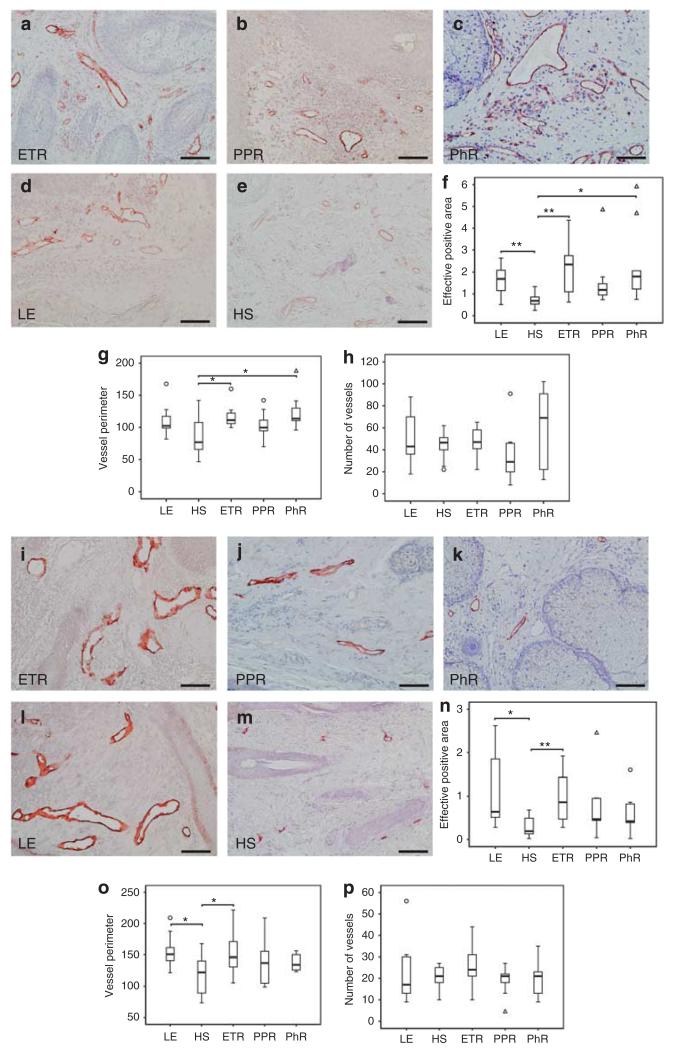
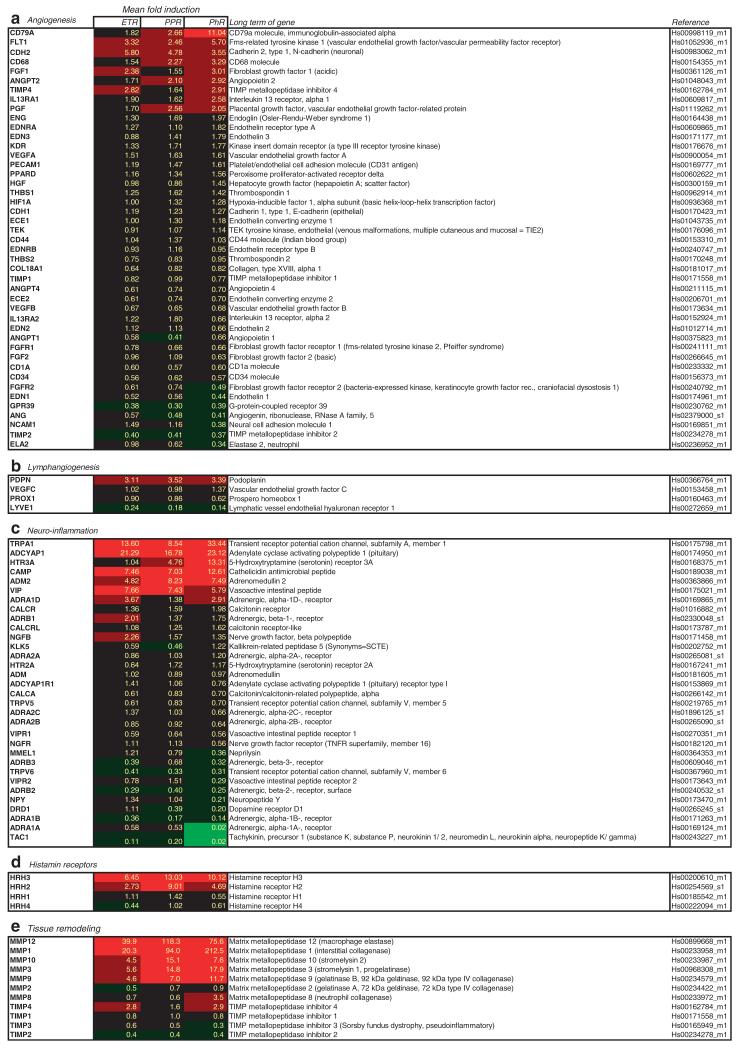
The aim of this study was to quantify vessel number and circumference in rosacea. Staining with CD31, a marker for blood vessel endothelium, showed grossly dilated vessels in all subtypes of rosacea (Figure 2a–h). Morphometrical analysis demonstrated statistically significant enhancement of CD31-positive tissue in ETR (P<0.01) and PhR (P<0.05), as well as perimeter enlargement (P<0.05). The number of vessels was not increased significantly when compared with healthy skin (HS). Accordingly, gene array studies combined with qRT-PCR revealed no upregulation of angiogenic key genes in ETR and PPR and only slight changes in PhR (Figure 6a).
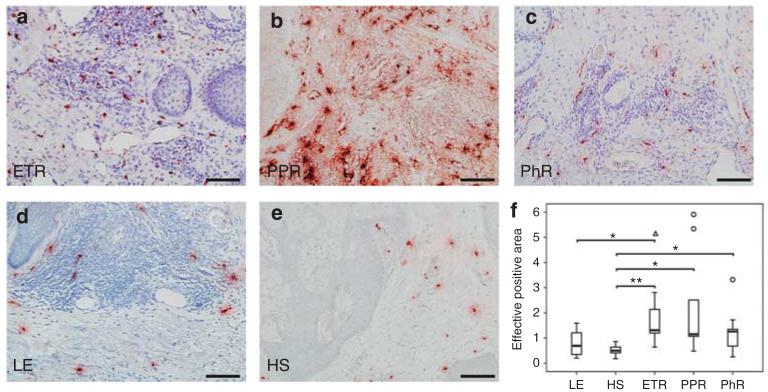
Figure 2. Density of blood and lymphatic vessels in human skin, as shown by immunohistochemistry and quantitative analysis of stained dermis.
Immunoreactivity for CD31 and podoplanin was observed in erythematous rosacea (ETR, n = 9), papulopustular rosacea (PPR, n = 9), phymatous rosacea (PhR, n = 9), lupus erythematosus (LE, n = 9), and healthy skin staining (HS, n = 10; bar = 100 μm; a–e and i–m). (f) PhR showed strongest statistically significant augmentation of CD31-positive tissue, followed by ETR (f–h; n–p; *P<0.05; **P<0.01). (g) Vessel perimeter measurement showed significant vasodilation in ETR and PhR, whereas the number of vessels (h) was not increased. A tendency toward angiogenesis was only observed in PhR. Augmentation in lymph vessel surface was statistically significant exclusively in ETR (n). Lymph vessel circumference measurements showed significant vasodilation in ETR (o). Number of lymphatic vessels was not increased in any subtype (p), unfilled triangles and circles represent outliers (f–h; n–p).
Figure 6. Detection of mRNA levels of genes relevant for neuroimmune and neurovascular interaction as determined by RT-PCR.
(a) Fold induction of genes involved in angiogenesis. Modulation of expression was detectable in phymatous rosacea (PhR, n = 6), but not in erythematous rosacea (ETR, n = 11) and papulopustular rosacea (PPR, n = 11). (b) Fold induction of genes involved in lymphangiogenesis. RT-PCR showed upregulation of podoplanin, but downregulation of LYVE1. (c) Fold induction of genes involved in neurovascular interaction. RT-PCR showed marked modulation of gene expression of different neuropeptides and their corresponding receptors in all subtypes. (d) Fold induction of histamine receptors. HRH3 was upregulated. (e) Fold induction of genes involved in tissue remodeling. RT-PCR shows evidence of a strong enhancement of matrix metalloproteinases (MMPs), whereas their inhibitors are downregulated.
Dilatation of lymphatic vessels, not lymphangiogenesis, in rosacea
The task was to determine the number and circumference of lymphatic vessels in rosacea. Staining with podoplanin, a marker for lymphatic vessels, showed vasodilation in all subtypes (Figure 2i–p). Morphometric analysis showed a statistically significant augmentation in vessel surface (P<0.01) and perimeter (P<0.05) exclusively in ETR. The number of vessels was not altered significantly in any subtype of rosacea. Investigation of lymphangiogenic key genes by gene array analysis and qRT-PCR revealed upregulation of podoplanin in all subtypes, whereas LYVE1 was down-regulated and VEGFC or PROX1 were unchanged when compared with HS (Figure 6b).
Slightly enhanced number of myelinated nerve fibers in subtypes of rosacea
We next sought to address the question of whether myelinated (neurofilament-positive, NF-200) and/or unmyelinated (PGP9.5-positive) nerve fibers are anatomically closely associated with other skin structures in rosacea skin in comparison with lupus erythematosus (LE) and HS (Figure 3). Unfortunately, PGP staining is hard to interpret by light microscopy because of the thin, irregular positive staining under these conditions. Here, IF is the better technique. Therefore, we first determined whether myelinated nerves, which are ultimately involved in pain transmission, are found in increased numbers in rosacea patients. Double IF results for unmyelinated nerves (PGP9.5) were analyzed qualitatively and showed close anatomic association (Figure 1a–d, other data not shown). Staining with NF-200 showed a higher density of neural structures, especially in the upper dermis (Figure 3). Morphometrical analysis demonstrated an increase of nerves, especially in ETR, but not PPR and PhR. Although clear differences in nerve numbers were observed between rosacea subtypes and HS, the data did not reach statistical significance.
Figure 3. Localization and density of myelinated sensory nerves (NF200) in human skin, as shown by immunohistochemistry and quantitative analysis of stained dermis.
Immunoreactivity for neurofilament was observed in erythematous rosacea (ETR, n = 9), papulopustular rosacea (PPR; n = 9), phymatous rosacea (PhR; n = 9), lupus erythematosus (LE; n = 9), and healthy skin (HS; n = 10; bar = 100 μm; a–e). There was a marked but not statistically significant increase of nerves in ETR (× 2.34) followed by a gradual decrease. Increase of neurofilament-positive nerves was comparable in PPR and LE (unfilled circle represents outlier) (f).
Our analysis of expression levels of genes involved in neurovascular and neuroimmune interactions revealed upregulation in pituitary adenylate cyclase-activating polypeptide (PACAP), vasoactive intestinal peptide (VIP), HTR3A (5-hydroxytryptamine (serotonin) receptor 3A), nerve growth factor beta, adrenergic, alpha-1D-, receptor (ADRA1D), adrenomedullin 2, and cathelicidin antimicrobial peptide (Figure 6c). Downregulation was detected in VIP receptor-1, PACAP receptor-1, nerve growth factor receptor (trkA), kallikrein-related peptidase-5, diverse adrenergic receptors (ADRB2/3, ADRA2C/1B), neuropeptide Y, and tachykinin, precursor 1/substance P.
Increased MC numbers in all subtypes of rosacea
MCs are functionally closely associated with both blood vessels and nerves, and altogether form a so-called microvascular unit (Steinhoff et al., 2003). We sought to determine the number of MCs in the various subtypes of rosacea, and to analyze the potential increase of MC associated with vascular structures, nerves, and FBs (Figure 4).
Figure 4. Localization and density of mast cells in human facial skin as shown by immunohistochemistry and quantitative analysis of stained dermis.
Immunoreactivity for tryptase was observed in erythematous rosacea (ETR, n = 9), papulopustular rosacea (PPR, n = 9), phymatous rosacea (PhR, n = 9),lupus erythematosus (LE; n = 9), and healthy skin (HS, n = 10; bar = 100 μm; a–e). The increase in mast cell density was statistically significant for all subtypes (ETR × 3.98; PPR × 4.41; PhR × 2.54), whereas mast cell density did not increase in LE (*P<0.05, **P<0.01; unfilled circles and triangle represent outliers) (f).
Quantitative analysis of tryptase staining revealed a statistically significant increased density of MC in all subtypes of rosacea, especially in PPR (P<0.05) followed by ETR (P<0.01) and PhR (P<0.05).
To analyze the genes associated with MC function, we investigated the mRNA expression levels of histamine receptors (HRH1–4; Figure 6d). Our gene array data showed upregulation of HRH2 and HRH3. Gene expression of HRH1 was not altered, and HRH4 was downregulated.
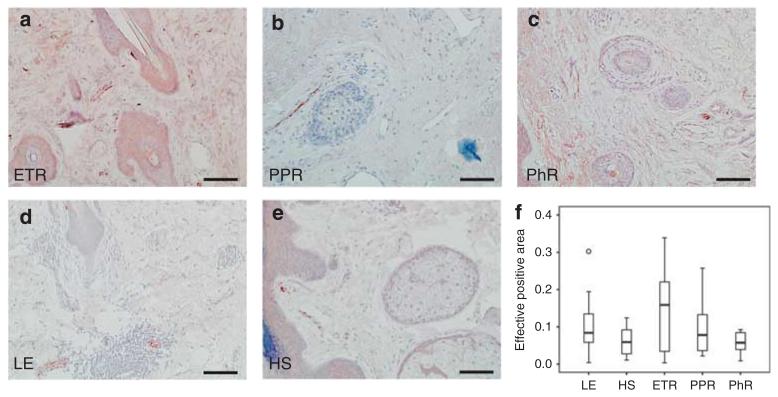
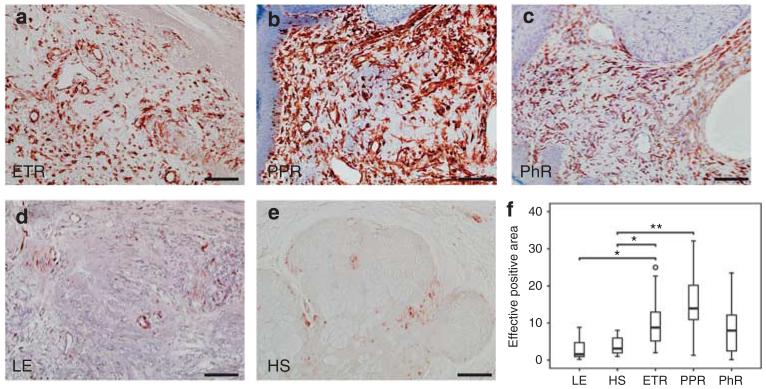
Increased staining of Vimentin-positive cells in rosacea
Fibrocytes/blasts have an essential role in the induction of skin fibrosis. Quantitative analysis of vimentin staining, a marker for fibrocytes/blasts and mesenchymal structures of blood vessels, demonstrated a statistically significant higher density of FB in PPR (P<0.01), followed by ETR (P<0.05) as compared with controls (Figure 5). Accordingly, qRT-PCR showed strong upregulation of genes involved in matrix remodeling as well, especially in PPR and PhR. Upregulation of matrix metalloproteinase (MMP)-1 and -12 was strongest, followed by upregulation of MMP-10, -3, and -9. All MMP inhibitors were downregulated at the RNA level (Figure 6e).
Figure 5. Localization and density of fibroblasts (FBs)/cytes and mesenchymal structures of blood vessels in human skin, as shown by immunohistochemistry and quantitative analysis of stained dermis.
Immunoreactivity for vimentin was observed in erythematous rosacea (ETR, n = 9), papulopustular rosacea(PPR, n = 9), phymatous rosacea (PhR, n = 9), lupus erythematosus (LE; n = 9), and healthy skin (HS; n = 10; bar = 100 μm; a–e). Density of FBs was increased in all subtypes, but significantly so in ETR (× 2.9) and PPR (× 3.94). Skin of lupus patients showed decreased density of FBs/cytes as compared with healthy human skin (*P<0.05; **P<0.01; unfilled circle represents outlier) (f).
DISCUSSION
The involvement of neuroimmune and neurovascular communication in the pathophysiology of rosacea is currently indicated only by clinical observation. Therefore, we examined the morphological and molecular correlation of various cells involved in the pathophysiology of rosacea and their specific pattern in the various subtypes of rosacea as compared with LE and HS. Our results show a close anatomic association of sensory nerves, blood vessels, and immune cells, as well as signs of neuroimmune and neurovascular communication such as vasodilation, rather than angiogenesis, dilated lymphatic vessels, and a strong increase in MC and FB numbers. In accordance with that finding, our qRT-PCR data demonstrate upregulation of receptors that are targets for mediators released by MC or sensory nerve endings. Taken together, our results strongly suggest substantial neurovascular and neuroimmune interaction in the pathophysiology of rosacea. In comparison with rosacea, LE also showed increased vasodilatation of blood vessels and lymphatic vessels, but no increase in nerves, MC, or FB numbers.
Our findings of significant vasodilatation in all subtypes of rosacea correlate well with symptoms such as flushing and erythema. Some authors proclaimed that angiogenesis has an important role in the pathogenesis, and suggested that the increase in vascular tissue, in particular, was due to this (Aroni et al., 2008; Gomaa et al., 2007). Morphologically, we could not find an increase in the vessel number in any subtype, whereas significant vasodilation was obvious. This finding correlates well with our gene array and qRT-PCR data, in which expression of angiogenic key genes was rarely modulated. PhR showed slight upregulation as well as downregulation of angiogenic genes as a sign of increased tissue remodeling.
The mechanism(s) that induce rapid flushing and erythema in rosacea are still unknown (Sobottka and Lehmann, 2009). Despite the prominent telangiectasia, blood vessels maintain their ability to respond to vasoactive stimuli (Guarrera et al., 1982), suggesting that changes are not structural or due to irreversible damage. However, our molecular investigation indicates a marked upregulation of genes that are involved in vasodilatation. Thus, sensory nerves may induce vasodilatation by activating high-affinity receptors for vasoregulatory neuropeptides on endothelial cells and/or smooth muscle cells surrounding vessels. In LE, lymphatic vessels were extremely dilated, whereas rapid flushing such as in rosacea is rarely observed in LE. This may explain the differences with respect to the strong neurovascular association in early rosacea but not in later subtypes and LE.
Recently, the lymphatic system has attracted growing interest as an important contributor during chronic inflammation (Huggenberger et al., 2010). Our results suggest that lymphatic vessels are already involved in the initiation process of rosacea but not in later subtypes, although clinically visible signs of edema are described at later stages (Crawford et al., 2004). Although the early involvement of lymphatic tissue was suggested before, augmentation of lymphatic tissue was previously attributed mainly to lymphangiogenesis (Gomaa et al., 2007). Our morphometric results show no enhancement in vessel number when compared with HS. Furthermore, our RT-PCR results showed that most of the genes involved in growth and elongation of lymphatic capillaries were only slightly or not at all upregulated. LYVE1, a gene having a key role in metabolism, binding, and transport of hyaluronic acid from tissues to lymphatic vessels and in transplacement of leukocytes in lymphatic vessels and lymph nodes (Jackson, 2009), was even downregulated (Figure 6b).
Little data exist about a possible influence of the nervous system on lymph vascular tissue. Some studies showed that neuropeptides affect the function of lymphatic vessels. For example, substance P induces the reduction in diastolic and systolic vessel diameter, stroke volume, and increase in contraction frequency (Amerini et al., 2004), and calcitonin gene-related peptide dose dependently inhibits the vasomotion of lymph vessels (Hosaka et al., 2006). Which of the neuropeptides involved in rosacea pathophysiology affect lymphatic function has to be clarified in detailed studies.
A recent theory that received greater emphasis in the pathophysiology of rosacea suggests the involvement of mechanisms of neurogenic inflammation, which may reflect the early and late clinical features of the disease including flushing, erythema, and induction of leukocyte infiltration, especially of MCs (Steinhoff et al., 2003; Roosterman et al., 2006; Reich et al., 2010). Although statistically not significant, we found a clear tendency of increased nerve density, especially in ETR, followed by a decrease in PhR. Reflecting those morphometrical findings, sensations such as “pricking, burning, or pain” are predominantly known in ETR and PPR, followed by less sensation in PhR (Crawford et al., 2004; Sobottka and Lehmann, 2009).
To further investigate the role of sensory nerves in the context of intercellular communication, we performed double IF staining, which showed a close anatomical association of sensory nerves, especially with blood vessels and MCs. Pathways of neurovascular and neuroimmune interactions in the pathophysiology of rosacea are still unknown; therefore, we analyzed gene expression levels of neuromediators and neuroreceptors at the RNA level. Note that these genes must be expressed by non-neuronal cells, because the neuronal genes are located in the dorsal root ganglia.
So far, suggested communicating neuropeptides involved in rosacea include calcitonin gene-related peptide and substance P (tachykinin, precursor 1; Powell et al., 1993; Lonne-Rahm et al., 2004). Unexpectedly, neither of these neuropeptides nor their receptors was upregulated at the RNA level, but were rather downregulated (Figure 6c). According to our results, their relevance in rosacea is rather marginal. In contrast, we recognized some new molecular pathways, which we do not believe to have been linked to the pathophysiology of rosacea until now.
Gene analysis showed upregulation of serotonin receptor HTR3A. Serotonin (5-hydroxytryptamine) is an important inflammatory and neurosensory mediator that is released from platelets and MCs, thereby contributing to nociception and vasoregulation (Oliveira et al., 2007). Expressed by primary afferent nerve fibers, HTR3A conveys their excitation and sensitization. 5-Hydroxytryptamine and the responding receptors have already been investigated for other inflammatory skin diseases (Nordlind et al., 2006), but, to our knowledge, contribution of 5-hydroxytryptamine in the pathophysiology of rosacea has not been studied. It has been reported that HTR3A antagonist Ondansetron led to good remissions of cutaneous symptoms in rosacea patients (Wollina, 1999). Thus, HTR3A antagonization may be a promising future therapeutic approach.
Cathelicidin antimicrobial peptide, a recently described antimicrobial peptide, was upregulated in all subtypes. Processed by kallikrein-5, cathelicidin antimicrobial peptide has proinflammatory activity, and promotes angiogenesis and chemotaxis. Yamasaki et al, (2007, 2011) suggest cathelicidin antimicrobial peptide to be one factor eliciting an exacerbated response of the innate immune system (Morizane et al., 2010).
Moreover, our molecular studies demonstrated that vasoactive neuropeptides such as PACAP, VIP, or adrenomedullin (ADR2) were significantly enhanced. PACAP has already been linked to the pathophysiology of psoriasis and atopic dermatitis, where it may mediate vasodilatation and plasma extravasation, and influence neurogenic inflammation via activation of VPAC receptors (Steinhoff et al., 1999; Seeliger et al., 2010). Both PACAP and VIP are capable of stimulating MC degranulation (Peters et al., 2006; Lenti et al., 2009). Upon stimulation, neuromediators such as PACAP can also be released by endothelial cells under inflammatory conditions, suggesting an important role of PACAP, VIP, or transient receptor potential channels, as well as adrenergic receptors, in rosacea pathophysiology.
MCs have long been known to be key effector cells in neurogenic inflammation, immune defense, and fibrosis (Metz and Maurer, 2007). A recent study discussed the important role of MCs in rosacea’s evolution to a chronic stage (Aroni et al., 2008).
Our results clearly indicate a marked upregulation of MC density in all subtypes of rosacea, with the greatest increase in PPR. In LE, MCs were not significantly increased compared with MCs in HS, which indicates that the high density of MCs in rosacea is more than a sign of skin inflammation in general.
Being activated by neuropeptides such as PACAP or VIP, MCs could be important interconnecters amplifying the neural impulses and conveying them via histamine or tryptase release to vasculature and immune cells (Steinhoff et al., 2003). Our morphometric and gene analysis data, however, indicate a strong interaction between nerves and MCs. HRH2, a receptor that mediates vasodilatory effects of histamine, was upregulated in rosacea, especially in PhR, when, according to our immunohistochemical findings, vasodilatation is most impressive. In addition, we observed a positive correlation between the increase of MCs and FBs in dermal structures of rosacea tissues, suggesting a strong interaction, e.g., by MC tryptase, which is known to have a chemotactic and mitotic effect on FBs (Gruber, 2003). Recent studies show that MC/FB communication appears to be involved in skin fibrosis (Monument et al., 2010).
Skin fibrosis and phymatous changes are characteristics of late-stage rosacea. In our study, we observed a significantly higher distribution of vimentin-positive cells in rosacea patients, with a maximum in PPR patients. This finding is consistent with clinical symptoms such as phymatic changes and previous histological findings (Jansen and Plewig, 1997). Activation of FB seems to be a specific feature in rosacea, as number of FBs is significantly different from that found in LE. Clinically, the skin in ETR is described to be fine in texture without obvious signs of fibrosis and fibrotic chances occur particularly in PhR (Crawford et al., 2004). Unexpectedly, however, according to our data FB occurrence in skin is vice versa predominantly in ETR and PPR. This observation leads us to conclude that fibrotic processes in rosacea start much earlier than is expected on the basis of clinical observations.
Our qRT-PCR experiments showed massive upregulation of MMP, especially in PPR and PhR, indicating that a strong process of remodeling is taking place. MMP inhibitors were found rather downregulated (Figure 6e). In addition to their involvement in tissue destruction and fibrosis, MMPs have recently been found to be involved in angiogenesis and apoptosis (Amălinei et al., 2010). In rosacea, MMP-9 and -2 are known to be involved in the development of neuropathic pain (Kawasaki et al., 2008) and fibrotic processes. Taken together, morphometrical and molecular results, effective inhibition of MMP, e.g., by tetracyclines (Gu et al., 2010; Lipowsky et al., 2011), may provide a novel therapeutic approach for the treatment of fibrosis and pain (stinging and burning) associated with rosacea. The unexpected early detection of FB activation is very important with respect to timing rosacea treatment. Here, detailed studies using various markers of the different FB differentiation stages are necessary to further dissect the role of FB activation.
In summary, our combined morphological and molecular study indicates a meaningful role of neurovascular and neuroimmune networks in the development of rosacea. We demonstrated, both at the histological and molecular levels, that neurogenic inflammation is an important part of the pathophysiology, resulting in vasodilation, but not in angiogenesis, and contributes to the fibrotic processes observed in this chronic inflammatory disease. Furthermore, our study detected some promising pathways of conduction, which remain to be clarified and validated in detailed studies. To gradually get to know the different involved parameters and interaction pathways opens a wide range of new and finally cause-related pharmacological targets for therapeutic intervention.
MATERIALS AND METHODS
For detailed descriptions see Supplementary Material online. The antibodies used are listed in Supplementary Material S1 online. The ready-to-use TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) are listed in Figure 6.
Skin collection
All facial skin material was obtained from previously taken diagnostic biopsies (Rosacea, Lupus) and plastic surgery (HS) in the Department of Dermatology, University Hospital Münster, Germany. The clinical diagnosis of rosacea subtypes was performed according to the classification system of the National Rosacea Society (Wilkin et al., 2002). On the basis of that, we investigated five different groups of patients: for morphometric stainings ETR (n = 9), PPR (n = 9), PhR (n = 9), HS (n = 10), LE (n = 9); and for gene analytic studies we investigated the following groups: ETR (n = 11), PPR (n = 11), PhR (n = 6), HS (n = 12, face). Patients were informed about the possible use of tissue leftover from surgery for investigation, and gave their written consent. Permission for human studies was given by the Ethical Committee of the University of Münster Germany, in accordance with the ethical standards of the 1964 Declaration of Helsinki Principles.
Double immunofluorescence
Histological staining with double IF was completed according to the standard protocol (Collins et al., 2002). Frozen sections of skin samples were processed for double IF staining. Blocking was performed with Target Retrieval Buffer, pH 6.1 (S1699, DAKO, Hamburg, Germany) at 90 °C for 40 minutes. Antibody pools were incubated overnight at 4 °C. After secondary antibodies (1:400) were washed, they were applied for 60 minutes at room temperature.
Immunohistochemical analysis
Immunohistochemistry was performed as described (Rattenholl et al., 2007). Paraffin sections of skin samples were deparaffinized and processed for immunohistochemical staining. Blocking was performed according to the specific characteristics of the different markers. Antibody pools were incubated for 1 hour. After secondary antibodies were washed, they were applied for 30 minutes at room temperature.
Image analysis
Using × 200 magnification, we took five pictures within each section, moving from epidermis to dermis. The positive stained area of the dermis was analyzed quantitatively by using specific image analysis software (Cell D 2.6 (Build 1200), Olympus Soft Imaging Solutions GmbH; Münster, Germany).
Quantitative RT-PCR
qRT-PCR was performed as previously described (Ständer et al., 2004). In short, mRNA expression was evaluated using semiquantitative PCR technology (qRT-PCR–Taqman Low Density Arrays). After extraction of total RNA, cDNA was synthesized using high-capacity cDNA archive kits (Applied Biosystems). Gene expression analysis was performed using TLDA arrays containing PCR primers for genes of interest and housekeeping genes. Synthesized cDNA (50 ng of cDNA per column) was added to the PCR master mix, and PCR reactions were performed on ABI 7900HT (Applied Biosystems).
Statistical analysis
Statistical analyses for morphometry were performed using SPSS for Windows, version 17.0 (SPSS, Chicago, IL). Statistical significance was determined by using t-tests. Differences were considered significant at a P-value ≤0.05.
The fold modulation of gene expression of rosacea samples versus samples of healthy volunteers was defined as 2(mean CtHV − mean CtRo), with CtHV and CtRo depicting the Ct values of healthy volunteer and rosacea samples, respectively.
To identify gene modulation between rosacea subtypes, one-way analysis of variance with Benjamini–Hochberg multiplicity correction was performed using JMP 7.0.1 (SAS Institute, Cary, NC) and irMF 3.5 (National Institute of Statistical Sciences, NISS, Triangle Park, NC) software.
Supplementary Material
ACKNOWLEDGMENTS
The skillful technical help of Heike Hinte, Andrea Poppe, Karin Baer, Pascale Reiniche, Luigi Russo and Sandy Wise is gratefully acknowledged. This work was supported by the National Rosacea Society, West Haven Foundation (UCSF), DFG 1014/2-2, SFB 492 (to MS), DFG Ce165/1-1 (to FC).
Abbreviations
- ETR
erythematous rosacea
- FB
fibroblast
- HS
healthy skin
- HTR3A
5-hydroxytryptamine (serotonin) receptor 3A
- LE
lupus erythematosus
- MC
mast cell
- MMP
matrix metalloproteinase
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PhR
phymatous rosacea
- PPR
papulopustular rosacea
- qRT-PCR
quantitative real-time RT-PCR
- SP
substance P
- VIP
vasoactive intestinal peptide
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
This article was published as part of a supplement sponsored by Galderma.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Amălinei C, Căruntu ID, Giuşcă SE, et al. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51:215–28. [PubMed] [Google Scholar]
- Amerini S, Ziche M, Greiner ST, et al. Effects of substance P on mesenteric lymphatic contractility in the rat. Lymphat Res Biol. 2004;2:2–10. doi: 10.1089/1539685041690409. [DOI] [PubMed] [Google Scholar]
- Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroni K, Tsagroni E, Kavantzas N, et al. A study of the pathogenesis of rosacea: how angiogenesis and mast cells may participate in a complex multifactorial process. Arch Dermatol Res. 2008;300:125–31. doi: 10.1007/s00403-007-0816-z. [DOI] [PubMed] [Google Scholar]
- Aroni K, Tsagroni E, Lazaris AC, et al. Rosacea: a clinicopathological approach. Dermatology. 2004;209:177–82. doi: 10.1159/000079886. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Steinhoff A, Homey B, et al. Neuroimmune interactions in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2007;7 doi: 10.1097/ACI.0b013e3282a644d2. [DOI] [PubMed] [Google Scholar]
- Collins AB, Colvin RB, Nousari HC, et al. Immunofluorescence methods for diagnosis of renal and skin diseases. In: Rose NR, Hamilton RG, Detrick B, editors. Manual of Clinical Laboratory Immunology. 6th ed American Society of Microbiology Press; Washington, DC: 2002. pp. 393–402. [Google Scholar]
- Crawford G, Pelle M, James W. Rosacea. I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327–41. doi: 10.1016/j.jaad.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Gomaa AH, Yaar M, Eyada MM, et al. Lymphangiogenesis and angiogenesis in non-phymatous rosacea. J Cutan Pathol. 2007;34 doi: 10.1111/j.1600-0560.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- Gruber BL. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5:147–53. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- Gu Y, Lee HM, Sorsa T, et al. Doxycycline [corrected] inhibits mononuclear cell-mediated connective tissue breakdown. FEMS Immunol Med Microbiol 58:218-25. FEMS Immunol Med Microbiol. 2010;2010;59(1):117. doi: 10.1111/j.1574-695X.2009.00625.x. Published erratum appears in. [DOI] [PubMed] [Google Scholar]
- Guarrera M, Parodi A, Cipriani C, et al. Flushing in rosacea: a possible mechanism. Arch Dermatol Res. 1982;272:311–6. doi: 10.1007/BF00509061. [DOI] [PubMed] [Google Scholar]
- Guzman-Sanchez DA, Ishiuji Y, Patel T, et al. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800–5. doi: 10.1016/j.jaad.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Hosaka K, Rayner SE, von der Weid PY, et al. Calcitonin gene-related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am J Physiol Heart Circ Physiol. 2006;290:H813–22. doi: 10.1152/ajpheart.00543.2005. [DOI] [PubMed] [Google Scholar]
- Huggenberger R, Ullmann S, Proulx ST, et al. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. Exp Med. 2010;207:2255–69. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, et al. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–47. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230:216–31. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144–50. doi: 10.1177/014107689709000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157 doi: 10.1111/j.1365-2133.2007.08028.x. [DOI] [PubMed] [Google Scholar]
- Lenti L, Zimmermann A, Kis D, et al. PACAP and VIP differentially preserve neurovascular reactivity after global cerebral ischemia in newborn pigs. Brain Res. 2009;1283:50–7. doi: 10.1016/j.brainres.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky HH, Sah R, Lescanic A. Relative roles of doxycycline and cation chelation in endothelial glycan shedding and adhesion of leukocytes. Am J Physiol Heart Circ Physiol. 2011;300:H415–22. doi: 10.1152/ajpheart.00923.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonne-Rahm S, Nordlind K, Edström DW, et al. Laser treatment of rosacea: a pathoetiological study. Arch Dermatol. 2004;140:1345–9. doi: 10.1001/archderm.140.11.1345. [DOI] [PubMed] [Google Scholar]
- Marks R, Harcourt-Webster JN. Histopathology of rosacea. Arch Dermatol. 1969;100:683–91. [PubMed] [Google Scholar]
- Marks R. Rosacea: hopeless hypotheses, marvellous myths, and dermal disorganization. In: Marks R, Plewig G, editors. Acne and Related Disorders. Martin Dunitz; London: 1989. pp. 293–9. [Google Scholar]
- Metz M, Maurer M. Mast cells—key effector cells in immune responses. Trends Immunol. 2007;28:234–41. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Monument M, Hart D, Befus A, et al. The mast cell stabilizer ketotifen fumarate lessens contracture severity and myofibroblast hyperplasia: a study of a rabbit model of posttraumatic joint contractures. Bone Joint Surg Am. 2010;92:1468–77. doi: 10.2106/JBJS.I.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane S, Yamasaki K, Kabigting FD, et al. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010;130:1297–306. doi: 10.1038/jid.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlind K, Thorslund K, Lonne-Rahm S, et al. Expression of serotonergic receptors in psoriatic skin. Arch Dermatol Res. 2006;298:99–106. doi: 10.1007/s00403-006-0652-6. [DOI] [PubMed] [Google Scholar]
- Oliveira MC, Pelegrini-da-Silva A, Parada CA, et al. 5-HT acts on nociceptive primary afferents through an indirect mechanism to induce hyperalgesia in the subcutaneous tissue. Neuroscience. 2007;145:708–14. doi: 10.1016/j.neuroscience.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Peters EM, Ericson ME, Hosoi J, et al. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol. 2006;126:1937–47. doi: 10.1038/sj.jid.5700429. [DOI] [PubMed] [Google Scholar]
- Powell F, Corbally N, Powell D. Substance P and rosacea. J Am Acad Dermatol. 1993;28:132–3. doi: 10.1016/s0190-9622(08)80863-8. [DOI] [PubMed] [Google Scholar]
- Powell FC. Clinical practice. Rosacea. N Engl J Med. 2005;352:793–803. doi: 10.1056/NEJMcp042829. [DOI] [PubMed] [Google Scholar]
- Rattenholl A, Seeliger S, Buddenkotte J, et al. Proteinase-activated receptor-2 (PAR2): a tumor suppressor in skin carcinogenesis. J Invest Dermatol. 2007;127:2245–52. doi: 10.1038/sj.jid.5700847. [DOI] [PubMed] [Google Scholar]
- Reich A, Wójcik-Maciejewicz A, Slominski AT. Stress and the skin. G Ital Dermatol Venereol. 2010;145:213–9. [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, et al. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- Seeliger S, Buddenkotte J, Schmidt-Choudhury A, et al. Pituitary adenylate cyclase activating polypeptide: an important vascular regulator in human skin in vivo. Am J Pathol. 2010;177:2563–75. doi: 10.2353/ajpath.2010.090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobottka A, Lehmann P. Rosacea 2009: new advances in pathophysiology, clinical staging and therapeutic strategies. Hautarzt. 2009;60:999–1009. doi: 10.1007/s00105-009-1825-y. [DOI] [PubMed] [Google Scholar]
- Ständer S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–39. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, McGregor GP, Radleff-Schlimme A, et al. Identification of pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP type 1 receptor in human skin: expression of PACAP-38 is increased in patients with psoriasis. Regul Pept. 1999;80:49–55. doi: 10.1016/s0167-0115(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2–11. doi: 10.1038/jidsymp.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Ständer S, Seeliger S, et al. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–88. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–8. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Whitfeld M, Gunasingam N, Leow LJ, et al. Staphylococcus epidermidis: a possible role in the pustules of rosacea. J Am Acad Dermatol. 2011;64:49–52. doi: 10.1016/j.jaad.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584–7. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- Wollina U. The response of erythematous rosacea to ondansetron. Br J Dermatol. 1999;140:561–2. doi: 10.1046/j.1365-2133.1999.02744.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688–97. doi: 10.1038/jid.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.