Abstract
Taste buds are the transducing endorgans of gustation. Each taste bud comprises 50–100 elongated cells, which extend from the basal lamina to the surface of the tongue, where their apical microvilli encounter taste stimuli in the oral cavity. Salts and acids utilize apically located ion channels for transduction, while bitter, sweet and umami (glutamate) stimuli utilize G protein coupled receptors (GPCRs) and second messenger signaling mechanisms. This review will focus on GPCR signaling mechanisms. Two classes of taste GPCRs have been identified, the T1Rs for sweet and umami (glutamate) stimuli, and the T2Rs for bitter stimuli. These low affinity GPCRs all couple to the same downstream signaling effectors that include Gβγ activation of PLCβ2, IP3-mediated release of Ca2+ from intracellular stores, and Ca2+-dependent activation of the monovalent selective cation channel, TrpM5. These events lead to membrane depolarization, action potentials, and release of ATP as a transmitter to activate gustatory afferents. The Gα subunit, α-gustducin, activates a phosphodiesterase to decrease intracellular cAMP levels, although the precise targets of cAMP have not been identified. With the molecular identification of the taste GPCRs, it has become clear that taste signaling is not limited to taste buds, but occurs in many cell types of the airways. These include solitary chemosensory cells, ciliated epithelial cells, and smooth muscle cells. Bitter receptors are most abundantly expressed in the airways, where they respond to irritating chemicals and promote protective airway reflexes, utilizing the same downstream signaling effectors as taste cells.
Keywords: GPCR signaling, taste cell, solitary chemosensory cell, gustducin, TrpM5, purinergic signaling
Introduction
Taste buds are the transducing elements of gustatory sensation. Housed in connective papillae on the tongue and scattered throughout the epithelium of the soft palate and larynx, these onion shaped end organs detect nutrients in foods and guard against ingestion of toxic substances and spoiled foods. Unlike the olfactory system, which can discriminate thousands of individual chemicals, the gustatory system can discriminate only 5 basic taste qualities-- sweet, sour, salty, bitter, and umami (the taste of glutamate and other L-amino acids). The appetitive taste qualities are sweet, for detection of sugars and sweeteners; salty, primarily for detection of Na+; and umami, for detection of L-amino acids—each required by the body for energy balance, ionic homeostasis, or building proteins. The aversive qualities are sour, which detects acids in unripe fruit and spoiled foods, and bitter, which detects a variety of plant alkaloids, many of which are toxic. Thus, detection of aversive tastes guards the entrance of the alimentary canal against the ingestion of potential toxins.
Because taste stimuli represent both ionic and complex compounds, different mechanisms have evolved for their detection. Salts and acids are detected primarily by apically-located ion channels, while chemicals that elicit bitter, sweet, and umami tastes are detected by G protein-coupled receptors (GPCRs) and 2nd messenger signaling pathways. In keeping with the theme of the symposium, this review will focus on GPCR signaling mechanisms. With the molecular identification of the taste receptors and their signaling effectors, it is now clear that taste receptor signaling is not limited to taste buds but occurs in a variety of tissues, including chemosensory cells of the alimentary tract (see Iwatsuki et al., this volume), pancreas (Nakagawa et al., 2009), brain (Ren et al., 2009, Singh et al., 2011), and several cell types of the airway epithelium (Finger et al., 2003, Tizzano et al., 2010, Shah et al., 2009, Deshpande et al., 2010). In all cases, these receptors detect similar compounds using similar signaling effectors, but elicit very different effects in the different tissues. This review will focus on taste receptor signaling in taste buds and airway epithelia, where these processes have been most extensively studied.
Taste buds, cell types, and innervation
Taste buds are onion shaped aggregates of approximately 50–100 elongate cells that extend from the basal lamina to the surface of the tongue, where their apical microvilli extend through an opening in the epithelium to contact sapid chemicals in the oral cavity. Each taste bud contains 3 types of elongate taste cells and a renewing population of basal cells (Figure 1). Type II, or “receptor” cells are the focus of this review, as they contain the G protein-coupled receptors (GPCRs) and downstream signaling effectors for bitter, sweet, and umami taste stimuli (Clapp et al., 2004, DeFazio et al., 2006). Interestingly, Type II cells lack voltage-gated Ca2+ channels and other presynaptic specializations, although they associate closely with afferent nerve processes (Clapp et al., 2006). Recent data have shown that these cells release ATP as a transmitter, which activates P2X receptors on afferent nerve fibers (Finger et al., 2005) and P2Y receptors on adjacent taste cells (Huang et al., 2009, Hayato et al., 2007). Type I, or “glial-like” cells are believed to play primarily a support function in the taste bud, as their membranes wrap around other cells in a glial like fashion and they express enzymes for the degradation of the ATP released from the Type II cells (Bartel et al., 2006). Type I cells also express the epithelial Na+ channel ENaC (Vandenbeuch et al., 2008), so they may also play a role in the transduction of salt taste. Type III or “presynaptic” cells transduce sour (acidic) stimuli (Huang et al., 2006, Huang et al., 2008b) and also respond to ATP released from receptor cells (Huang et al., 2009). These cells contain and release several transmitters, including serotonin (Huang et al., 2005), norepineprine (Huang et al., 2008a), and possibly GABA (DeFazio et al., 2006, Starostik et al., 2010). Serotonin appears to modulate release of ATP from Type II cells (Huang et al., 2009), but the role of the other transmitters is not yet clear. In addition to modulating adjacent taste cells, Type III cells are the only cells in the taste bud to make conventional synapses with sensory afferents (Murray and Murray, 1971, Royer and Kinnamon, 1991). However, the role of these transmitters in activating nerve fibers is unclear, since the double knockout of P2X2 and P2X3 eliminates all taste-evoked neural activity (Finger et al., 2005).
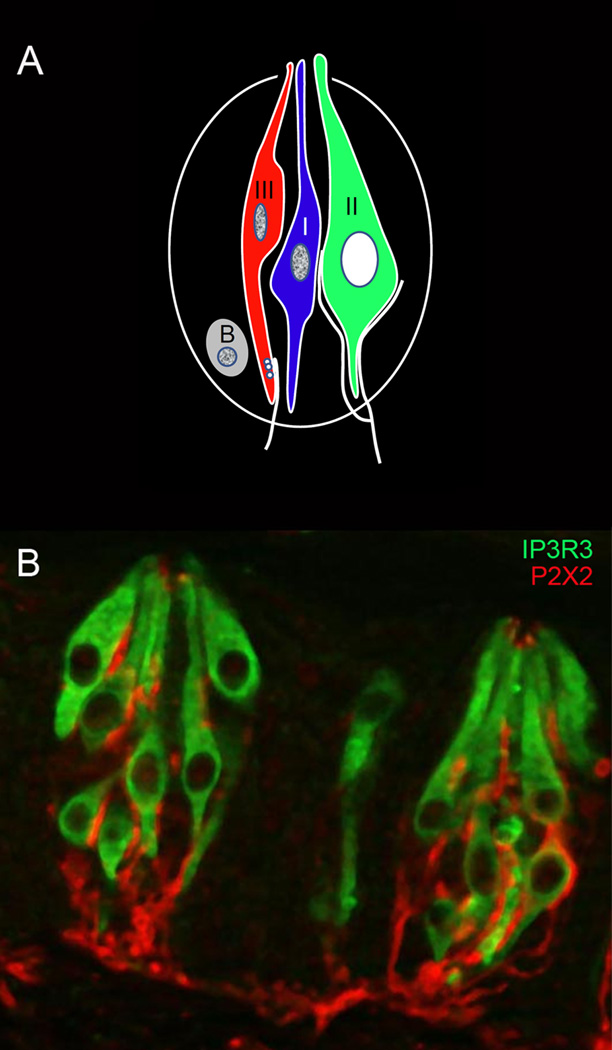
Figure 1.
A. Diagrammatic illustration of a taste bud, showing the 3 types of taste cells and a renewing population of basal (B) cells. Type II cells contain the GPCRs signaling effectors for bitter, sweet, and umami stimuli, and are the focus of this review. Type I cells are generally considered to have a support function, while Type III cells respond to sour stimuli and form prominent synapses with afferent nerve fibers. Type II cells also associate closely with afferent nerve fibers, but do not form conventional synapses.
B. Image of two taste buds, showing Type II cells stained with an antibody against the Type III IP3 receptor, and nerve fibers stained with an antibody against the purinergic receptor P2X2. Apical staining of P2X2 may represent non-specific binding. (Image courtesy of A. Montoya and J. Kinnamon, University of Denver).
Taste buds on the anterior two thirds of the tongue are housed in fungiform papillae, which in rodents contain 1–2 taste buds each and are innervated by the chorda tympani branch of the facial nerve. Taste buds on the posterior tongue are housed in foliate and circumvallate papillae, each of which contains hundreds of taste buds that are innervated primarily by the glossopharyngeal nerve. Taste buds on the soft palate are innervated by another branch of the lingual nerve, the greater superficial petrosal nerve, while taste buds on the epiglottis and larynx are innervated by superior laryngeal nerve, a branch of the vagus nerve. Despite differences in their location and innervation, the structure of taste buds in different regions of the oral cavity is remarkably conserved. In addition, despite what is often published in textbooks, there is no distinct map of chemosensitivity on the tongue, although variations in thresholds exist in the different taste fields.
G protein-coupled taste receptors
Two classes of taste GPCRs have been identified molecularly, T1Rs and T2Rs. T1Rs mediate sweet and umami (glutamate) taste, while T2Rs mediate bitter taste. In general, taste receptors are low affinity receptors compared to other GPCRs, with binding affinities in the high µM to mM range. These concentrations, however, are similar to the concentration of most nutrients in foods. Because of their low affinity, the taste GPCRs were all cloned by mapping the human and mouse genomes, using information obtained from the linkage analysis of taste polymorphisms. A detailed discussion of the genetic origins of these taste receptors is reviewed in (Bachmanov and Beauchamp, 2007).
T1Rs
T1Rs are classical “C” type receptors, with large N-terminal ligand binding domains that exhibit a venus fly trap ligand binding module similar to the metabotropic glutamate receptors, the GABAB receptor, and the calcium sensing receptor. Three different T1Rs have been identified, which are products of the Tas1R genes: T1R1, T1R2, and T1R3 (Max et al., 2001, Montmayeur et al., 2001, Nelson et al., 2002, Nelson et al., 2001, Sainz et al., 2001). These receptors are functional only as heterodimers, with T1R3 serving as an obligate partner for both the umami receptor (T1R1 + T1R3) and the sweet receptor (T1R2 + T1R3). In heterologous systems, the umami receptor responds broadly to all L-amino acids in rodents (Nelson et al., 2002), but only to L-glutamate in humans (Li et al., 2002). Ligand binding occurs in the N-terminal domain of the T1R1 monomer. Importantly, the umami receptor in both rodents and humans is strongly potentiated by 5’-ribonucleotides such as inosine-5- monophosphate (IMP) and guanosine-5- monophosphate (GMP), which bind in an allosteric fashion to the T1R1 venus fly trap domain and stabilize the closed (active) state of the receptor (Zhang et al., 2008). The sweet receptor T1R2 + T1R3 binds sugars, D-amino acids, synthetic sweeteners and some sweet proteins-- basically all compounds that are recognized as sweet (Nelson et al., 2001). Although most ligand binding for small sweeteners occurs in the N-terminal domain of T1R2, some binding, particularly for large sweet proteins, also occurs in regions of T1R3 (Nie et al., 2005, Jiang et al., 2005, Jiang et al., 2004). None of the T1R binding domains has been successfully crystallized, so structural details of ligand binding remain to be elucidated. T1R3 null mice have been generated in two different labs, with differing results. In the Zuker lab, genetic elimination of T1R3 abolished responses to virtually all sweet and umami compounds, suggesting that T1R1 + T1R3 and T1R2 + T1R3 are the only receptors to mediate these qualities (Zhao et al., 2003). In contrast, the Margolskee lab found that while T1R3 knockout mice fail to respond to most sweet stimuli, considerable responses remain to umami compounds, suggesting the existence of multiple receptors for umami stimuli (Damak et al., 2003, Yoshida et al., 2009b, Delay et al., 2006, Maruyama et al., 2006). Candidate receptors are taste-specific isoforms of two metabotropic glutamate receptors, mGluR1 (San Gabriel et al., 2009a) and mGlurR4 (Chaudhari et al., 1996). However, knockouts will be required to verify a role in taste transduction.
T2Rs
Bitter taste is mediated by the T2R GPCRs, products of the Tas2R gene family. T2Rs are classical “A” type receptors that are similar in structure to the opsins and the olfactory receptors (Adler et al., 2000, Chandrashekar et al., 2000). They have short N-terminal domains, with ligand binding in the extracellular loops and transmembrane domains. Recent data suggest that carboxy terminal regions are particularly important for agonist selectivity (Brockhoff et al., 2010). The family consists of about 30 members in mammals, each of which binds structurally similar bitter compounds. While some receptors are rather narrowly tuned, others are broadly tuned and respond to many bitter compounds (Meyerhof et al., 2010). These receptors have been considered to function as monomers, but recent data suggest that they can also form functional oligomers (Kuhn et al., 2010). Although the role of oligomerization in T2R function has not been clearly elucidated, literally thousands of structurally diverse molecules taste bitter and oligomers would greatly increase the repertoire of stimuli that can activate a given receptor. Molecular studies have shown that bitter responsive taste cells express most or all of the receptors, which is not surprising since bitter taste evolved to avoid ingestion of toxic substances, and bitter compounds are not readily distinguished psychophysically in humans (Adler et al., 2000). However, physiological (Caicedo and Roper, 2001) and molecular (Behrens et al., 2007, Matsunami et al., 2000) studies of bitter responsive taste cells have shown that not all taste cells express all bitter receptors, suggesting that some bitter compounds could be discriminated.
Other taste GPCRs
Although T1Rs and T2Rs are generally considered to be the primary taste receptors, other GPCRs have been identified in taste buds and likely contribute to detection of nutrients, even though they do not appear to mediate a distinct taste quality. These include GPR40 and GPR120, both of which are expressed in subsets of taste cells and detect medium and long chain fatty acids (Cartoni et al., 2010). Another GPCR that is expressed abundantly in taste buds is the calcium sensing receptor, CaSR (San Gabriel et al., 2009b, Bystrova et al., 2010). Recent studies in humans have shown that agonists of the CaSR, including calcium and glutathione, elicit the so-called “kokumi” taste, which results in a potentiation of sweet, salty, and umami tastes (Ohsu et al., 2010). Thus, CaSR and the fatty acid GPCRs appear to modulate tastes rather than elicit a specific taste quality, and both appear to selectively enhance the appetitive taste qualities.
Downstream signaling effectors
Gβγ-mediated signaling
In general, T2Rs and T1Rs, as well as T1R1 and T1R2 are expressed in largely non-overlapping subsets of Type II taste cells, suggesting that these qualities can all be distinguished. However, T1Rs and T2Rs generally activate the same downstream signaling effectors in Type II taste cells (Zhang et al., 2003) (Figure 2). Taste receptor binding leads to activation of a heterotrimeric G protein, which consists in most cells of Gα–gustducin (McLaughlin et al., 1992) and it’s βγ partners, β3γ13 (Huang et al., 1999). The dominant leg of the pathway is mediated by the βγ partners, which activate phospholipase Cβ2 (PLCβ2) (Rossler et al., 1998) to convert the membrane lipid PIP2 into the second messengers 1,4,5- inositol trisphosphate (IP3) and diacylglycerol (DAG). While the function of DAG remains unclear, IP3 binds to the Type III IP3 receptor (IP3R3) (Clapp et al., 2001, Miyoshi et al., 2001), causing release of Ca2+ from intracellular stores and subsequent Ca2+-dependent activation of a monovalent selective cation channel, transient receptor potential channel M5 (TrpM5) (Perez et al., 2002, Zhang et al., 2007). This leads to membrane depolarization, action potential generation (Vandenbeuch and Kinnamon, 2009, Yoshida et al., 2009a), and release of ATP through gap junction hemichannels, likely composed of pannexin-1 (Dando and Roper, 2009, Huang et al., 2007, Romanov et al., 2007, Murata et al., 2010). Recent evidence suggests that Type II taste cells also express the vesicular ATP transporter, VNUT. This leaves open the possibility that ATP may also be released in a vesicular manner (Iwatsuki et al., 2009). Evidence for this signaling pathway comes from immunocytochemical and molecular studies showing that the component signaling effectors are co-expressed in both bitter and sweet/umami responsive Type II taste cells (Clapp et al., 2001, Clapp et al., 2004, DeFazio et al., 2006). Further, stimulation of isolated Type II taste cells with bitter, sweet, or umami taste stimuli elicits increases in intracellular Ca2+ that do not require extracellular Ca2+, are blocked by the PLC inhibitor U73122, and are sensitive to thapsigargin, which inhibits the Ca2+ ATPase that refills intracellular Ca2+ stores (Ogura et al., 2002, Hacker et al., 2008). Finally, knockout of PLCβ2, TrpM5 or IP3R3 strongly reduces or eliminates afferent nerve responses to most bitter, sweet, and umami taste stimuli (Zhang et al., 2003, Damak et al., 2006, Hisatsune et al., 2007).
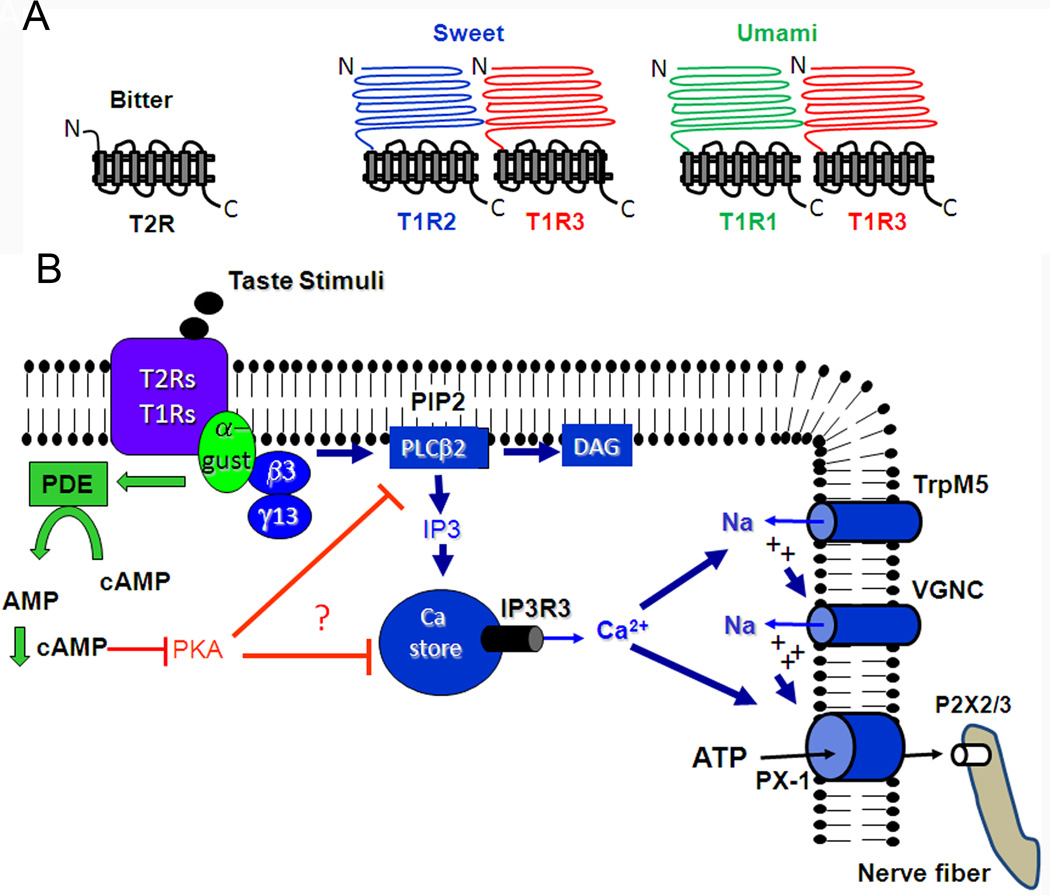
Figure 2.
Diagrammatic representation of taste GPCRs (top panel) and downstream signaling effectors (bottom panel). Receptor binding leads to Gβγ activation of PLCβ2, production of IP3, release of Ca2+ from intracellular stores, Ca2+ dependent activation of TrpM5, depolarization, activation of voltage-gated Na+ channels (VGNC), and release of ATP through pannexin-1 hemichannels. The released ATP activates purinergic receptors on afferent nerve fibers. Alpha gustducin tonically regulates cAMP levels via activation of a phosphodiesterase (PDE), which subsequently prevents phosphorylation and desensitization of Ca2+ signaling effectors.
The apparent role of TrpM5 in this process is to translate the Ca2+ that is released from the intracellular stores into a membrane depolarization that is sufficient to activate voltage-gated Na+ channels and elicit action potentials, which may be required to open the pannexin hemichannels. In support of this hypothesis, knockout of TrpM5 or inhibition of voltage gated Na+ channels with tetrodotoxin inhibits ATP release (Murata et al., 2010, Huang and Roper, 2010). Further, loose patch recordings from identified Type II taste cells during taste stimulation showed that ATP release was proportional to the frequency of action potentials elicited by the taste stimulus (Murata et al., 2010).
Gα-mediated signaling
All T2R receptors, and the T1R receptors in the anterior tongue and palate, are co-expressed with the Gα subunit, gustducin (Adler et al., 2000, Kim et al., 2003, Stone et al., 2007). Gα-gustducin (α-gust) was the first protein to be molecularly identified in taste cells (McLaughlin et al., 1992), but its role in taste signal transduction is still not completely understood. Gustducin has considerable sequence homology to transducin, which is also expressed in taste buds (McLaughlin et al., 1994). By analogy to the visual system, both α-gust and α-transducin are expected to activate a phosphodiesterase (PDE) and decrease intracellular cAMP levels. Biochemical studies have shown that bitter stimuli do decrease intracellular cAMP levels, and the decrease is inhibited by antibodies to α-gust (Yan et al., 2001). Cyclic AMP is also decreased in taste tissue in response to umami stimuli (Abaffy et al., 2003). However, many studies have shown that sugars increase cAMP levels in taste tissue (Bernhardt et al., 1996) and the increase is not simply a secondary consequence of Gβγ mediated release of Ca2+ from intracellular stores (Trubey et al., 2006). Gustducin knockout mice are significantly compromised to bitter, sweet, and umami stimuli, but the effect for sweet is much less than for bitter and umami (Wong et al., 1996, Ruiz et al., 2003, Glendinning et al., 2005). Thus the role of α-gust in sweet taste is much less clear than for bitter and umami taste. Part of the lack of effect of the gustducin knockout on sweet taste is that the sweet receptor T1R3 is not generally co-expressed with α-gust in posterior tongue. Instead, T1R3 is usually co-expressed with a Gq family protein, Gα14 in circumvallate and foliate taste buds (Tizzano et al., 2008, Shindo et al., 2008). Whether Gα14 mediates sweet transduction in these taste fields awaits studies in Gα14 knockout mice.
What is the role of the decreased cAMP in taste signaling? Although molecular evidence has indicated expression of a cyclic nucleotide gated channel in taste buds (Misaka et al., 1997), there is no physiological evidence for cyclic nucleotide-gated currents in taste cells. To determine other possible functions of the gustducin-mediated decrease in cAMP, biochemical assays were performed on isolated circumvallate taste buds of gustducin knockout mice. Interestingly, the knockout mice were found to have highly elevated levels of cAMP relative to wildtype mice (Clapp et al., 2008). These levels were elevated in the absence of any taste stimuli, suggesting that the taste receptors and/or G protein have tonic activity in the absence of taste ligands. The elevated cAMP likely activates Protein Kinase A to phosphorylate and inhibit PLC signaling effectors, since H-89, a specific Protein Kinase A inhibitor, rescued responses to bitter stimuli in the taste cells of gustducin knockout mice (Clapp et al., 2008). These data suggest that gustducin tonically regulates cAMP levels in taste cells to keep phosphorylation levels low and prevent chronic adaptation to bitter taste stimuli. Whether α-gust plays a similar role in the transduction of umami and sweet taste has not been determined.
Taste receptor signaling in the airways
The cloning of taste signaling effectors and the production of several lines of transgenic mice expressing GFP from their promoters, particularly α-gust, TrpM5, and T1R3 has revealed an extensive expression in the airways, from the upper airways to the lungs. T2R signaling has been most extensively studied, but T1Rs are also expressed in a number of regions of the airways. The cell types expressing these signaling effectors include solitary chemosensory (also called chemoreceptor) cells (Finger et al., 2003, Sbarbati et al., 2004, Lin et al., 2008, Kaske et al., 2007, Ogura et al., 2010, Tizzano et al., 2011, Tizzano et al., 2010), ciliated epithelial cells (Shah et al., 2009), and most recently, smooth muscle cells lining the airways (Deshpande et al., 2010).
Solitary chemosensory cells
Solitary chemosensory cells (SCCs) were first described in aquatic vertebrates (Whitear, 1992, Kotrschal and Whitear, 1988), where they are extensively expressed on the gill arches and skin. Each SCC has an apical process with microvilli that reaches the surface of the epithelium and a basolateral membrane that is densely supplied with nerve endings, suggesting a sensory function. The morphology of SCCs resembles that of taste receptor cells, but unlike taste cells, which are aggregated in taste buds, SCCs are scattered singly in the epithelium (Figure 3). In air breathing vertebrates, SCCs are found scattered in the airway epithelium, suggesting a respiratory function. Although SCCs have not been extensively studied in non-mammalian terrestrial vertebrates, SCCs have been described in the airways of alligators, where they are scattered among the olfactory receptor cells (Hansen, 2007). SCCs appear to be restricted to the vertebrate lineage, as none have been identified in invertebrate species (Finger, 2006).
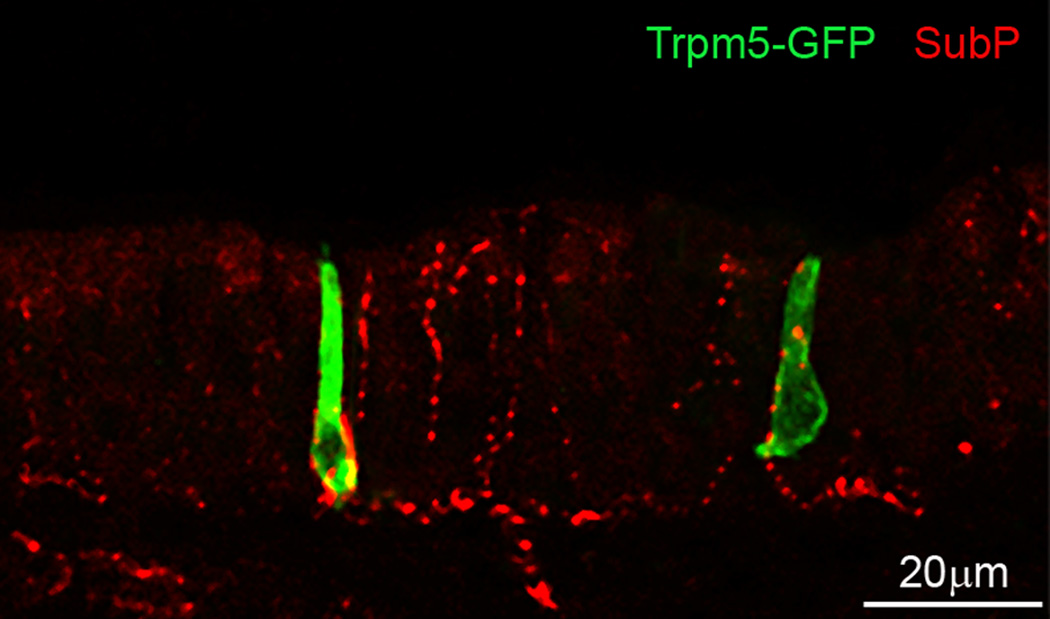
Figure 3.
Laser scanning confocal image of two TrpM5-GFP labeled solitary chemosensory cells of the mouse nasal epithelium. Nerve endings are stained with an antibody against substance P, a transmitter expressed in peptidergic trigeminal nerve fibers. (Image courtesy of M. Tizzano, University of Colorado Denver).
In rodents, SCCs are found in the upper airways, scattered in the nasal respiratory epithelium (Finger et al., 2003, Tizzano et al., 2010), at the entrance of the vomeronasal duct (Ogura et al., 2010), and on the larynx (Tizzano et al., 2011). In the lower airways, they are found in the trachea, the bronchi, and larger bronchioles, but not in the smaller bronchioles or the alveoli (Tizzano et al., 2010). In the respiratory epithelium and the larynx, the SCCs are heavily innervated by peptidergic fibers of the trigeminal and vagus nerves, respectively. Unlike Type II taste receptor cells that lack conventional synapses with sensory afferents (Clapp et al., 2004, Clapp et al., 2006), the SCCs innervated by peptidergic fibers appear to have classical synapses with pre and postsynaptic specializations (Finger et al., 2003). A recent study suggests that a subset of SCCs expresses cholinergic markers and contacts nerve fibers expressing nicotinic acetylcholine receptors (Krasteva et al., 2010), suggesting acetylcholine may be the transmitter. In contrast to the upper airways, SCCs in the trachea and lower airways are much more sparsely innervated, suggesting these SCCs may mediate different functions from the SCCs in the upper airway epithelia.
The SCCs of the nasal respiratory epithelium have been studied most extensively. These cells express both T2Rs and T1Rs, but T1Rs are much less abundantly expressed than T2Rs. Unlike taste cells where T1Rs and T2Rs are expressed in non-overlapping subsets of cells, SCCs in the airways often express both, suggesting different classes of compounds (i.e., sweet and bitter) may elicit similar effects in the airways (Tizzano et al., 2011, Ohmoto et al., 2008). The downstream signaling effectors are identical to taste receptor cells, with Gβγ stimulation of PLCβ2, production of IP3, release of Ca2+ from intracellular stores, activation of TrpM5, depolarization, and release of transmitter. The only difference is that SCCs appear to use conventional vesicular synaptic transmission to activate the afferent nerves, while taste cells release ATP via non-vesicular mechanisms. SCCs have been isolated from α–gust-GFP mice and TrpM5-GFP mice and studied with Ca2+ imaging. GFP labeled SCCs respond rather selectively to the bitter stimulus denatonium, suggesting these cells are more narrowly tuned than bitter responsive taste cells, which respond to a variety of bitter compounds. As in taste cells, the response is inhibited by the PLC inhibitor U73122 (Gulbransen et al., 2008). In addition to denatonium, SCCs also respond to homoserine lactones (HSLs) that are produced by gram negative pathogenic bacteria as quorum signaling molecules. When the concentration of these molecules reaches a critical level, the bacteria become pathogenic and form a biofilm, attacking the epithelium. SCCs detect the HSLs at the appropriate concentration and activate the trigeminal nerve to produce a pronounced apnea that protects the airway from further inhalation. These responses are absent in both TrpM5 and α-gust knockout mice, suggesting that activation of the trigeminal nerve by HSLs requires the integrity of the SCCs (Tizzano et al., 2010). Important questions that remain are the nature of the stimuli that activate the T1Rs and the physiological role of the SCCs of the lower airways. Since most of the lower airway SCCs are not innervated, activation of these cells likely results in secretory functions or mucociliary clearance mechanisms, rather than protective nerve reflexes.
Ciliated epithelial cells
Cultured human airway epithelial cells have been shown to express T2Rs and some of their downstream signaling effectors (Shah et al., 2009). In this case, the receptors are found on the ciliated cells, rather than on the SCCs described above. Several T2Rs and α-gust are expressed on the motile cilia, with PLCβ2 expressed beneath the tight junctions. The cultured airway cells responded to several bitter compounds with increases in intracellular Ca2+ and a concomitant increase in the frequency of ciliary beating. Unlike the SCCs, TrpM5 does not appear to be expressed, which is not surprising since epithelial cells are not generally electrically excitable. The authors suggest that the ciliated cells detect noxious inhaled substances or products of bacterial infection and increase ciliary beating to remove the harmful substances from the airways. It is of interest that subsequent studies using immunocytochemical probes and transgene expression have failed to find evidence of T2R signaling on ciliated airway cells of rodents (Tizzano et al., 2011). It is possible that this represents a species difference, or possibly an effect of cell culture conditions. It will be important to document the presence of these receptors on freshly isolated human airway epithelia.
Airway smooth muscle cells
T2R signaling has recently been reported on cultured human smooth muscle cells that line the airway. Several T2Rs were expressed in the muscle cells, along with α-gust (Deshpande et al., 2010). Stimulation with a variety of bitter compounds caused an increase in intracellular Ca2+, which was blocked or reduced by pharmacological inhibitors of Gβγ, PLCβ2, and IP3. Interestingly, stimulation with bitter compounds caused a potent relaxation of the smooth muscle. This was unexpected, since acetylcholine, which causes an increase in intracellular Ca2+ via activation of Gαq, evokes muscle contraction. The authors found that the bitter taste mediated increase in intracellular Ca2+ was accompanied by a Ca2+-dependent increase in K+ conductance mediated by the BK channel, which resulted in membrane hyperpolarization and relaxation. This suggests that the T2R signaling cascade is in a membrane compartment that is distinct from the acetylcholine receptor signaling cascade. The authors suggest that inhaled bitter compounds could be used in a therapeutic manner to treat airway diseases, such as asthma and chronic pulmonary obstructive disease. One concern, however, is how these compounds pass from the airway epithelium to the smooth muscle to mediate the effects. Again, as was the case with ciliated epithelial cells discussed above, recent studies have failed to document expression of T2R downstream signaling effectors in smooth muscle of the rodent airway (Tizzano et al., 2011). It will be important to document that these signaling effectors are present in vivo.
Summary and future directions
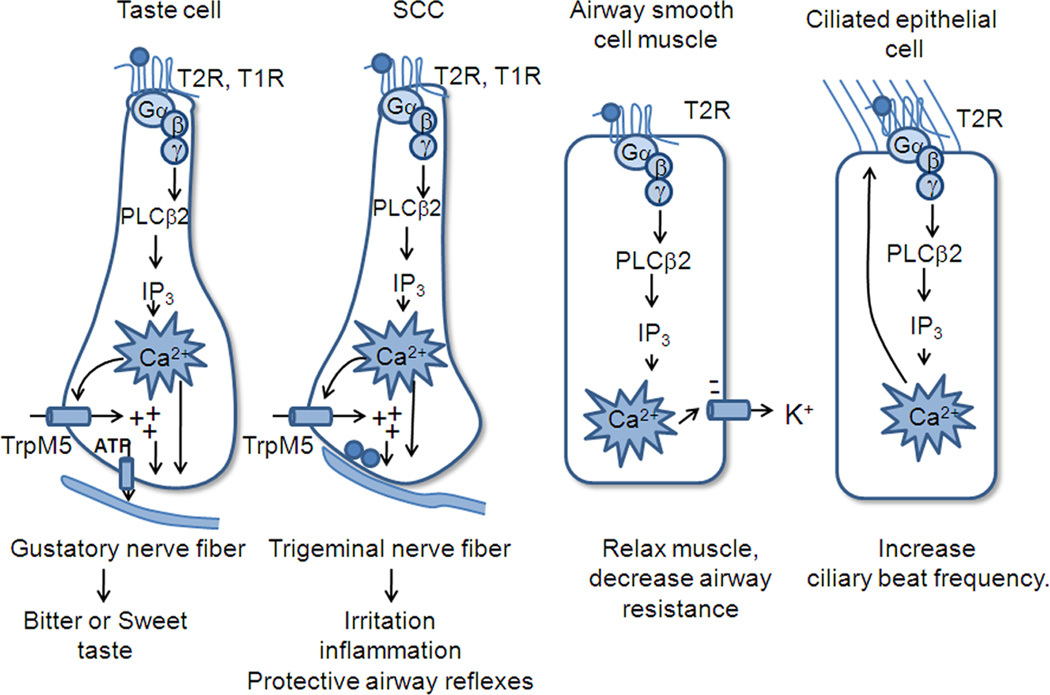
Taste receptor signaling is remarkably conserved in tissues ranging from taste receptor cells to several cell types in the airways, and several cell types in the gastrointestinal epithelium (see review by Iwatsuki, this volume). In all cases, the stimuli bind to low affinity receptors that activate Gβγ, causing stimulation of PLCβ2, production of IP3, and release of Ca2+ from intracellular stores. Following the increase in intracellular Ca2+, the downstream events vary, depending on the cell type (Figure 4). In taste cells and solitary chemoreceptor cells, the Ca2+ activates TrpM5 to depolarize the cell and evoke release of transmitter, while in smooth muscle cells the Ca2+ activates the Ca2+ dependent K+ channel to hyperpolarize the cell and cause muscle relaxation. In ciliated epithelial cells that are not electrically excitable or dependent on membrane potential, the increase in Ca2+ simply causes an increase in ciliary beat frequency, a Ca2+ dependent process. Thus similar upstream effectors activate different physiological processes in different systems to evoke different effects. Interestingly, the “bitter” compounds all appear to evoke processes that favor their expulsion from the body-- in taste cells they evoke an unpleasant taste, in SCCs they evoke protective airway reflexes such as sneezing or coughing, and lower in the airway they enhance ciliary beating and relaxation of the airway, both of which would promote their expulsion.
Figure 4.
Diagrammatic illustration of differences in signaling effectors in taste cells, SCCs of the airway, ciliated epithelial cells, and smooth muscle cells lining the airways. In all cases taste GPCRs activate the downstream PLC signaling effectors, but the effects of increased Ca2+ differ among the different cell types.
Several unanswered questions remain, about signaling in taste cells as well as airway epithelium. First, what is the role of gustducin in sweet taste? Gustducin should decrease cAMP levels, but previous biochemical studies have shown that most sugars increase cAMP in taste tissue. Studies examining cAMP signaling in individual taste cells will be required to resolve the role of cAMP in sweet taste. Further, what is the role of T1Rs in the airway epithelium? What compounds activate them and what is the effect? Do other natural compounds, including bacterial or viral gene products activate T2Rs in vivo? Finally, what is the role of taste signaling in human airways in vivo? The two human studies described above both used cultured airway cells and culture conditions can affect gene expression. Fresh biopsies of human tissue will be required to address this question, as well as whether human airway epithelium has trigeminally innervated SCCs similar to rodents.
Acknowledgements
The author thanks Dr. John Kinnamon for the unpublished image in Figure 1 and Dr. Marco Tizzano for the unpublished image in Figure 3. Drs. Aurelie Vandenbeuch and Thomas Finger provided helpful comments on the manuscript.
Funding: This was supported in part by NIH grants DC00766, DC006021, DC007495, DC009820, and P30DC004657.
Footnotes
Conflict of interest: none
References
- Abaffy T, Trubey KR, Chaudhari N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am J Physiol Cell Physiol. 2003;284:C1420–C1428. doi: 10.1152/ajpcell.00556.2002. [DOI] [PubMed] [Google Scholar]
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 2007;27:12630–12640. doi: 10.1523/JNEUROSCI.1168-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996;490(Pt 2):325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhoff A, Behrens M, Niv MY, Meyerhof W. Structural requirements of bitter taste receptor activation. Proc Natl Acad Sci U S A. 2010;107:11110–11115. doi: 10.1073/pnas.0913862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrova MF, Romanov RA, Rogachevskaja OA, Churbanov GD, Kolesnikov SS. Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J Cell Sci. 2010;123:972–982. doi: 10.1242/jcs.061879. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci. 1996;16:3817–3826. doi: 10.1523/JNEUROSCI.16-12-03817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Trubey KR, Vandenbeuch A, Stone LM, Margolskee RF, Chaudhari N, Kinnamon SC. Tonic activity of Galpha-gustducin regulates taste cell responsivity. FEBS Lett. 2008;582:3783–3787. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 2006;31:351–357. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE. Evolution of taste. In: Kaas J, editor. Evolution of the Nervous System. Oxford, UK: Elsevier Press; 2006. pp. 423–441. [Google Scholar]
- Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol. 2008;99:2929–2937. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 2008;99:1503–1514. doi: 10.1152/jn.00892.2007. [DOI] [PubMed] [Google Scholar]
- Hansen A. Olfactory and solitary chemosensory cells: two different chemosensory systems in the nasal cavity of the American alligator, Alligator mississippiensis. BMC Neurosci. 2007;8:64. doi: 10.1186/1471-2202-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayato R, Ohtubo Y, Yoshii K. Functional expression of ionotropic purinergic receptors on mouse taste bud cells. J Physiol. 2007;584:473–488. doi: 10.1113/jphysiol.2007.138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2007;282:37225–37231. doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008a;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008b;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Roper SD. Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010;588:2343–2350. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Jiang P, Cui M, Zhao B, Snyder LA, Benard LM, Osman R, Max M, Margolskee RF. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J Biol Chem. 2005;280:34296–34305. doi: 10.1074/jbc.M505255200. [DOI] [PubMed] [Google Scholar]
- Jiang P, Ji Q, Liu Z, Snyder LA, Benard LM, Margolskee RF, Max M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem. 2004;279:45068–45075. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]
- Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun. 2003;312:500–516. doi: 10.1016/j.bbrc.2003.10.137. [DOI] [PubMed] [Google Scholar]
- Kotrschal K, Whitear M. Chemosensory anterior dorsal fin in rocklings (Gaidropsarus and Ciliata, Teleostei, Gadidae): somatotopic representation of the ramus recurrens facialis as revealed by transganglionic transport of HRP. J Comp Neurol. 1988;268:109–120. doi: 10.1002/cne.902680111. [DOI] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Veres T, Papadakis T, Hartmann P, Muchlfield C, Schiecker K, Hans K, Tallini YN, Braun Aea. Tracheal bursh cells are neuronally connected cholinergic sensory cells (abstract).) Society for Neuroscience. 2010 [Google Scholar]
- Kuhn C, Bufe B, Batram C, Meyerhof W. Oligomerization of TAS2R bitter taste receptors. Chem Senses. 2010;35:395–406. doi: 10.1093/chemse/bjq027. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99:1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci. 2006;26:2227–2234. doi: 10.1523/JNEUROSCI.4329-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Spickofsky N, Danho W, Margolskee RF. Molecular cloning of G proteins and phosphodiesterases from rat taste cells. Physiol Behav. 1994;56:1157–1164. doi: 10.1016/0031-9384(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Misaka T, Kusakabe Y, Emori Y, Gonoi T, Arai S, Abe K. Taste buds have a cyclic nucleotide-activated channel, CNGgust. J Biol Chem. 1997;272:22623–22629. doi: 10.1074/jbc.272.36.22623. [DOI] [PubMed] [Google Scholar]
- Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 2001;26:259–265. doi: 10.1093/chemse/26.3.259. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF, Ninomiya Y. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol. 2010;104:896–901. doi: 10.1152/jn.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RG, Murray A. Relations and possible significance of taste bud cells. Contrib Sens Physiol. 1971;5:47–95. doi: 10.1016/b978-0-12-151805-9.50008-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Ogura T, Krosnowski K, Zhang L, Bekkerman M, Lin W. Chemoreception regulates chemical access to mouse vomeronasal organ: role of solitary chemosensory cells. PLoS One. 2010;5:e11924. doi: 10.1371/journal.pone.0011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Margolskee RF, Kinnamon SC. Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol. 2002;87:3152–3155. doi: 10.1152/jn.2002.87.6.3152. [DOI] [PubMed] [Google Scholar]
- Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol Cell Neurosci. 2008;38:505–517. doi: 10.1016/j.mcn.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Ohsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T, Maruyama Y, Miyamura N, Eto Y. Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem. 2010;285:1016–1022. doi: 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. Embo J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Royer SM, Kinnamon JC. HVEM serial-section analysis of rabbit foliate taste buds: I. Type III cells and their synapses. J Comp Neurol. 1991;306:49–72. doi: 10.1002/cne.903060105. [DOI] [PubMed] [Google Scholar]
- Ruiz CJ, Wray K, Delay E, Margolskee RF, Kinnamon SC. Behavioral evidence for a role of alpha-gustducin in glutamate taste. Chem Senses. 2003;28:573–579. doi: 10.1093/chemse/bjg049. [DOI] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr. 2009a;90:743S–746S. doi: 10.3945/ajcn.2009.27462I. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Uneyama H, Maekawa T, Torii K. The calcium-sensing receptor in taste tissue. Biochem Biophys Res Commun. 2009b;378:414–418. doi: 10.1016/j.bbrc.2008.11.060. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Crescimanno C, Osculati F. Identification and characterization of a specific sensory epithelium in the rat larynx. J Comp Neurol. 2004;475:188–201. doi: 10.1002/cne.20172. [DOI] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y, Miura H, Carninci P, Kawai J, Hayashizaki Y, Ninomiya Y, Hino A, Kanda T, Kusakabe Y. G alpha14 is a candidate mediator of sweet/umami signal transduction in the posterior region of the mouse tongue. Biochem Biophys Res Commun. 2008;376:504–508. doi: 10.1016/j.bbrc.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun. 2011 doi: 10.1016/j.bbrc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Starostik MR, Rebello MR, Cotter KA, Kulik A, Medler KF. Expression of GABAergic receptors in mouse taste receptor cells. PLoS One. 2010;5:e13639. doi: 10.1371/journal.pone.0013639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and Gustducin in Palatal Taste Buds of Mice. Chem Senses. 2007;32:255–262. doi: 10.1093/chemse/bjl053. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in Solitary Chemosensory Cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Dvoryanchikov G, Barrows JK, Kim S, Chaudhari N, Finger TE. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neurosci. 2008;9:110. doi: 10.1186/1471-2202-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trubey KR, Culpepper S, Maruyama Y, Kinnamon SC, Chaudhari N. Tastants evoke cAMP signal in taste buds that is independent of calcium signaling. Am J Physiol Cell Physiol. 2006;291:C237–C244. doi: 10.1152/ajpcell.00303.2005. [DOI] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Kinnamon SC. Why do taste cells generate action potentials? J Biol. 2009;8:42. doi: 10.1186/jbiol138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitear M. Solitary Chemoreceptor Cells. In: Hara TJ, editor. Chemoreception in Fishes. 2 ed. London: Elsevier Press; 1992. [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand JG, Spielman AI. Bitter taste transduced by PLC-beta(2)-dependent rise in IP(3) and alpha-gustducin-dependent fall in cyclic nucleotides. Am J Physiol Cell Physiol. 2001;280:C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 2009a;587:4425–4439. doi: 10.1113/jphysiol.2009.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Yasumatsu K, Shirosaki S, Jyotaki M, Horio N, Murata Y, Shigemura N, Nakashima K, Ninomiya Y. Multiple receptor systems for umami taste in mice. Ann N Y Acad Sci. 2009b;1170:51–54. doi: 10.1111/j.1749-6632.2009.03902.x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci U S A. 2008;105:20930–20934. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao Z, Margolskee RF, Liman ER. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]