Abstract
Objective
To assess the effect of aging on the immunological response to antiretroviral therapy (ART) in the West African context.
Methods
The change in CD4 T-cell count was analysed according to age at the time of ART initiation among HIV-infected patients enrolled in the International epidemiological Database to Evaluate AIDS (IeDEA) Collaboration in the West African region. CD4 gain over 12 months of ART was estimated using linear mixed models. Models were adjusted for baseline CD4 cell count, sex, baseline clinical stage, calendar period and ART regimen.
Results
The total number of patients included was 24 107, contributing for 50 893 measures of CD4 cell count in the first year of ART. The baseline median CD4 cell count was 144 cells/μl [interquartile range (IQR) 61–235]; median CD4 cell count reached 310 cells/μl (IQR 204–443) after 1 year of ART. The median age at treatment initiation was 36.3 years (10th–90th percentiles=26.5–50.1). In adjusted analysis, the mean CD4 gain was significantly higher in younger patients (P < 0.0001). At 12 months, patients below 30 years recovered an additional 22 cells/μl on average [95% confidence interval (CI) 2–43] compared to patients at least 50 years.
Conclusion
Among HIV-infected adults in West Africa, the immunological response after 12 months of ART was significantly poorer in elderly patients. As the population of treated patients is likely to get older, the impact of this age effect on immunological response to ART may increase over time.
Keywords: age, antiretroviral treatment, HIV/AIDS, immunological response, linear mixed models, West Africa
Introduction
Effective antiretroviral therapy (ART) has significantly increased the life expectancy of HIV-infected patients [1–5] in developed and resource-limited countries. Ageing of the general population and large-scale access to ART in resource-limited countries have both contributed to the overall ageing of the HIV population. A study estimated at 3 million the number of people aged at least 50 years and living with HIV in 2007 in sub-Saharan Africa [6].
Medical care addressing the specific needs of this ageing HIV population is needed [7]. Data from Europe and the USA reported a lower immune response to ART in older patients [8–19]. This age effect, which remained moderate with no obvious threshold effect [19,20], was partly explained by the decreased thymic output over age [20,21]. Other studies, with smaller sample size, did not find any effect of age on the immune response to ART [22,23].
The effect of age on the response to ART in sub-Saharan Africa has not been well documented so far. And yet, due to several immunological and demographic specificities of sub-Saharan Africa, the effect of age on immune restoration may differ from that observed in high-income countries. On one hand, HIV-infected patients in Africa are presenting a higher level of T-cell activation compared to those in high-income countries [24]; whereas an increased T-cell activation has been related to a lower CD4 gain after ART initiation [25]. Hence, a decrease of de-novo production of CD4 (potentially related to age) might have a greater impact when the loss of CD4 is accelerated by a persistent immune activation. On the other hand, life expectancy being far lower in sub-Saharan Africa compared to high-resource settings (e.g. 57 years in Cote d'Ivoire compared to 81 years in France in 2009), HIV patients who survive beyond 50 years may be selected patients presenting specific characteristics of a slower progression of the infection.
The objective of this analysis was to estimate the impact of age on the immunological response to ART 12 months after treatment initiation, in HIV-infected adults followed in the West-African context.
Methods
The IeDEA West Africa collaboration
To better describe the current epidemiology of HIV infection and especially care patterns and treatment, a prospective and observational multicohort collaboration located in West Africa was established in 2006 within the International epidemiologic Database to Evaluate AIDS (IeDEA) Collaboration of the US National Institutes of Health (http://www.iedeawestafrica.org/) [26]. Fifteen HIV/AIDS adult clinics located within seven countries – Benin (n=1), Burkina Faso (n=1), Côte d'Ivoire (n=6), Gambia (n=1), Mali (n=2), Nigeria (n=2) and Senegal (n=2) – participated in this analysis. Every 18 months, each cohort submits information to the central coordinating centre in Abidjan, Côte d'Ivoire, using a standardized data format. The data collected capture demographic, therapeutic, clinical and biological information.
Inclusion criteria
All naive HIV-infected patients aged 16 years or older at ART starting date, with documented sex, date of birth, date of ART initiation and CD4 T-cell count measured at ART initiation (or 6 months before) were eligible for this analysis.
Statistical analysis
Analyses were performed on an intent-to-continue basis; ART modifications were not taken into account. Baseline was defined as the date of ART initiation and the end point of the study was 12 months after baseline. The CD4 change from baseline was analysed with linear mixed models. The effect of age on CD4 change was adjusted for sex, baseline HIV clinical stage (Centers for Disease Control (CDC) or WHO), the year of ART initiation, ARTregimen and baseline CD4 cell count. A linear effect of age was compared to a class effect (5-year categories). Fixed effects on CD4 change were estimated using Wald tests. Residual homoscedasticity and normality were checked graphically. Robustness analyses were conducted using absolute CD4 cell count (after square root transformation). We considered a patient as lost to follow-up if he/she was not known to have died and was not seen at least 3 months prior to the cohort closure date. Results of the primary analysis were confirmed by performing a sensitivity analysis excluding patients who died or were lost to follow-up within the first 12 months.
Results
Population characteristics
The IeDEA West Africa database included a total of 33 815 patients as of January 2010. One thousand nine hundred and twenty-two patients with missing data were excluded (ART initiation after closure date, unknown date of ART initiation, missing demographic data), 1756 patients were previously under ART and 6030 patients (17.8%) had no baseline CD4 measurement available. In the present study 24 107 patients were finally eligible (Fig. 1) and the number of patients enrolled per site ranged between 216 and 3769.
Fig. 1.
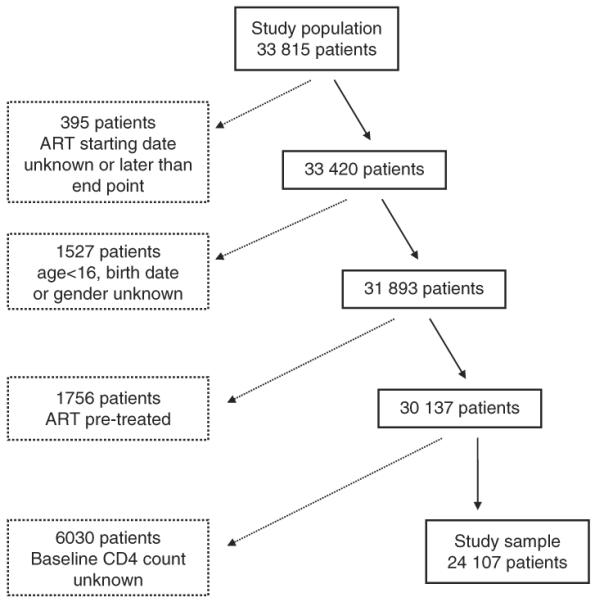
Flow diagram of the selection of the study sample (IeDEA West Africa Collaboration).
The characteristics of the study sample and of the excluded patients are described in Table 1. In the study sample, the median age at ART initiation was 36.3 [interquartile range (IQR) 30.5–43.2], 63.6% of the patients were women and the baseline clinical stage was WHO stage I or II, or CDC stage A or B for 44.1% of the patients. Most patients started ART in 2004 or later (93.0%). Most patients (86.9%) initiated a non-nucleoside reverse transcriptase inhibitor (NNRTI) ART-based regimen. The most common ART combinations were stavudine + lamivudine + nevirapine for 7663 patients (31.8%), zidovudine + lamivudine + efavirenz for 3696 patients (15.3%) and zidovudine + lamivudine + nevirapine for 3496 patients (14.5%). Baseline median CD4 cell count was significantly lower (P < 0.0001) in the study sample compared to excluded patients [144 cells/μl (IQR 61–235) and 183 cells/μl (IQR 82–336), respectively]. Within the study sample, the baseline median CD4 cell count was 117 cells/μl (IQR 43–212) for patients lost to follow-up, 55 cells/μl (IQR 15–143) for deceased patients and 156 cells/μl (IQR 73–245) for patients who remained alive.
Table 1.
Baseline and follow-up characteristics for study sample (n = 24 107) compared to patients not included in the analysis (n = 9708).
| Variables | Patients excluded | Study sample |
|---|---|---|
| Total number of patients | 9708 | 24107 |
| Baseline CD4 cell count (cells/μl) | ||
| Median (IQR) | 183 (82–336) | 144 (61–235) |
| Unknown (%) | 7288 (75.1) | – |
| Age at baseline (years) | ||
| Median (IQR) | 36.7 (30.7–43.7) | 36.3 (30.5–43.2) |
| Unknown (%) | 1714 (17.7) | – |
| <25 years (%) | 558 (5.8) | 1486 (6.2) |
| 25–29 | 1166 (12.0) | 3885 (16.1) |
| 30–34 | 1621 (16.7) | 5192 (21.5) |
| 35–39 | 1716 (17.7) | 4939 (20.5) |
| 40–45 | 1184 (12.2) | 3716 (15.4) |
| 45–50 | 835 (8.6) | 2459 (10.2) |
| 50–54 | 490 (5.1) | 1370 (5.7) |
| ≥55 | 424 (4.4) | 1060 (4.4) |
| Sex (%) | ||
| Women | 5804 (59.8) | 15322 (63.6) |
| Men | 3457 (35.6) | 8785 (36.4) |
| Unknown | 447 (4.6) | – |
| Baseline clinical stage (%) | ||
| CDC stage A, B, WHO I, II | 3796 (39.1) | 10637 (44.1) |
| AIDS stage, WHO III, IV | 3036 (31.3) | 9702 (40.2) |
| Unknown | 2876 (29.6) | 3768 (15.7) |
| Year of ART initiation (%) | ||
| Prior to 2004 | 847 (8.7) | 1683 (7.0) |
| 2004 to 2009 | 8521 (87.8) | 22424 (93.0) |
| Unknown | 340 (3.5) | – |
| Initial ART regimen (%) | ||
| NNRTI-based | 7804 (80.4) | 20938 (86.9) |
| PI-based | 700 (7.2) | 1331 (5.5) |
| Others | 750 (7.7) | 1739 (7.2) |
| Unknown | 454 (4.7) | 99 (0.4) |
| Follow-up characteristics | ||
| Died (%) | 188 (1.9) | 779 (3.2) |
| Lost to follow-up (%) | 2602 (26.8) | 5690 (23.6) |
| Number of CD4 measurements | 8915 | 50893 |
| Median number of CD4 measurements (IQR) | 0 (0–2) | 2 (1–3) |
IeDEA West Africa Collaboration.
ART, antiretroviral treatment; CDC, Centers for Disease Control; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
CD4 response to treatment
Within the study sample, the median number of CD4 measurements available during the study period was 2 (IQR 1–3). The median CD4 cell count was 277 cells/μl (IQR 177–403) and 310 cells/μl (IQR 204–443) 6 and 12 months after starting ART, respectively. At baseline, the median CD4 cell count was 150 cells/μl (IQR 64–241) for patients under 30 years and 150 cells/μl (IQR 69–240) for those at least 50 years. Twelve months after starting ART 42.3% of the patients had a CD4 cell count available, the median CD4 cell count was 332 cells/μl (IQR 216–473) for those aged 16–30 years and 305 cells/μl (IQR 208–416) for patients aged at least 50 years.
Table 2 presents adjusted estimates of mean CD4 change after 12 months of ART for the following reference group of patients: women who started a NNRTI-based ART regimen within the year 2004 or later, who initiated the treatment at clinical stage A, B or WHO I, II, with an initial CD4 cell count equal to 180 cells/μl and were aged between 16 and 30 years. The mean CD4 changes were adjusted for initial CD4 cell count, ART regimen, sex, initial clinical stage and year of ART initiation.
Table 2.
Mean CD4 change (cells/μl) after 12 months of ART, estimated by multivariable linear mixed model.
| Mean CD4 change estimated (cells/μl) | 95% Confidence interval | P value | |
|---|---|---|---|
| Age at baselinea (years) | <0.0001 | ||
| [16–30] | +251b | +239; +263 | |
| [30–35] vs. [16–30] | +3 | −12; +18 | |
| [35–40] vs. [16–30] | −17 | −33; −2 | |
| [40–45] vs. [16–30] | −20 | −37; −3 | |
| [45–50] vs. [16–30] | −34 | −54; −15 | |
| [50–81] vs. [16–30] | −22 | −43; −2 | |
| Baselinea CD4 cell count | <0.0001 | ||
| For 50 cells/μl lower | +19 | +18; +20 | |
| Sex | <0.0001 | ||
| Women vs. men | +30 | +21; +39 | |
| Baselinea clinical stage | <0.0001 | ||
| A, B/I, II vs. AIDS/WHO III, IV | +17 | +6; +28 | |
| Year of ART initiation | <0.0001 | ||
| Prior to 2004 vs. up to 2003 | −48 | −64; −31 | |
| Initial ART regimen | <0.0001 | ||
| PI-based vs. NNRTI-based | −36 | −54; −18 | |
| Others regimen vs. NNRTI-based | −45 | −60; −30 |
IeDEA West Africa Collaboration.
ART, antiretroviral treatment; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Baseline: initiation of ART.
For the reference group: 16–30-year-old women, initiating NNRTI regimen after 2003 at Centers for Disease Control clinical stage A, B or WHO I, II and with 180 CD4 cells/μl at baseline.
The overall mean effect of age on the CD4 gain was significant (P<0.0001). Compared to patients below 30 years [251 cells/μl; 95% confidence interval (CI) 239; 263], the average CD4 change was not significantly different (P=0.7017) in patients between 30 and 34 years (254 cells/μl, 95% CI 242; 266). For patients aged at least 35 years, the mean CD4 change at 12 months was significantly lower compared to patients aged below 30 years (−17 cells/μl, 95% CI −33; −2 for 35–40 years; −20 cells/μl, 95% CI −37; −3 for 40–45 years; −34 cells/μl, 95% CI −54; −15 for 45–50 years and −22 cells, 95% CI −43; −2 for patients aged ≥50 years).
The average CD4 gain was significantly higher among women than men (+30 cells/μl, 95% CI +21; +39), for patients with a nonadvanced clinical stageat baseline (A, B, I, II) compared to advanced (AIDS, III, IV; +17 cells/μl, 95% CI +6 +28), for patients who initiated ART in 2004 or later compared to patients starting prior 2004 (+48 cells/μl, 95% CI +31; +64) and for patients treated with NNRTI ART-based regimen compared to protease inhibitor ART-based regimen (+36 cells/μl, 95% CI +18; +54). The effect of initial CD4 cell count on mean CD4 change was also significant (P<0.0001), +19 cells/μl (95% CI +18; +20) for each 50 cells/μl CD4 cell count lower at baseline.
As a robustness analysis, the linear mixed model was also conducted by removing patients lost to follow-up (n=5690) and deceased patients (n=779) during the first year of ART. Fig. 2 illustrates the mean CD4 change estimated on these two subsets. Although the average CD4 gain was slightly higher when considering lost to follow-up and deceased patients, the overall results were similar. Also, a continuous linear effect of age led to a similar fit of the data (AIC linear 614759 vs. AIC categories 614767).
Fig. 2.
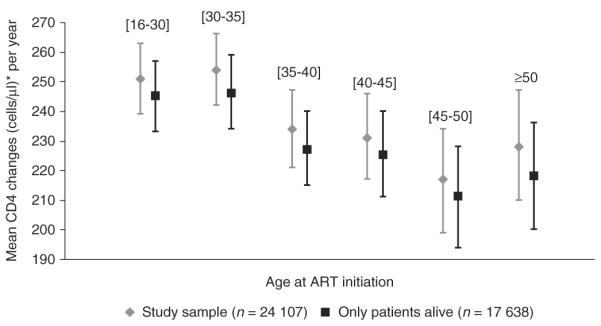
Mean CD4 changes with 95% confidence intervals (cells/μl) after 12 months of ART according to age, estimated by adjusted linear mixed models in the study population, with and without deceased and lost to follow-up patients (n = 24 107 and n = 17 638, respectively) (IeDEA West Africa Collaboration). *For the reference group: women, initiating NNRTI regimen after 2003 at Centers for Disease Control clinical stage A, B or WHO I, II and with 180 CD4 cells/μl at baseline.
Discussion
In a large collaboration of observational cohorts of HIV-infected patients in West Africa, we found a significant impact of age on the immune response during the first 12 months of ART with a −20 to −34 cells/μl reduction in CD4 gain among patients older than 40 compared to patients younger than 30 years. This impairment in CD4 gain may have serious clinical and public health consequences, life expectancy being related to the time spent with higher CD4 cell counts [27].
Data on the effect of age in Africa are very scarce but always showed a poorer ART response in older patients [2,3,28]. We confirmed the effect of age on CD4 responses in sub-Saharan Africa; however, we were not able to explore the possible causal factors. Thymic output may be compromised by malnutrition and infections [29] and higher level of T-cell activation [24] may also participate to an increased turnover of T cells. A poor immunological response in older patients is particularly problematic in this context where HIV RNA viral load measurement and new line of ART are rarely available [30]. Therefore, an improvement in the CD4 response among older patients should be achieved by improving modifiable risk factors of poor immunological response such as HIV replication, concomitant infections or malnutrition.
An interesting result is related to the absence of clear threshold effect of age in our study. It is difficult to conclude on the existence of a clear threshold from results published so far because the cut-points varied [9,18,28] and the justification of a nonlinear effect was often lacking. Our interpretation is that there is a continuous effect of age (following thymic atrophy) that has a substantial impact on CD4 response as early as from 40 years.
Our study presents several limitations that need to be discussed. No adjustment on virological response could be performed in the present study because HIV-RNA viral load was not widely available in the study clinics like in most settings in resource-limited countries. However, older patients are known to show a better virological response [18] even after adjustment for time from seroconversion [31] that could be linked to treatment adherence. By not taking into account a better virological response in older patients we may have in fact underestimated the deleterious effect of age on the CD4 response and the observed relationship may be minimal. A methodological drawback regularly encountered in cohorts established in resource-limited countries is the high rate of losses to follow-up [32]. The consequence may be a biased estimate of the CD4 gain. Statistical approaches have been proposed to correct this type of bias [33]. However, these approaches also have their limitations and one of them is the common assumption that patients lost to follow-up are homogeneous. For instance, one can assume that they are all in a poorer health status than those followed [34]. A recent active survey performed in East Africa [35] reported that actually one-third of lost-to-follow-up patients were dead but others had been transferred or had travelled because they had an improved health status. In our study, we observed a trend towards a better immunological response when including patients lost to follow-up in the analyses, which may be due to a better outcome in these patients. Although our conclusions were robust to the inclusion/exclusion of patients lost to follow-up and deceased, additional information on these patients would lead to more accurate estimates.
In conclusion, CD4 gain after ART initiation was significantly poorer in elderly patients in this West African setting. As the population of treated HIV-infected patients in this part of the world is likely to get older, thanks to the overall effectiveness of ART and considering that the scaling-up of ART will continue, the impact of this age effect on patients' immunological response is likely to increase over time.
Acknowledgements
We thank the fieldwork team, study sites and participants for their effort.
Source of funding: The International epidemiological Database to Evaluate AIDS in West Africa (IeDEA West Africa) is supported by: the National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Allergy And Infectious Diseases (NIAID) as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) (grant no. 5U01AI069919-01 to 05).
The International epidemiological Database to Evaluate AIDS in West Africa (IeDEAWest Africa) is supported by the National Institute of Health (NIH).
Footnotes
Contributors: E.B. and R.T. led the process of data analysis, interpretation, and paper writing. S.P.E., P.S.S., M.C., A.M. and J.D. are local co-investigators, they managed the fieldwork and co-interpreted the data and co-wrote the article. A.L. managed local fieldwork, conducted previous analysis and co-wrote the article. F.D. is the primary investigator of the IeDEA West Africa Collaboration and provided advice during data interpretation and writing. D.K.E. is the project manager, he managed the fieldwork and co-interpreted the data and co-wrote the article.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Conflicts of interest There are no conflicts of interest.
Additional study sites and participants The IeDEA West Africa Adults Group is constituted as follows:
Primary investigator: Pr François Dabis (INSERM U897, ISPED, Bordeaux, France).
Co-investigators: Samuel Ajayi, Clarisse Amani-Bosse, Franck Olivier Ba-Gomis, Emmanuel Bissagnene, Man Charurat, Eric Delaporte, Joseph Drabo, Serge-Paul Eholié, Serge-Olivier Koulé, Moussa Maiga, Eugène Messou, Albert Minga, Benson Okwara, Kevin Peterson, Papa Salif Sow, Hamar Traoré, Marcel D Zannou.
Other members: Gérard Allou, Xavier Anglaret, Jean-Claude Azani, Alain Azondékon, Eric Balestre, Jules Bashi, Patrick Coffie, Ye-Diarra, Didier K Ekouévi, Jean-François Etard, Antoine Jaquet, Alain Kouakoussui, Valériane Leroy, Charlotte Lewden, Karen Malateste, Lorna Renner, Annie Sasco, Haby Signaté Sy, Rodolphe Thiébaut, Marguerite Timité-Konan, Hapsatou Touré.
Adult Clinical centres:
Service de Médecine Interne et Tropicale (SMIT), CHU de Treichville, Abidjan, Côte d'Ivoire.
Unité de Soins Ambulatoires et de Conseil (USAC), Abidjan, Côte d'Ivoire.
Centre Médical de Suivi de Donneurs de Sang/CNTS/PRIMO-CI, Abidjan, Côte d'Ivoire.
ACONDA-MTCT-Plus, Abidjan, Côte d'Ivoire.
ACONDA-CePReF, Abidjan Côte d'Ivoire.
Centre Intégré de Recherche Bioclinique d'Abidjan (CIRBA), Abidjan, Côte d'Ivoire.
Service des Maladies Infectieuses, CHU de FANN/ISAARV, Dakar, Sénégal.
ANRS 1215 Cohort, Dakar, Senegal.
Service d'Hépato-Gastro-Entérologie, Hôpital Gabriel Touré, Bamako, Mali.
Centre de Prise en Charge des Personnes vivant avec le VIH, Hôpital du Point G, Bamako, Mali.
Fajara Cohort, Banjul, Gambia.
Service de Médecine Interne, CNHU Hubert Maga, Cotonou, Benin.
Service de Médecine Interne, CHU Yalgado, Ouagadougou, Burkina-Faso.
University of Abuja Teaching Hospital, Federal Capital Territory, Nigeria.
University of Benin Teaching Hospital, Edo State, Nigeria.
Coordinating centres.
Programme PAC-CI, CHU de Treichville, Abidjan, Côte d'Ivoire.
ISPED, Université Bordeaux Segalen, France.
References
- 1.Egger M, May M, Chêne G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 2.Seyler C, Anglaret X, Dakoury-Dogbo N, Messou E, Touré S, Danel C, et al. Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Côte d'Ivoire. Antivir Ther. 2003;8:385–393. [PubMed] [Google Scholar]
- 3.Laurent C, Ngom Gueye NF, Ndour CT, Gueye PM, Diouf M, Diakhaté N, et al. Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr. 2005;38:14–17. doi: 10.1097/00126334-200501010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 5.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in ressource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 6.Negin J, Cumming RG. HIV infection in older adults in sub-Saharan Africa: extrapolating prevalence from existing data. Bull World Health Organ. 2010;88:847–853. doi: 10.2471/BLT.10.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills EJ, Rammohan A, Awofeso N. Ageing faster with AIDS in Africa. Lancet. 2011;377:1131–1133. doi: 10.1016/S0140-6736(10)62180-0. [DOI] [PubMed] [Google Scholar]
- 8.Le Moing V, Chêne G, Carrieri MP, Besnier JM, Masquelier B, Salamon R, et al. Clinical, biologic, and behavioral predictors of early immunologic and virologic response in HIV-infected patients initiating protease inhibitors. J Acquir Immune Defic Syndr. 2001;27:372–376. doi: 10.1097/00126334-200108010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Grabar S, Kousignian I, Sobel A, Le Bras P, Gasnault J, Enel P, et al. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18:2029–2038. doi: 10.1097/00002030-200410210-00007. [DOI] [PubMed] [Google Scholar]
- 10.Nogueras M, Navarro G, Antón E, Sala M, Cervantes M, Amengual M, et al. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis. 2006;6:159. doi: 10.1186/1471-2334-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita TE, Phair JP, Muñoz A, Margolick JB, Detels R, O'Brien SJ, et al. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. AIDS. 2001;15:735–746. doi: 10.1097/00002030-200104130-00009. [DOI] [PubMed] [Google Scholar]
- 12.Viard JP, Mocroft A, Chiesi A, Kirk O, Røge B, Panos G, et al. Influence of age on CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: evidence from the EuroSIDA study. J Infect Dis. 2001;183:1290–1294. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi RT, Spritzler J, Chan E, Asmuth DM, Rodriguez B, Merigan TC, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–434. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 15.Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 16.Florence E, Lundgren J, Dreezen C, Fisher M, Kirk O, Blaxhult A, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the Euro-SIDA study. HIV Med. 2003;4:255–262. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 17.Marimoutou C, Chêne G, Mercié P, Neau D, Farbos S, Morlat P, et al. Prognostic factors of combined viral load and CD4R cell count responses under triple antiretroviral therapy, Aquitaine cohort, 1996–1998. J Acquir Immune Defic Syndr. 2001;27:161–167. doi: 10.1097/00126334-200106010-00011. [DOI] [PubMed] [Google Scholar]
- 18.Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–1473. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- 19.Althoff KN, Justice AC, Gange SJ, Deeks SG, Saag MS, Silverberg MJ, et al. Virologic and immunologic response to HAART, by age and regimen class. AIDS. 2010;24:2469–2479. doi: 10.1097/QAD.0b013e32833e6d14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen Stuart J, Hamann D, Borleffs J, Roos M, Miedema F, Boucher C, et al. Reconstitution of naive T cells during antiretroviral treatment of HIV-infected adults is dependent on age. AIDS. 2002;16:2263–2266. doi: 10.1097/00002030-200211220-00005. [DOI] [PubMed] [Google Scholar]
- 21.Bains I, Thiébaut R, Yates AJ, Callard R. Quantifying thymic export: combining models of naive T cell proliferation and TCR excision circle dynamics gives an explicit measure of thymic output. J Immunol. 2009;183:4329–4336. doi: 10.4049/jimmunol.0900743. [DOI] [PubMed] [Google Scholar]
- 22.Tumbarello M, Rabagliati R, de Gaetano Donati K, Bertagnolio S, Montuori E, Tamburrini E, et al. Older age does not influence CD4 cell recovery in HIV-1 infected patients receiving highly active antiretroviral therapy. BMC Infect Dis. 2004;4:46. doi: 10.1186/1471-2334-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlando G, Meraviglia P, Cordier L, Meroni L, Landonio S, Giorgi R, et al. Antiretroviral treatment and age-related co-morbidities in a cohort of older HIV-infected patients. HIV Med. 2006;7:549–557. doi: 10.1111/j.1468-1293.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 24.Rizzardini G, Trabattoni D, Saresella M, Piconi S, Lukwiya M, Declich S, et al. Immune activation in HIV-infected African individuals. Italian-Ugandan AIDS cooperation program. AIDS. 1998;12:2387–2396. doi: 10.1097/00002030-199818000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4R T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2011 doi: 10.1093/ije/dyr080. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The `Mortalité 2000 and 2005' surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 28.Mutevedzi PC, Lessells RJ, Rodger AJ, Newell ML. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLoS One. 2011;6:e21795. doi: 10.1371/journal.pone.0021795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br J Nutr. 2007;(Suppl 1):S11–S16. doi: 10.1017/S0007114507832880. [DOI] [PubMed] [Google Scholar]
- 30.ART-LINC of IeDEA Study Group. Keiser O, Tweya H, Boulle A, Braitstein P, Schecter M, Brinkhof MW, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Moing V, Thiébaut R, Chêne G, Sobel A, Massip P, Collin F, et al. Long-term evolution of CD4 count in patients with a plasma HIV RNA persistently <500 copies/mL during treatment with antiretroviral drugs. HIV Med. 2007;8:156–163. doi: 10.1111/j.1468-1293.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Spycher BD, Sidle J, Weigel R, Geng EH, Fox MP, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8:e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiébaut R, Jacqmin-Gadda H, Babiker A, Commenges D, CASCADE Collaboration Joint modelling of bivariate longitudinal data with informative dropout and left-censoring, with application to the evolution of CD4R cell count and HIV RNA viral load in response to treatment of HIV infection. Stat Med. 2005;24:65–82. doi: 10.1002/sim.1923. [DOI] [PubMed] [Google Scholar]
- 34.De Gruttola V, Tu XM. Modelling progression of CD4-lymphocyte count and its relationship to survival time. Biometrics. 1994;50:1003–1014. [PubMed] [Google Scholar]
- 35.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. J Am Med Assoc. 2008;300:506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]


