Abstract
Background
Cervical cancer, a rare outcome of high-risk human papillomavirus (HPV) infection, disproportionately affects African American women, who are about twice more likely than European American women to die of the disease. Most cervical HPV infections clear in about one year. However, in some women HPV persists, posing a greater risk for cervical dysplasia and cancer. The Carolina Women’s Care Study (CWCS) was conducted to explore the biological, genetic, and lifestyle determinants of persistent HPV infection in college-aged European American and African American women. This paper presents the initial results of the CWCS, based upon data obtained at enrollment.
Methods
Freshman female students attending the University of South Carolina were enrolled in the CWCS and followed until graduation with biannual visits, including two Papanicolaou tests, cervical mucus collection, and a questionnaire assessing lifestyle factors. We recruited 467 women, 293 of whom completed four or more visits for a total of 2274 visits.
Results and conclusion
CWCS participants were 70% European American, 24% African American, 3% Latina/Hispanic, and 3% Asian. At enrollment, 32% tested positive for any HPV. HPV16 infection was the most common (18% of infections). Together, HPV16, 66, 51, 52, and 18 accounted for 58% of all HPV infections. Sixty-four percent of all HPV-positive samples contained more than one HPV type, with an average of 2.2 HPV types per HPV-positive participant. We found differences between African American and European American women in the prevalence of HPV infection (38.1% African American, 30.7% European American) and abnormal Papanicolaou test results (9.8% African-American, 5.8% European American). While these differences did not reach statistical significance at enrollment, as the longitudinal data of this cohort are analyzed, the sample size will allow us to confirm these results and compare the natural history of HPV infection in college-aged African American and European American women.
Keywords: human papillomavirus, Carolina Women’s Care Study, papillomavirus persistence, race, ethnicity, health disparities
Introduction
Because of effective Papanicolaou screening, the US has a lower incidence of cervical cancer than many other countries in the world. However, it is estimated that 12,340 women will get cervical cancer (8.1 per 100,000) and 4030 will die of the disease (2.4 per 100,000) in the US in 2013. Cervical cancer incidence and mortality rates are higher for African American than for European American women. African American women are 1.2 times more likely to be diagnosed with cervical cancer, and have mortality rates twice those of European American women.1,2
Human papillomavirus (HPV) is the etiologic agent for cervical cancer.3 The natural history of cervical HPV infection is characterized by a transient asymptomatic infection that usually resolves without producing cytologic abnormalities.4,5 About 10% of HPV infections persist, leading to cytologic abnormalities of the cervical squamous epithelium, yet the majority of these infections will also resolve if left untreated.6 A small fraction of HPV infections lead to severe dysplasia that may, in turn, lead to invasive cervical cancer.3–5
It is often suggested that cervical cancer disparities may be a consequence of lack of access to Papanicolaou screening and follow-up.7,8 In addition, biological mechanisms, including those that would increase risk of HPV persistence, may play a role. Therefore, we designed a longitudinal cohort study, the Carolina Women’s Care Study (CWCS), that would control for access to health care, and allow for an exploration of biological and genetic determinants of HPV persistence in African American and European American women. This paper describes the design of the CWCS and compares HPV prevalence in European American and African American participants at enrollment.
Materials and methods
Study design
The CWCS was a prospective longitudinal study, with enrollment commencing in November 2004 and follow-up continuing through April 2011, on the Columbia campus of the University of South Carolina. The goals of the study were to gather information on the incidence and persistence of cervical HPV infections, and to elucidate behavioral and biological determinants of the natural history of HPV in a cohort of college-aged women attending the University of South Carolina. In addition to identification of HPV status and type, potential biological determinants of HPV persistence to be explored included germ line single nucleotide polymorphisms in blood-derived DNA, cytokine protein expression profiles in cervical mucus, and gene expression profiles in exfoliated cervical cells. Lifestyle cofactors considered include diet, stress, physical activity, depression, feelings of social discrimination, and sexual history. Approval for the study was obtained from the University of South Carolina institutional review board on July 29, 2004 and renewed annually thereafter.
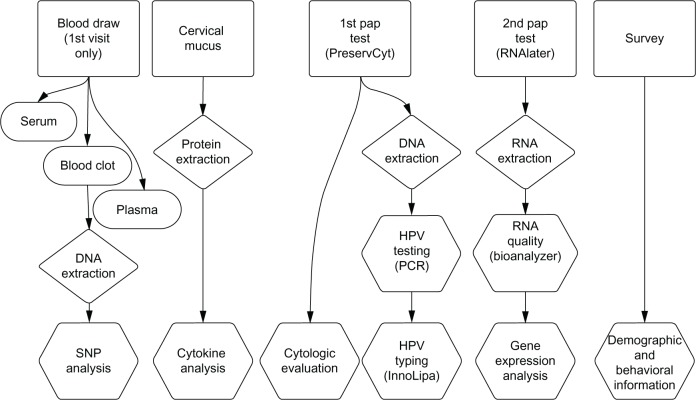
The overall design of the CWCS is shown in Figure 1. During the initial visit, blood specimens were obtained from study participants in collection tubes with and without EDTA. These samples were processed and stored as serum, plasma, and clot. Participants returned for biannual visits, one each fall and spring semester, during their time of enrollment at the university. At each visit, participants were given pelvic examinations, which included collection of cervical mucus and two Papanicolaou test samples. At the end of each study visit, participants completed a self-administered survey assessing their nutritional, exercise, and sexual history, as well as their emotional/psychosocial profile.
Figure 1.
Overall design of the Carolina Women’s Care Study.
Abbreviations: HPV, human papillomavirus; SNP, single nucleotide polymorphism; PCR, polymerase chain reaction; RNA, ribonucleic acid; DNA, deoxyribonucleic acid.
Recruitment and informed consent
All women between the ages of 18 and 22 years, enrolled for a minimum of 12 credit hours at the University of South Carolina, and classified as freshmen, were eligible for participation. Eligible participants were recruited through informational sessions, posters, pamphlets and a CWCS website, and through direct patient contact while visiting the campus-based Women’s Care Clinic located in the Thomson Student Health Center. At the initial visit, the study nurse practitioner described the study to the students. Those interested in joining the study signed two documents, ie, a written informed consent and a Health Insurance Portability and Accountability Act compliance document. Participants were given a copy of each document for future reference. A unique identifier with the corresponding barcode (Code 39, Barcodesinc, Chicago, IL, USA) was assigned to each new participant, and this number and barcode were used on the biological samples and surveys throughout the study. All of the clinical information, including contact information and unique participant identifier, was entered into a password-protected Microsoft Access 2003 database located in the Women’s Care Clinic.
Withdrawal and inclusion
Participants could remove themselves from the study at any time. Inclusion required continued full-time enrollment at the University. Participants were automatically withdrawn from the study if they were no longer enrolled full time, if they repeatedly missed their scheduled study visits, or if study personnel were unable to contact them.
Study population
A cohort of 467 female freshmen aged 18–25 years was included in the study, with staggered enrollment from November 2004 through May 2009. These study participants were followed through April 2011. Within this cohort, 293 women completed four or more visits, for a total of 2274 visits.
Study visits
The first study visit included a one-time blood draw with a single venous puncture used to collect two vials of blood. A BD Vacutainer® tube containing EDTA (BD Biosciences, Franklin Lakes, NJ, USA) was collected and used to isolate plasma, and a silicone-coated BD Vacutainer tube (BD Biosciences) was collected and used to isolate serum and clotted blood, the latter of which was used for genomic DNA extraction. When a blood sample could not be obtained, the study participant was asked to supply a saliva sample for genomic DNA isolation (DNA Genotek Inc, Ottawa, Canada).
At each visit, biometric measures were obtained by the nurse practitioner including height, weight, temperature, and blood pressure. Clinically apparent infections were noted if observed during the pelvic examination and appropriately treated. The pelvic examination included first the collection of cervical mucus and then two Papanicolaou test samples. Cervical mucus was obtained using a Merocel Eye Spear (Medtronic, Minneapolis, MN, USA). The sponge was held in contact with the cervical os for 30 seconds, and subsequently placed into a barcode-labeled empty transport vial that was immediately frozen at −20°C.
Following cervical mucus sampling, two cervical exfoliated cell samples were obtained using standard endocervical and ectocervical sampling methods. The first Papanicolaou test sample was collected in 20 mL of PreservCyt solution (Cytyc Corporation, Marlborough, MA, USA). Five mL of PreservCyt solution containing exfoliated cervical cells were removed and placed into a barcoded 15 mL conical tube for HPV detection and typing, and the remaining sample was sent for cytologic evaluation. The second cervical sample was collected in 2 mL of RNAlater (Ambion Inc, Carlsbad, CA, USA), placed in a 15 mL conical tube labeled with the participant’s barcode, and stored at −20°C.
Participants in the CWCS received treatment in accordance with the American Society for Colposcopy and Cervical Pathology guidelines of 2004, which recommended annual Papanicolaou tests for females 18 years of age and older, or within three years of the onset of sexual activity, whichever occurs first. Women with an abnormal cytologic finding, including atypical squamous cells of undetermined significance (ASCUS), a low-grade squamous intraepithelial lesion (LSIL), or a high-grade squamous intraepithelial lesion (HSIL), were referred to colposcopy. If abnormalities were found during colposcopic examination of the cervix, biopsies were taken and submitted for histologic interpretation. A diagnosis of cervical intraepithelial neoplasia 1 was followed with repeat Papanicolaou testing in six months and a diagnosis of cervical intraepithelial neoplasia 2 or above with ablative or excisional treatment. The American Society for Colposcopy and Cervical Pathology guidelines were revised in 2006, recommending that the first Papanicolaou test be performed at 21 years of age regardless of sexual activity. Additionally, the revised guidelines recommend colposcopy following cytologic diagnosis of HSIL, but not for ASCUS or LSIL, for females under 21 years of age. To reflect these changes, the study protocol was modified to refer participants to colposcopy as directed by the new guidelines.
A self-administered survey was given to each participant at the end of the physical examination to complete before leaving the Women’s Care Clinic. The completed survey was sealed in an envelope marked only with the participant’s numeric bar-coded label and returned to study personnel. This survey is described in detail further on in this section.
Study samples
DNA was extracted from exfoliated cervical cells collected in PreservCyt using sodium dodecyl sulfate/proteinase K digestion, followed by phenol/chloroform extraction and ethanol precipitation. Purified DNA was resuspended in 0.1 mL of 10 mM Tris HCL pH 8.0.
The presence of HPV was determined by real-time polymerase chain reaction (PCR) against the L1 region of the HPV genome using PGMY09/11 primers.9 Each sample was amplified using a final concentration of 200 pM of each primer in the presence of 1× BV buffer (16.6 mM ammonium sulfate, 67 mM Tris-HCl pH 8.8, 6.7 mM MgCl2, 10 mM β-mercaptoethanol), 1 mM each of dNTP and 6% dimethyl sulfoxide, 1 unit Platinum Taq (Invitrogen, Carlsbad CA, USA), and 1× Sybr dye (Invitrogen). Amplifications were performed on a MyIQ thermocycler (BioRad Hercules, CA, USA) with initial incubation at 94°C for two minutes, then 50 cycles of 94°C for 10 seconds, 57°C for 50 seconds, and 68°C for 50 seconds. The melt curve initiated at 52°C for 10 seconds, increasing by 0.5°C to 92°C. A 1.5 μL aliquot of each real-time PCR was separated by electrophoresis on a 1.5% agarose gel to visualize the 450 bp product expected in HPV-positive samples. In addition to the HPV PCR primers, PCR primers that amplified line DNA (F 5′-TTTTGAGTTAGGTGTGGGATATA-3′, R 5′-AAAATCAAAAAATTCCCTTTC-3′) or the human beta-globin gene (GH20 and PC04)9 were used to confirm the presence of amplifiable human DNA in the samples.
The INNO-LiPa HPV AMP kit (INNO-LiPA, Innogenetics, Gent, Belgium) and the INNO-LiPA genotyping extra kit (INNO-LiPA) were used to determine the type of HPV present in the samples found to be positive by real-time PCR screening. This reverse hybridization line blot assay is designed to detect and identify 27 HPV types utilizing amplification of the L1 region of the HPV genome. This test allows for the identification of seven low-risk (HPV 6, 11, 40, 43, 44, 54, and 70) and 15 high-risk (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82) HPV types. It also detects five HPV types of probable high-risk (HPV26, 53, 66, 69, and 74). In addition to the type-specific probes, the linear array contains two “generic” HPV probe mixtures that hybridize with virtually any HPV-derived amplification product. Samples that tested positive for one or both of the generic HPV bands on the linear array, but were negative for the type-specific bands, were considered HPV-positive and included in the analysis for total HPV infections, but were excluded from analysis of low-risk and high-risk HPV infections.
Self-administered survey
A self-administered survey was completed at each study visit. The initial survey included general demographic questions, such as ethnicity, parental education, and standardized college entrance examination scores, as well as behavioral questions involving alcohol, tobacco and marijuana use, physical exercise frequency, and duration. A history of the sexual behavior of the participants was obtained, including age at first sexual intercourse, number of sexual partners, birth control methods used, parity, and any history of abnormal Papanicolaou tests. Relationship histories, such as exposure to partner violence, were also included. A psychologic inventory was obtained using well established scales to assess feelings of stress, depression, or social discrimination.10–12 Fruit and vegetable intake was also assessed. The survey used at all subsequent visits (follow-up survey) included all questions from the initial survey with the exception of the static demographic questions. An additional fat intake screener was included in the follow-up survey.13,14
Participants were given a self-administered survey that asked questions designed to quantify underlying levels of stress, depression, or feelings of social discrimination. Three complete scales were used. The first, ie, The Global Measure of Perceived Stress (14 questions) is an instrument widely used to measure perceived stress,10 and although it is a non-diagnostic tool, it is valuable in revealing relative differences in perceived stress among populations.
The Center of Epidemiologic Study on Depression scale was presented in 1977 by Lenore Radloff11 and has been widely used in the field of psychiatric epidemiology to assess depression. Cutoff values validated for this scale were used in assessing depression in our participants, with a total score <15 indicating no depression, 15–21 indicating mild to moderate depression, and >21 indicating possible major depression. The Everyday Discrimination scale was added to assess feelings of discrimination.12 This assessment tool has been widely validated.15,16
At each visit, participants were asked to provide information about their diet. We used the Eating at America’s Table Survey to measure fruit and vegetable intake. This survey was developed to calculate the amount of antioxidants and vitamins ingested over the previous month. This scale was validated by Thompson et al.17 At all visits (except for the initial visit) a questionnaire was used to determine fat intake.13,14
Database management
Study data collected from the Women’s Care Clinic at the Thomson Student Health Center, University of South Carolina, were entered into and stored in a database written in Microsoft Access 2003. Deidentified study parameters were exported from the clinical database using Structured Query Language queries and imported into the main CWCS study database (CWCSdb). All laboratory data generated by the analysis of the study samples was entered or imported into the CWCSdb. The CWCSdb contains no protected health information. The CWCSdb is a relational database written in Microsoft Access 2003 and located on a dedicated computer housed in the laboratory of KC. The computer that housed the CWCSdb was set up as a non-networked, password-protected local workstation, and the database is password-protected using security on both the data storage and user interface sides of the database.
Statistical methods
Statistical analyses were carried out using the statistical programming language, R version 2.12.2.18 Descriptive analyses were performed using means, standard deviations, frequencies, and percentages. Student’s t-tests and Chi-square tests were used to assess proportional differences between HPV-positive/HPV-negative samples and between African and European American participants. Additionally, Chi-square permutation tests were performed on the categorical HPV-positive/HPV-negative variables as related to abnormal Papanicolaou diagnoses, and Kruskal–Wallis permutation tests on the ranked order variables for the number of concurrent HPV types.
Results
Study population and HPV positivity at enrollment
Of the 467 women enrolled in the CWCS, 69.8% were European American, 24.2% were African American, 2.8% were Hispanic, and 2.6% were of Asian or Pacific Island ancestry (Table 1). The average age of the participants at enrollment was 18.8 years. At enrollment, 93% of participants were sexually active, with an average age at first sexual intercourse of 16.4 years, and on average 3.7 sexual partners. At enrollment, 31.7% of the CWCS participants were HPV-positive. As expected, among the demographic and life history characteristics of the study population, age at first sexual activity and number of vaginal sexual partners were strongly associated with HPV positivity at enrollment. Only 36 of the 467 women had received at least one dose of the HPV vaccine (Gardasil®, Merck and Co Inc, Whitehouse Station, NJ, USA) at the time of enrollment and therefore we could not determine a meaningful correlation between vaccination and HPV positivity at this time.
Table 1.
Demographics of HPV-negative and HPV-positive CWCS participants at enrollment
| Characteristics | Total | HPV-negative | HPV-positive | P-value |
|---|---|---|---|---|
| Participants, n (%) | 467 (100.0) | 319 (68.3) | 148 (31.7) | |
| Age at enrollment, mean (SD)* | 18.8 (1.0) | 18.8 (1.1) | 18.8 (0.7) | 1.0000 |
| Age of first sexual activity, mean (SD)* | 16.4 (1.2) | 16.5 (1.2) | 16.0 (1.2) | 0.0003 |
| Number of vaginal sexual partners, mean (SD)* | 3.7 (4.1) | 2.9 (3.4) | 5.3 (5.0) | <0.0003 |
| Body mass index, mean (SD)* | 23.6 (4.4) | 23.6 (4.5) | 23.6 (4.3) | 0.9960 |
| CES-D scale, (SD)* | 10.9 (3.9) | 10.8 (3.7) | 11.2 (4.2) | 0.3001 |
| Everyday depression scale, mean (SD)* | 12.6 (8.3) | 12.3 (7.7) | 13.3 (9.5) | 0.2282 |
| Discrimination scale, mean (SD)* | 11.3 (7.3) | 11.2 (7.1) | 11.5 (7.5) | 0.6777 |
| HPV vaccine, n (%)** | 36 (7.7) | 28 (8.8) | 8 (5.4) | 0.2040 |
| Birth control method, n (%) | ||||
| Condom | 289 (61.9) | 205 (64.3) | 84 (56.8) | 0.1202 |
| Hormonal | 169 (36.2) | 108 (33.9) | 61 (41.2) | 0.1236 |
| Withdrawal | 97 (20.8) | 57 (17.9) | 40 (27.0) | 0.0232 |
| No birth control method used | 54 (11.6) | 46 (14.4) | 8 (5.4) | 0.0046 |
| Other/unknown | 20 (4.3) | 15 (4.7) | 5 (3.4) | 0.5344 |
| Ethnicity, n (%) | 0.1167 | |||
| European American | 326 (69.8) | 226 (70.8) | 100 (67.6) | |
| African American | 113 (24.2) | 70 (21.9) | 43 (29.1) | |
| Hispanic | 13 (2.8) | 10 (3.1) | 3 (2.0) | |
| Asian | 12 (2.6) | 11 (3.4) | 1 (0.7) | |
| Other/unknown | 3 (0.6) | 2 (0.6) | 1 (0.7) | |
| Papanicolaou result, n (%) | <0.0001 | |||
| Negative | 411 (88.0) | 308 (96.9) | 103 (69.6) | |
| ASCUS | 23 (4.9) | 6 (1.9) | 17 (11.5) | |
| LSIL | 27 (5.8) | 2 (0.6) | 25 (16.9) | |
| HSIL (including ASC-H) | 5 (1.1) | 2 (0.6) | 3 (2.0) | |
| No result | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Tobacco use, n (%) | 0.1933 | |||
| None | 332 (71.1) | 236 (74.0) | 96 (64.9) | |
| <25 cigarettes/month | 82 (17.6) | 54 (16.9) | 28 (18.9) | |
| ≥25 cigarettes/month | 51 (10.9) | 29 (9.1) | 22 (14.9) | |
| Unknown | 2 (0.4) | 0 (0.0) | 2 (1.4) | |
Notes:P-value reflects differences between African American and European American participants. Student t-test was used with variables designated with an asterisk (*). Chi-square test was used to assess proportional differences of the other variables; **at least one dose of the HPV vaccine (Gardasil®).
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; CES-D, Center for Epidemiologic Studies Depression Scale; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus; CWCS, Carolina Women’s Care Study; SD, standard deviation; ACT-H, atypical squamous cells with possible HSIL.
HPV positivity was not associated with the use of barrier methods of contraception (such as condoms) or hormonal treatments including the pill, patch, ring, and Depo Provera® (Pfizer Inc, New York, NY, USA) (Table 1). HPV positivity was associated with withdrawal. HPV-negative status was associated with no use of birth control. The CWCS enrolled 31 women who had not yet become sexually active. Therefore, it was not surprising that participants who reported no use of birth control were more likely to be HPV-negative.
Distribution of HPV type at enrollment
We observed 26 different types of HPV in our study population at the first visit (Table 2). A total of 364 HPV infections were detected at the first visit, 353 of which could be typed using the INNO-LiPa system. Of these 353 infections where the HPV type could be positively identified, 308 were high-risk and 45 were low-risk HPV types. HPV16 was the single most common type, accounting for 17.6% of all infections observed. Five high-risk HPV types (HPV16, 66, 51, 52, and 18) accounted for 58.4% of all HPV infections detected at enrollment and HPV6 (7.4% of all infections) was the most common low-risk HPV type observed. Interestingly, no HPV11 was detected.
Table 2.
Distribution of HPV types detected in CWCS participants at enrollment
| HPV | HR/LR | n | % |
|---|---|---|---|
| 16 | HR | 62 | 17.6 |
| 66 | HR | 53 | 15.0 |
| 51 | HR | 43 | 12.2 |
| 52 | HR | 24 | 6.8 |
| 18 | HR | 24 | 6.8 |
| 53 | HR | 20 | 5.7 |
| 31 | HR | 15 | 4.2 |
| 39 | HR | 14 | 4.0 |
| 73 | HR | 13 | 3.7 |
| 59 | HR | 9 | 2.5 |
| 33 | HR | 5 | 1.4 |
| 35 | HR | 5 | 1.4 |
| 56 | HR | 5 | 1.4 |
| 58 | HR | 4 | 1.1 |
| 68 | HR | 4 | 1.1 |
| 82 | HR | 3 | 0.8 |
| 69 | HR | 2 | 0.6 |
| 74 | HR | 2 | 0.6 |
| 45 | HR | 1 | 0.3 |
| 26 | HR | 0 | 0.0 |
| 6 | LR | 26 | 7.4 |
| 40 | LR | 9 | 2.5 |
| 54 | LR | 4 | 1.1 |
| 43 | LR | 2 | 0.6 |
| 44 | LR | 2 | 0.6 |
| 70 | LR | 2 | 0.6 |
| Total | 353 | 100 |
Abbreviations: HPV, human papillomavirus; CWCS, Carolina Women’s Care Study; HR, high-risk, LR, low-risk.
About 20% of participants, or 63.5% of the HPV-positive first-visit samples, were infected with multiple HPV types (Table 3). The average number of HPV types per HPV-infected individual at enrollment was 2.2. Of the participants infected with HPV at enrollment, about 22% had two HPV types and about 16% had three HPV types (Figure 2). At least one high-risk HPV type was detected in 90% of samples testing positive for any HPV.
Table 3.
HPV positivity, Papanicolaou test results, and tobacco use among African American and European American CWCS participants at enrollment
| Characteristics | Total | EA + AA | EA | AA | P-value |
|---|---|---|---|---|---|
| HPV positivity | |||||
| Any HPV n (%) | 148 (31.7) | 143 (32.6) | 100 (30.7) | 43 (38.1) | 0.1840 |
| HR HPV n (%) | 133 (28.5) | 129 (29.6) | 90 (27.6) | 39 (34.5) | 0.2050 |
| LR HPV n (%) | 38 (8.1) | 36 (8.2) | 24 (7.4) | 12 (10.6) | 0.3740 |
| Multiple types n (%) | 94 (20.1) | 90 (20.5) | 63 (19.3) | 27 (23.9) | 0.8120 |
| Number of HR types per | 2.2 (1.5) | 2.2 (1.5) | 2.2 (1.6) | 2.0 (1.3) | 0.4250 |
| HPV positive person, mean (SD)* | |||||
| HPV vaccine n (%)** | 36 (7.7) | 36 (8.2) | 28 (8.6) | 8 (7.1) | 0.6143 |
| Papanicolaou result n (%) | 0.5678 | ||||
| Negative | 411 (88.0) | 385 (87.7) | 289 (88.7) | 96 (84.9) | |
| ASCUS | 23 (4.9) | 23 (5.3) | 17 (5.2) | 6 (5.3) | |
| LSIL | 27 (5.8) | 25 (5.7) | 16 (4.9) | 9 (8.0) | |
| HSIL (including ASC-H) | 5 (1.1) | 5 (1.1) | 3 (0.9) | 2 (1.8) | |
| No result | 1 (0.2) | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Tobacco use n (%) | <0.0001 | ||||
| None | 332 (71.1) | 313 (71.3) | 209 (64.1) | 104 (92.0) | |
| <25 cigarettes/month | 82 (17.6) | 76 (17.3) | 69 (21.2) | 7 (6.2) | |
| ≥25 cigarettes/month | 51 (10.9) | 48 (10.9) | 46 (14.1) | 2 (1.8) | |
| Unknown | 2 (0.4) | 2 (0.5) | 2 (0.6) | 0 (0) | |
Notes: Proportional differences were assessed between African American and European American participants. The Student’s t-test was used for variables designated with an (*) and the Chi-square test was used to assess proportional differences of the other variables; **at least one dose of the HPV vaccine (Gardasil®).
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus; CWCS, Carolina Women’s Care Study; SD, standard deviation; ACT-H, atypical squamous cells with possible HSIL; HR, high-risk; LR, low-risk; EA, European American; AA, African American.
Figure 2.
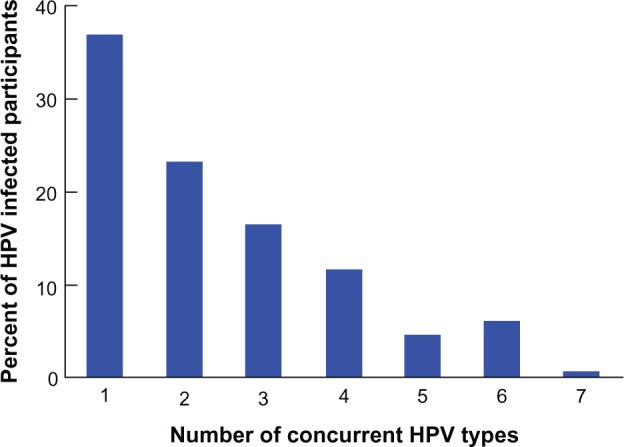
Distribution of HPV-positive study participants by number of concurrent HPV infections at enrollment.
Abbreviation: HPV, human papillomavirus.
HPV positivity in European American and African American participants at enrollment
African American women are 1.2 times more likely to be diagnosed with and about two times more likely to die of cervical cancer compared with European American women.1,2 Therefore, an important goal of the CWCS was to investigate differences in HPV infection between European American and African American women. At enrollment, 38.1% of the African American women were positive for any HPV, while 30.7% of European American women were positive (Table 3). The prevalence of high-risk HPV was 34.5% in African American women and 27.6% in European American women. While these data suggest a higher prevalence of HPV infection in African American women, the difference did not reach statistical significance.
Papanicolaou test results
The overall frequency of abnormal Papanicolaou test results (LSIL and HSIL, including a single case of atypical squamous cells with possible HSIL classified with HSIL) at entry was 6.9% (Table 1). As expected, a large difference (about 15-fold) in the frequency of Papanicolaou test abnormalities was observed between participants with and without HPV infection (Table 1 and Figure 3). There was no significant difference in abnormal Papanicolaou test frequency among HPV-positive participants, regardless of the number of concurrent HPV infections detected (Figure 3). The prevalence of abnormal Papanicolaou tests at enrollment was higher in African American women (9.8%) than in European American women (5.8%); however this difference did not reach statistical significance (Table 3).
Figure 3.
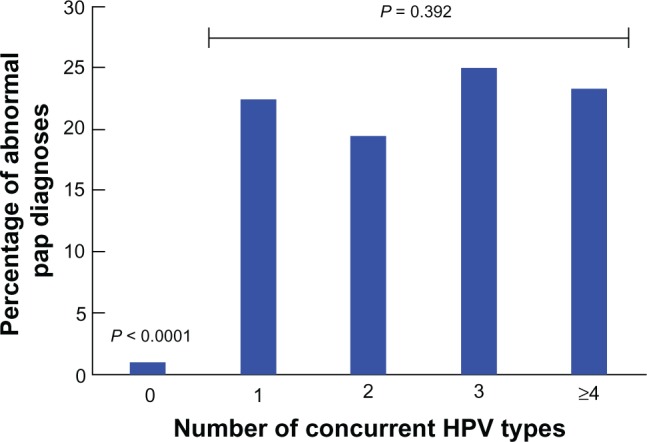
Multiple HPV infections and frequency of abnormal Papanicolaou tests. Percentage of Carolina Women’s Care Study participants with abnormal Papanicolaou tests (LSIL, HSIL) among HPV-negative (0 HPV) and HPV-positive participants grouped by number of concurrent HPV types detected at enrollment (1 HPV, 2 HPV, 3 HPV and ≥4 HPV).
Abbreviations: HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Other potential associations with HPV positivity at enrollment
Risk factors assessed in the CWCS that may be associated with HPV infection can be divided into the following categories: metabolic status, emotional distress, and smoking, with the rationale that all of these factors may affect immune function and influence the acquisition and/or course of HPV infection. We used body mass index as an indicator of metabolic status. Our study population had an average body mass index of 23.6 (Table 1). Seventy percent of study participants had a body mass index within the normal range (18.5–25) for their age and gender. Five percent were underweight (<18.5) and 25% were overweight or obese (>25). In total, 138 individuals (30%) were outside the normal range of body mass index values.
To quantify emotional distress, we utilized scales to assess stress (Center of Epidemiologic Study on Depression Scale), depression (Everyday Depression Scale), and feelings of discrimination (Discrimination Scale). Average values for these scales are presented in Table 1, and we found no differences in these values between HPV-positive and HPV-negative women at enrollment.
Tobacco use (smoking) is associated with altered immune function. About 11% of CWCS participants regularly used cigarettes (≥25 per month), and 71% reported no cigarette use. At enrollment, 14.9% of HPV-positive women smoked regularly, while 9.1% of HPV-negative women reported cigarette smoking. However, this difference did not reach statistical significance with HPV (Table 1).
Discussion
Several previous studies have shown that genital HPV infections are common among college-aged women.19–24 In a study of HPV infection and risk factors in women attending the University of Washington, 19.7% of the women tested positive for any HPV at enrollment and the most common HPV type at first infection was HPV16 (10.4%).19 Similarly, in a study of female students from a state university in New Jersey, 26% of the women tested positive at baseline and the incidences of HPV types 16, 51, and 66 were the highest.21 In a study of university students in Montreal, the baseline overall prevalence of any HPV was 29% (21.8% for high-risk HPV and 14.8% for low-risk HPV) and the most common types at enrollment were HPV16 (7.0%), HPV53 (4.3%), and HPV84 (3.8%).22 In the Young Women’s Health Study (mean age at baseline, 24.2 years) conducted in Arizona, the most common types of HPV were 16, 39, 84, and 51.23 Our baseline finding of 31.7% positivity for any HPV (28.5% for high-risk HPV and 8.1% for low-risk HPV) in college-aged women attending the University of South Carolina is slightly higher than these previous reports of HPV positivity in college-aged women, which ranged from 19.7% to 29%.19,21,22 Similar to the study of Ho et al,21 we found that the three most prevalent high-risk HPV types detected at enrollment in the CWCS were 16, 66, and 51. It is important to note that the INNO-LiPA method that we used for HPV typing does not detect HPV84, which was found to be common in prior studies.22,23 Early in the CWCS study, we typed HPV in the Papanicolaou test samples by DNA sequencing of PCR products from the L1 region and found that HPV84 was common in our study population. However, because many of our samples contained multiple HPV infections, which make interpretation of DNA sequencing results difficult, we switched to the INNO-LiPA method for HPV typing and repeated the typing using INNO-LiPA for all samples.
The first HPV vaccine (Gardasil) was approved by the US Food and Drug Administration for use in the US on June 8, 2006 for prevention of disease caused by two low-risk (HPV6 and 11) and two high-risk (HPV16 and 18) types. We had enrolled 252 participants prior to the approval of the vaccine and 215 participants were enrolled after Gardasil became available. Given that 36 of the 215 participants who entered the study after the release of Gardasil had received at least one dose, vaccine uptake in these women was 16.7%. Gardasil uptake is about 30% in 13–17-year-old females and 9% in 18–26-year-old females in the US.25
Multiple high-risk HPV infections are common, especially in younger women.26 In a routine screening population in Scotland, the prevalence of multiple HPV infection was 43.3% in samples that were HPV-positive.26 Our findings were higher, with 63.5% of the HPV-positive samples containing multiple HPV types. Studies in cohorts of women of a broad age range reported a significant correlation between concurrent infection with multiple HPV types and severity of cervical intraepithelial neoplasia27 or squamous intraepithelial lesions.28 In contrast, we observed no significant correlation between infection with multiple HPV types and the presence of cervical abnormalities at enrollment in the college-aged CWCS cohort. However, there were only 32 cases (6.9%) of abnormal Papanicolaou tests (LSIL and HSIL) at enrollment. Therefore, it will be interesting to determine if there is any correlation between multiple HPV types and cytologic abnormalities as we continue the analysis of our longitudinal data. It should be noted that the distribution of Papanicolaou test results we observed in the CWCS at enrollment is consistent with what has been previously reported in other populations of young women, in which the rates of abnormal Papanicolaou tests were 3%–16%.29,30
African American women in South Carolina are more likely to get cervical cancer than European American women and about twice as likely to die of the disease.7 While some of this disparity in risk of cervical cancer may be explained by lack of early detection among women who are not or rarely screened, other unknown factors, including biological determinants, are likely to play a role. A major goal of the CWCS is to explore the incidence, prevalence, and persistence of HPV in African American and European American women attending the University of South Carolina, to identify factors that contribute ultimately to HPV persistence in this population. African American women are well represented in our CWCS study population (24%). At enrollment, African American women had a slightly higher prevalence of HPV infection and abnormal Papanicolaou tests than European American women. Given the size of the study population, the observed differences have not yet reached statistical significance. However, as analysis of the longitudinal data continues in this cohort, we will be able to explore this difference further with a much larger sample size and also compare the natural history of HPV infection in college-aged African American and European American women.
Acknowledgments
This study was supported by a grant (P20MD001770) from the National Institute on Minority Health and Health Disparities. The authors would like to thank the Women’s Care Clinic at the University of South Carolina, in particular, the study nurse practitioners, Michelle Zager (MSN, CFNP) and Julie Cuy-Castellanos (MSN, WHNP), for collecting the samples and providing care for the study participants, Debbie Beck, Ed.D. for access and use of the facility, and the faculty and staff of the Women’s Care Center for their assistance in ensuring the success of the study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cancer Facts and Figures 2013 Atlanta, GA: American Cancer Society; 2013Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdfAccessed May 10, 2013 [Google Scholar]
- 2.SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012Available from: http://seer.cancer.gov/csr/1975_2009_pops09/Accessed May 10, 2013 [Google Scholar]
- 3.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 4.Goodman MT, Shvetsov YB, McDuffie K, et al. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008;68(21):8813–8824. doi: 10.1158/0008-5472.CAN-08-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285(23):2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 6.Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113(1):18–25. doi: 10.1097/AOG.0b013e31818f5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt HM, Modayil MV, Hurley D, et al. Cervical cancer disparities in South Carolina: an update of early detection, special programs, descriptive epidemiology, and emerging directions. J S C Med Assoc. 2006;102(7):223–230. [PubMed] [Google Scholar]
- 8.Barnholtz-Sloan J, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity. Cancer Causes Control. 2009;20(7):1129–1138. doi: 10.1007/s10552-009-9317-z. [DOI] [PubMed] [Google Scholar]
- 9.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 11.Radloff LS. The CES-D Scale A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 12.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 13.Thompson FE, Midthune D, Subar AF, Kipnis V, Kahle LL, Schatzkin A. Development and evaluation of a short instrument to estimate usual dietary intake of percentage energy from fat. J Am Diet Assoc. 2007;107(5):760–767. doi: 10.1016/j.jada.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Williams GC, Hurley TG, Thompson FE, et al. Performance of a short percentage energy from fat tool in measuring change in dietary intervention studies. J Nutr. 2008;138(1):212S–217S. doi: 10.1093/jn/138.1.212S. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61(7):1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Taylor TR, Kamarck TW, Shiffman S. Validation of the Detroit Area Study Discrimination Scale in a community sample of older African American adults: the Pittsburgh Healthy Heart Project. Int J Behav Med. 2004;11(2):88–94. doi: 10.1207/s15327558ijbm1102_4. [DOI] [PubMed] [Google Scholar]
- 17.Thompson FE, Subar AF, Smith AF, et al. Fruit and vegetable assessment: performance of 2 new short instruments and a food frequency questionnaire. J Am Diet Assoc. 2002;102(12):1764–1772. doi: 10.1016/s0002-8223(02)90379-2. [DOI] [PubMed] [Google Scholar]
- 18.R: A Language and Environment for Statistical Computing[computer program]. Version 2.12.2. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 19.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 20.Moscicki AB, Ma Y, Wibbelsman C, et al. Risks for cervical intraepithelial neoplasia 3 among adolescents and young women with abnormal cytology. Obstet Gynecol. 2008;112(6):1335–1342. doi: 10.1097/AOG.0b013e31818c9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338(7):423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 22.Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12(6):485–490. [PubMed] [Google Scholar]
- 23.Giuliano AR, Harris R, Sedjo RL, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women’s Health Study. J Infect Dis. 2002;186(4):462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 24.Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357(9271):1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 25.Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. J Adolesc Health. 2009;45(5):453–462. doi: 10.1016/j.jadohealth.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Cuschieri KS, Cubie HA, Whitley MW, et al. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J Clin Pathol. 2004;57(1):68–72. doi: 10.1136/jcp.57.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bello BD, Spinillo A, Alberizzi P, et al. Cervical infections by multiple human papillomavirus (HPV) genotypes: prevalence and impact on the risk of precancerous epithelial lesions. J Med Virol. 2009;81(4):703–712. doi: 10.1002/jmv.21429. [DOI] [PubMed] [Google Scholar]
- 28.Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1274–1280. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 29.Simsir A, Brooks S, Cochran L, Bourquin P, Ioffe OB. Cervicovaginal smear abnormalities in sexually active adolescents. Implications for management. Acta Cytol. 2002;46(2):271–276. doi: 10.1159/000326721. [DOI] [PubMed] [Google Scholar]
- 30.Mount SL, Papillo JL. A study of 10,296 pediatric and adolescent Papanicolaou smear diagnoses in northern New England. Pediatrics. 1999;103(3):539–545. doi: 10.1542/peds.103.3.539. [DOI] [PubMed] [Google Scholar]