Abstract
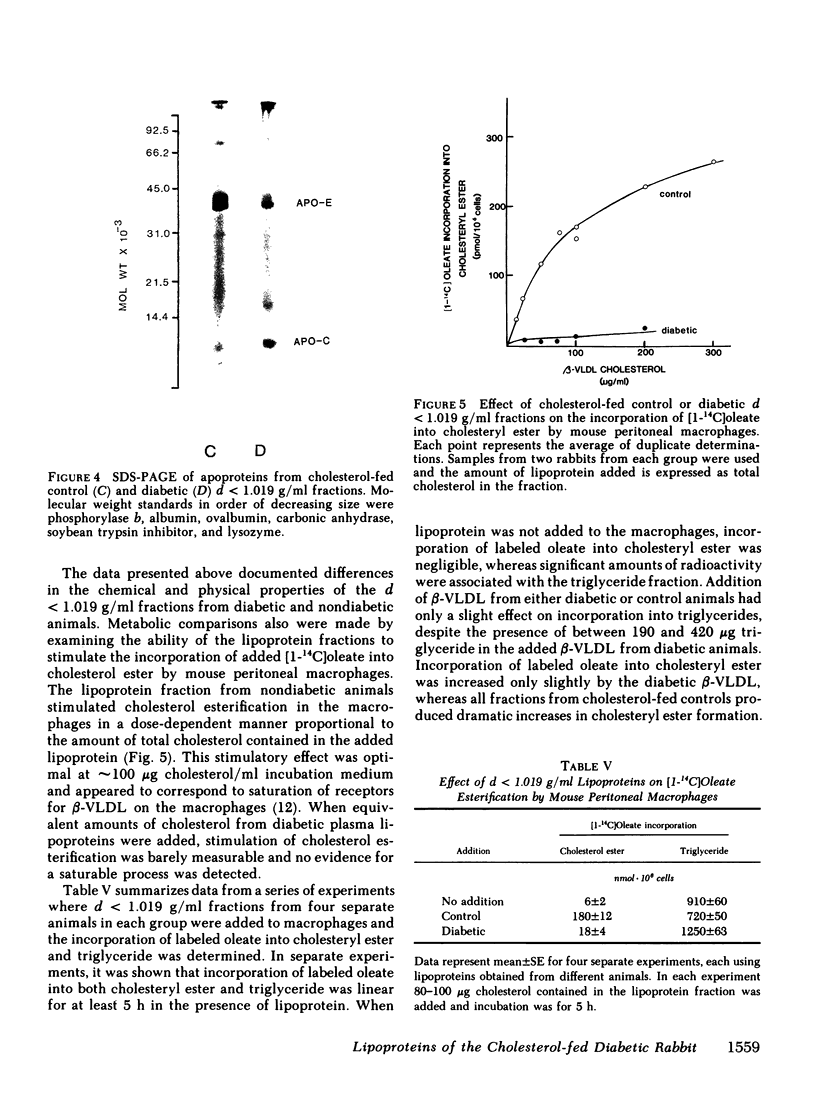
Alloxan-diabetic rabbits develop a pronounced hypercholesterolemia and hypertriglyceridemia in response to cholesterol feeding. Despite higher levels of plasma cholesterol, these animals have much less atherosclerosis than cholesterol-fed nondiabetics. To determine whether this effect is due to properties of the lipoproteins, we compared chemical, physical, and metabolic characteristics of a very low density lipoprotein (VLDL) fraction (d less than 1.019 g/ml) from the diabetic and nondiabetic cholesterol-fed rabbits. The molar ratio of triglyceride to cholesteryl ester in the particles from diabetic animals ranged from 2:1 to 6:1, and this ratio remained constant in subfractions from individual rabbits. Triglyceride from nondiabetic control animals was a minor component. Differential scanning calorimetry showed a distinct order-disorder phase transition for cholesteryl ester at approximately 42 degrees C in the fractions from control animals, whereas in fractions from most of the diabetics no such transition was observed, indicating that both triglyceride and cholesteryl ester are present in the core of the same particle. The relative amount of apoprotein E in particles from diabetic animals was much less than that of cholesterol-fed controls. The ability of the lipoproteins from both groups to stimulate cholesteryl ester formation in mouse peritoneal macrophages also was tested. Lipoproteins from cholesterol-fed controls stimulated cholesteryl ester formation in a dose-dependent manner, but particles from the diabetic group had little or no effect. The results suggest that the presence of unusual VLDL particles in diabetic cholesterol-fed rabbits is responsible, at least in part, for the reduced incidence of atherosclerosis in this animal model.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- COOK D. L., MILLS L. M., GREEN D. M. The mechanism of alloxan protection in experimental atherosclerosis. J Exp Med. 1954 Feb;99(2):119–124. doi: 10.1084/jem.99.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camejo G., Bosch V., López A. The very low density lipoproteins of cholesterol-fed rabbits. A study of their structure and in vivo changes in plasma. Atherosclerosis. 1974 Jan-Feb;19(1):139–152. doi: 10.1016/0021-9150(74)90050-1. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Cone J. T., Segrest J. P. Defective in vitro lipolysis of type IV hyperlipidemic human plasma by purified milk lipoprotein lipase. Studies by single vertical spin centrifugation. J Biol Chem. 1982 Jul 10;257(13):7472–7480. [PubMed] [Google Scholar]
- DOLE V. P., HAMLIN J. T., 3rd Particulate fat in lymph and blood. Physiol Rev. 1962 Oct;42:674–701. doi: 10.1152/physrev.1962.42.4.674. [DOI] [PubMed] [Google Scholar]
- DUFF G. L., BRECHIN D. J., FINKELSTEIN W. E. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. IV. The effect of insulin therapy on the inhibition of atherosclerosis in the alloxan-diabetic rabbit. J Exp Med. 1954 Oct 1;100(4):371–380. doi: 10.1084/jem.100.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFF G. L., PAYNE T. P. B. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. III. The mechanism of the inhibition of experimental cholesterol atherosclerosis in alloxan-diabetic rabbits. J Exp Med. 1950 Oct 1;92(4):299–317. doi: 10.1084/jem.92.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M. Structure and interactions of lipids in human plasma low density lipoproteins. J Biol Chem. 1977 Jan 25;252(2):744–754. [PubMed] [Google Scholar]
- Deckelbaum R. J., Tall A. R., Small D. M. Interaction of cholesterol ester and triglyceride in human plasma very low density lipoprotein. J Lipid Res. 1977 Mar;18(2):164–168. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fletcher M. J. A colorimetric method for estimating serum triglycerides. Clin Chim Acta. 1968 Nov;22(3):393–397. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- GOFMAN J. W., LINDGREN F. The role of lipids and lipoproteins in atherosclerosis. Science. 1950 Feb 17;111(2877):166–171. doi: 10.1126/science.111.2877.166. [DOI] [PubMed] [Google Scholar]
- Gianturco S. H., Bradley W. A., Gotto A. M., Jr, Morrisett J. D., Peavy D. L. Hypertriglyceridemic very low density lipoproteins induce triglyceride synthesis and accumulation in mouse peritoneal macrophages. J Clin Invest. 1982 Jul;70(1):168–178. doi: 10.1172/JCI110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianturco S. H., Brown F. B., Gotto A. M., Jr, Bradley W. A. Receptor-mediated uptake of hypertriglyceridemic very low density lipoproteins by normal human fibroblasts. J Lipid Res. 1982 Sep;23(7):984–993. [PubMed] [Google Scholar]
- Gianturco S. H., Gotto A. M., Jr, Hwang S. L., Karlin J. B., Lin A. H., Prasad S. C., Bradley W. A. Apolipoprotein E mediates uptake of Sf 100-400 hypertriglyceridemic very low density lipoproteins by the low density lipoprotein receptor pathway in normal human fibroblasts. J Biol Chem. 1983 Apr 10;258(7):4526–4533. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Brown M. S., Innerarity T. L., Mahley R. W. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J Biol Chem. 1980 Mar 10;255(5):1839–1848. [PubMed] [Google Scholar]
- Holdsworth G., Stocks J., Dodson P., Galton D. J. An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J Clin Invest. 1982 Apr;69(4):932–939. doi: 10.1172/JCI110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Kroon P. A., Seidenberg J. Organization of the core lipids of lipoproteins from normal and cholesterol-fed rabbits. A proton nuclear magnetic resonance study. Biochemistry. 1982 Dec 7;21(25):6483–6488. doi: 10.1021/bi00268a025. [DOI] [PubMed] [Google Scholar]
- Kroon P. A. The order-disorder transition of the core cholesteryl esters of human plasma low density lipoprotein. A proton nuclear magnetic resonance study. J Biol Chem. 1981 Jun 10;256(11):5332–5339. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lichtenstein A. H., Brecher P. Properties of acyl-CoA:cholesterol acyltransferase in rat liver microsomes. Topological localization and effects of detergents, albumin, and polar steroids. J Biol Chem. 1980 Oct 10;255(19):9098–9104. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- Mahoney E. M., Khoo J. C., Steinberg D. Lipoprotein lipase secretion by human monocytes and rabbit alveolar macrophages in culture. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1639–1642. doi: 10.1073/pnas.79.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGILL G. C., Jr, HOLMAN R. L. The influence of alloxan diabetes on cholesterol atheromatosis in the rabbit. Proc Soc Exp Biol Med. 1949 Oct;72(1):72–75. doi: 10.3181/00379727-72-17335. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Small D. M. Triolein-cholesteryl oleate-cholesterol-lecithin emulsions: structural models of triglyceride-rich lipoproteins. Biochemistry. 1983 Jan 18;22(2):443–451. doi: 10.1021/bi00271a030. [DOI] [PubMed] [Google Scholar]
- PIERCE F. T., Jr The relationship of serum lipoproteins to atherosclerosis in the cholesterol-fed alloxanized rabbit. Circulation. 1952 Mar;5(3):401–407. doi: 10.1161/01.cir.5.3.401. [DOI] [PubMed] [Google Scholar]
- RAABO E., TERKILDSEN T. C. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960;12(4):402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Snibson D. A. Clearance of chylomicron triacylglycerol and cholesteryl ester from the plasma of streptozotocin-induced diabetic and hypercholesterolemic hypothyroid rats. Metabolism. 1977 May;26(5):493–503. doi: 10.1016/0026-0495(77)90093-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. L., Ghiselli G. C., Torreggiani D., Sirtori C. R. Very low density lipoproteins in normal and cholesterol-fed rabbits: lipid and protein composition and metabolism. Part 1. Chemical composition of very low density lipoproteins in rabbits. Atherosclerosis. 1976 Jan-Feb;23(1):73–83. doi: 10.1016/0021-9150(76)90119-2. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J Clin Invest. 1980 Mar;65(3):652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore V. G., Shore B., Hart R. G. Changes in apolipoproteins and properties of rabbit very low density lipoproteins on induction of cholesteremia. Biochemistry. 1974 Apr 9;13(8):1579–1585. doi: 10.1021/bi00705a004. [DOI] [PubMed] [Google Scholar]
- Waugh D. A., Small D. M. Rapid method for determining cholesteryl ester transitions of apoB-containing lipoproteins. J Lipid Res. 1982 Jan;23(1):201–204. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]