Abstract
Neuropeptidergic signaling is widely adopted by animals for the regulation of physiology and behavior in a rapidly changing environment. The vasopressin/oxytocin neuropeptide family originates from an ancestral peptide precursor in the antecedent of protostomian and deuterostomian animals. In vertebrates, vasopressin and oxytocin have both hormonal effects on peripheral target tissues, such as in the regulation of reproduction and water balance, and neuromodulatory actions in the central nervous system controlling social behavior and cognition. The recent identification of vasopressin/oxytocin-related signaling in C. elegans reveals that this peptidergic system is widespread among nematodes. Genetic analysis of the C. elegans nematocin system denotes vasopressin/oxytocin-like peptides as ancient neuromodulators of neuronal circuits involved in reproductive behavior and associative learning, whereas former invertebrate studies focused on conserved peripheral actions of this peptide family. Nematocin provides neuromodulatory input into the gustatory plasticity circuit as well as into distinct male mating circuits to generate a coherent mating behavior. Molecular interactions are comparable to those underlying vasopressin- and oxytocin-mediated effects in the mammalian brain. Understanding how the vasopressin/oxytocin family fine-tunes neuronal circuits for social behavior, learning and memory poses a major challenge. Functional conservation of these effects in nematodes and most likely in other invertebrates enables the development of future models to help answering this question.
Keywords: vasopressin, oxytocin, neuropeptide, nematocin, C. elegans, neuromodulation, learning, reproductive behavior
Introduction
The neuropeptide hormones vasopressin and oxytocin are important regulators of animal physiology and behavior. Originally extracted from the mammalian pituitary, they were the first neuropeptides to be purified and sequenced in the 1950s.1,2 Since their discovery in mammals, vasopressin- and oxytocin-like peptides have been identified in most vertebrates and several invertebrate species,3-5 dating back their origin more than 700 million years. Vasopressin, or antidiuretic hormone, is mainly known for its actions in fluid homeostasis and the regulation of blood pressure,6,7 while oxytocin stimulates uterine contractions during birth and induces milk ejection from mammary glands.8 Besides peripheral hormonal effects, both peptides act as neuromodulators in the brain influencing social behavior, memory and learning.9,10 Vasopressin- and oxytocin-mediated effects in the central nervous system are consistent with the long-range diffusion of peptides from hypothalamic centers in addition to local peptidergic release from neuronal fibers projecting to specific brain areas.11 Nevertheless, the precise neural mechanisms by which these neuropeptides affect our behavior remain unclear. Here we review the evolution of vasopressin and oxytocin peptides and their functions and integrate new insights from the recent genetic analysis of a related signaling system in the nematode Caenorhabditis elegans.12,13
Phylogeny of the Vasopressin/Oxytocin Signaling System
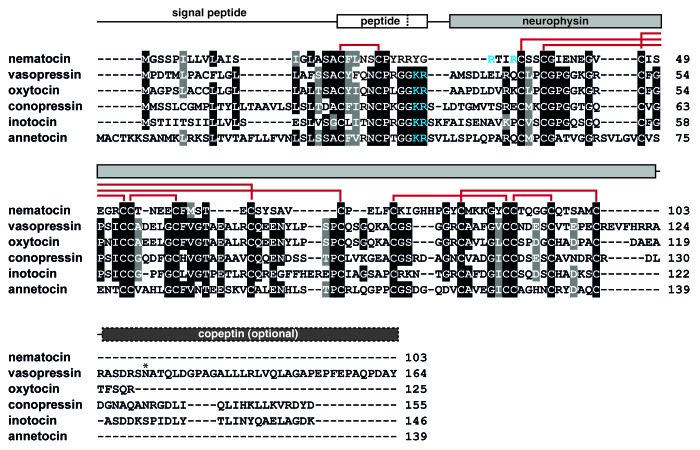
Vasopressin- and oxytocin-related peptides are present in representatives of the protostomian (most invertebrates) and deuterostomian (some invertebrates and all vertebrates) lineages,3-5 indicating that this hormonal system originated early in evolution. Invertebrates, with a few exceptions, have only one peptide homolog, whereas most vertebrates have two: a vasopressin- and oxytocin-like peptide (Table 1). Based on structural and positional similarities of the vasopressin and oxytocin genes, it is thought they originate from duplication of a common ancestral gene, which likely occurred after the radiation of the jawless fish about 500 million years ago.3,14 Rapid evolution following gene amplification continued the diversification of the vertebrate vasopressin/oxytocin family (Table 1).3,14 The amino acid at position eight in the nonapeptide sequence represents a distinguishing feature for the biological activity of these peptides: oxytocin-like sequences generally carry a neutral residue, whereas the same position is occupied by a basic residue in vasopressin-like peptides.14 The discovery of related invertebrate hormones in annelids, insects and mollusks among others reveals a more diverse sequence repertoire, for which classification according to the eighth amino acid no longer holds (Table 1).4,15,16 Despite the variety of peptide sequences identified in vertebrate and invertebrate species, all members of the vasopressin/oxytocin family share a similar, cyclized architecture at both the peptide and precursor level. The precursor protein typically contains a signal peptide, immediately followed by the mature peptide, a basic cleavage site and a cysteine-rich neurophysin domain (Fig. 1). The latter is thought to facilitate the folding and trafficking of vasopressin/oxytocin-like peptides through the secretory pathway.6 In mammalian vasopressin precursors, an additional glycoprotein (copeptin) is present at the C-terminus (Fig. 1), which is also found as a highly divergent extension of the neurophysin domain in lower vertebrates.3
Table 1. Vertebrate and invertebrate members of the vasopressin/oxytocin peptide family.
| Peptide name | Sequencea | Sourceb |
|---|---|---|
|
Vertebrate vasopressin-related peptides | ||
| arg-vasopressin |
CYFQNCPRG-NH2 |
mammals |
| lys-vasopressin |
CYFQNCPKG-NH2 |
pig, some marsupials |
| phenypressin |
CFFQNCPRG-NH2 |
some marsupials |
| vasotocin |
CYIQNCPRG-NH2 |
non-mammalian vertebrates |
|
Vertebrate oxytocin-related peptides | ||
| oxytocin |
CYIQNCPLG-NH2 |
mammals |
| P8-oxytocin |
CYIQNCPPG-NH2 |
New World monkeys |
| mesotocin |
CYIQNCPIG-NH2 |
non-mammalian vertebratesc |
| isotocin |
CYISNCPIG-NH2 |
bony fishes |
| glumitocin |
CYISNCPQG-NH2 |
cartilaginous fishes (rays)d |
| valitocin |
CYIQNCPVG-NH2 |
cartilaginous fishes (sharks)e |
| aspargtocin |
CYINNCPLG-NH2 |
cartilaginous fishes (sharks)e |
| asvatocin |
CYINNCPVG-NH2 |
cartilaginous fishes (sharks)f |
| phasvatocin |
CYFNNCPVG-NH2 |
cartilaginous fishes (sharks)f |
|
Invertebrate vasopressin/oxytocin-related peptides | ||
|
A. Urochordates |
|
|
|
Styela OT-like peptide |
CYISDCPNSRFWST-NH2 |
Styela plicata |
|
Ciona VP/OT-like peptide |
CFFRDCSNMDWYR |
Ciona intestinalis |
|
B. Echinoderms |
|
|
| echinotocin |
CFISNCPKG-NH2 |
Strongylocentrotus purpuratus |
|
C. Mollusks |
|
|
| lys-conopressin |
CFIRNCPKG-NH2 |
various mollusks |
| arg-conopressin |
CIIRNCPRG-NH2 |
Conus striatus |
| cephalotocin |
CYFRNCPIG-NH2 |
Octopus vulgaris |
| octopressin |
CFWTSCPIG-NH2 |
Octopus vulgaris |
|
D. Arthropods |
|
|
| crustacean VP/OT-like peptide |
CFITNCPPG-NH2 |
Daphnia pulex |
| inotocin |
CLITNCPRG-NH2 |
various insects |
| |
CLIVNCPRG-NH2 |
Camponotus floridanus |
|
E. Annelids |
|
|
| annetocin |
CFVRNCPTG-NH2 |
Eisenia fetida |
| lys-conopressin |
CFIRNCPKG-NH2 |
Erpobdella octoculata |
| hirudotocin |
CFIRNCPLG-NH2 |
Hirudo medicinalis |
|
F. Nematodes |
|
|
| nematocin |
CFLNSCPYRRY-NH2 |
Caenorhabditis elegans |
| |
|
Caenorhabditis remanei |
| |
|
Caenorhabditis brenneri |
| |
|
Caenorhabditis briggsae |
| |
|
Caenorhabditis japonica |
| |
|
Pristionchus pacificus |
| |
|
Necator americanus |
| |
|
Ancylostoma caninum |
| |
CFLNSCPFRRY-NH2 |
Strongyloides stercoralis |
| CFLNSCPYRRI-NH2 | Bursaphelenchus xylophilus | |
a Identical and similar amino acids in 70% of all sequences are indicated in bold or underlined, respectively; bFor references see 3–5,11,13,15,29,36clungfishes, amphibians, reptiles, birds and some marsupials; dRaia clavata; eSqualus acanthias; fScyliorhinus caniculus. VP, vasopressin; OT, oxytocin.
Figure 1. Schematic architecture and sequence alignment of vasopressin/oxytocin-related precursor proteins. Amino acid sequences are aligned for representative precursors (with GenBank accession numbers) from mammals, ecdyzozoans and lophotrochozoans: C. elegans nematocin (AFJ42491.1), human vasopressin (AAA61291.1) and oxytocin (AAA59977.1), Lymnea stagnalis conopressin (AAB35220.1), Tribolium castaneum inotocin (NP_001078831.1) and Eisenia fetida annetocin (CAD20057.2). Identical residues are highlighted in black and similar amino acids in gray. Disulfide bridges in the peptide and neurophysin domain are indicated with red lines and cleavage sites for proprotein convertases in blue. An additional glycopeptide, copeptin, is cleaved from the human vasopressin precursor, but probably not from other depicted sequences. Glycosylation of copeptin occurs at the asterisk-marked residue.
Recently, a novel vasopressin/oxytocin family member has been identified in the nematode C. elegans. The structure of the nematocin precursor is reminiscent of the vasopressin and oxytocin precursors (Fig. 1),5,12,13 but the mature peptide has two additional residues at its C-terminus compared with related invertebrate nonapeptides (Table 1).12,13 Supported by similar findings in urochordates (Table 1A),17,18 a sister group to vertebrates, this denotes high pressure on conserving the cyclic structure of vasopressin/oxytocin-like peptides rather than the peptide length. Nematocin contains a putative internal dibasic cleavage site; nevertheless, the full-length neuropeptide is present in vivo12 and the C-terminal Arg and Tyr residues are crucial for activation of its receptor.13 The signaling system is widespread among free-living and parasitic nematodes and peptide sequences are invariant, except for minor changes in the human parasite Strongyloides stercoralis and the pine wood nematode Bursaphelenchus xylophilus (Table 1F).13 Most published genomes of nematodes belonging to the Rhabditina (clade V) and Tylenchina (clade IV) groups, with few exceptions, contain the nematocin gene. Remarkably, nematocin is probably absent in the sequenced genomes of Brugia malayi and Ascaris suum, both members of the Spirurina group (clade III) and in Trichinella spiralis, classified in the Dorylaimia (clade I). In this context, it is interesting to note that the life cycle of these parasites is not characterized by an active, free-living larval stage outside the vector or host, unlike most free-living and parasitic species in which nematocin is present (Table 1F). A possible explanation for the absence of the nematocin system in some nematode species thus might be that it was lost during adaptation to different lifestyles. The discontinuous conservation of vasopressin/oxytocin-related peptides has been reported previously in arthropods as well. In contrast to crustaceans and basal insects, related sequences are missing from the genomes of the fruit fly, the silkworm, the honey bee and others.4 In these species, it is hypothesized that competing hormonal systems have taken over the function of vasopressin/oxytocin signaling.4 The sequencing and completion of additional nematode genomes together with refined insights in nematode phylogeny will allow better understanding of the early evolution of vasopressin/oxytocin-related signaling.
In vertebrates, four types of vasopressin/oxytocin receptors are classified with distinct expression patterns and biological effects: the oxytocin receptor and the vasopressin receptors V1a, V1b and V2.6 A fifth receptor type (V2b) is thought to be present in fish and some tetrapods.19,20 The number of invertebrate receptor homologs varies from one up to three in a given species,13 but the evolutionary history of receptor subtypes remains unclear. The vasopressin/oxytocin receptor family is most closely related to the superfamily of gonadotropin-releasing hormone receptors, which have also been found in a wide variety of deuterostomian and protostomian lineages including C. elegans.21,22 Phylogenetic analysis suggests a shared ancestry with deep roots between these two G protein-coupled receptor (GPCR) families.21 Besides these, many neuropeptidergic systems are suggested to have an ancient origin. Well studied examples are the tachykinin, neuropeptide Y, somatostatin and galanin systems.23,24 C. elegans also contains a number of evolutionary conserved neuropeptide GPCRs including cholecystokinin, vasoactive intestinal peptide and neuromedin U-related receptors.25-28 Most likely, many other conserved neuropeptide-GPCR systems will be discovered in the near future.
Evidence for the Conservation of Vasopressin- and Oxytocinergic Brain Centers
In the vertebrate brain, vasopressin- and oxytocin-like peptides are mainly synthesized by distinct neuronal populations in the hypothalamic paraventricular, supraoptic and accessory nuclei.6,11 Axonal projections from magnocellular neurons in these brain regions shuttle nonapeptides to the posterior lobe of the pituitary, where they are stored and released into the peripheral circulation. Alternatively, release from neuronal sites in- and outside the hypothalamus results in the local delivery and subsequent diffusion of nonapeptides in the brain.6 In invertebrates, vasopressin- and oxytocin-related peptides are similarly produced by neurons with cell bodies located in cerebral ganglia or occasionally in peripheral ganglia.13,29 These neurons are often characterized by projections to the brain as well as long projecting axons that reach distant parts of the body and in some cases couple directly to the circulatory system.16,30,31 Evolutionary conservation extends further with the hypothesis that the cells responsible for producing vasopressin- and oxytocin-related peptides are located in similar neurosecretory brain centers, which are characterized by a typical “molecular fingerprint.” Tessmar-Raible et al. show that neurons producing the vasopressin homolog in annelids and fish express common tissue-restricted microRNAs and a cell-type-specific combination of transcription factors orthologous to the vertebrate orthopedia, retina homeobox and nk2.1 genes.32 These gene regulatory features specify the identity of an ancient vasopressin-/oxytocinergic neuronal cell type that most likely possesses dual sensory-neurosecretory properties. Co-expression of vasopressin-like peptides and opsins in the zebrafish and annelid nervous system suggests the direct coupling of peptide secretion to light cycles.32 In C. elegans, although not exclusively, nematocin is strongly expressed in neurons that are able to sense thermal or mechanical cues.12,13 Sensory-neurosecretory cells may thus represent an ancient neuronal architecture for the vasopressin/oxytocin-related signaling system, which can directly convey sensory input to changes in physiology or behavior through peptidergic secretion.
Vasopressin and Oxytocin Functions Throughout Evolution: Peripheral and Central Actions
Pleiotropic effects of the vasopressin/oxytocin family in homeostatic regulation include the control of stress responses, metabolism and circadian rhythms among others;6,33 here, we focus on those functions most studied in invertebrates as well. Vasopressin and oxytocin are myoactive peptides that stimulate contractions in a variety of tissues. In mammals, vasopressin causes vasoconstriction through V1a receptors on vascular smooth muscle,6 and oxytocin elicits contractions during parturition and lactation through oxytocin receptors on myometrial cells of the uterus and myoepithelial cells of mammary glands.8 Myoactivity is one of the best conserved functions of vasopressin- and oxytocin-related peptides;5,15 for example, the octopus homolog elicits contractions in reproductive and cardiovascular tissues, expressing the octopressin receptor.29
In vertebrates, vasopressin is also dedicated to fluid homeostasis. Activation of V2-type receptors in the mammalian kidney causes antidiuresis by stimulating water reabsorption from the renal collecting ducts and in non-mammalian tetrapods, vasotocin reduces glomerular filtration rates.7 Despite several indications for putative anti- or diuretic effects of vasopressin/oxytocin-related peptides in invertebrates, their role in osmoregulation remains uncertain. Diuretic activity of inotocin, the insect homolog, is questioned by contradictory reports in locusts4 and only minor inotocin receptor expression was found in excretory tissues of the red flour beetle,4 suggesting indirect effects on water balance.30 Annetocin reduces body-weight of leeches34 and evokes contractions of the earthworm’s excretory nephridia,15 but these effects are more likely to be interpreted as reproduction-related actions.34,35 A role in primitive osmoregulation is found in the sea squirt, where a vasopressin/oxytocin-related peptide induces siphon closure to prevent the influx of dilute seawater.17
Similar to the effects of oxytocin on mammalian reproduction,8 members of the vasopressin/oxytocin family influence egg-laying in invertebrates.15,16,36 Besides classical effects in reproduction, vasopressin- and oxytocin-like peptides have been found to regulate reproductive behaviors such as mating in vertebrates and invertebrates.12,37 In medicinal leeches, conopressin induces a stereotypical twisting of the body that resembles spontaneous mate exploration by acting on a central pattern generator of oscillating neurons in reproductive midbody ganglia.36 In Lymnea stagnalis, the same peptide is expressed in male neurons that innervate the penis complex and vas deferens. Here, peptidergic release results in muscular contractions of the vas deferens expressing the conopressin receptor.16,38 Similarly, vasopressin and oxytocin induce contractions within rat and rabbit ejaculatory tissues in addition to regulating sexual behavior in the central nervous system.37,39
Hormonal vasopressin/oxytocin-related effects such as on fluid homeostasis and reproduction are mediated by receptors on peripheral target tissues, reached by peptides released into the blood stream or from long-distance projecting axons. Vasopressin and oxytocin receptors are also found in the vertebrate brain and are directly targeted by peptidergic release within the central nervous system. The central vasopressin-/oxytocinergic system is indicative of the neuromodulatory functions of these neuropeptides, which have been extensively reviewed elsewhere.9-11,40 Oxytocin and also vasopressin, are particularly important for the expression of affiliative behaviors such as parental care, pair bonding and partner preference, which rely on the formation of a social memory from visual, auditory or olfactory cues.10 Both neuropeptides also moderate physiological stress responses and anxious behavior, often with opposing effects.11 Vasopressin/oxytocin signaling in the mammalian hippocampus and lateral septum is important for learning and memory.11,40 In a social context, central administration of vasopressin increases social memory in rats.11 The modulatory effects on non-social learning and memory were established by the pioneering work of de Wied and colleagues.40 Vasopressin- and oxytocin-related receptors are present in the central nervous system of several invertebrates,29,38 but little or no information is available on their actions, keeping the evolutionary origin of central vasopressin and oxytocin effects in the dark.
Vasopressin/Oxytocin-related Signaling in C. elegans: A Conserved Regulator of Reproductive Behavior
Despite the characterization of many vasopressin/oxytocin-related peptides and their receptors from invertebrates, evidence on their biological function is limited, partly because of the lack of established genetic tools for studying gene function in these species. Recent genetic studies on vasopressin/oxytocin-like signaling in C. elegans provide evidence for the presumed neuromodulatory function of this peptide family in the nervous system of invertebrates. A limited number of neurons with predominantly sensory-neurosecretory properties express C. elegans nematocin.12,13 Among these, the NSM neurosecretory motor neurons have sensory endings and processes from which secretion to the pseudocoelomic fluid might occur.41 Two vasopressin/oxytocin-related receptors (NTR-1 and NTR-2) are present in neuronal, but also in peripheral muscle tissues.12,13
Sexual dimorphism represents an important feature of C. elegans nematocin signaling, complementing observations in many vertebrates.6,10 Garrison et al. report expression of the nematocin peptide and receptors in a shared set of hermaphrodite and male neurons, but with additional male-specific expression at sites implicated in reproductive behavior (Table 2).12 In line with this observation, nematocin signaling has no apparent function in hermaphrodite reproduction but is important for expanding the full mating potential of males.12 C. elegans males display a pattern of stereotyped mating behaviors, controlled by distinct cellular and molecular sub-circuits.42-45 Males deficient in nematocin signaling perform poorly at multiple mating stages including mate search, mate recognition and mating itself.12 Garrison et al. show that the motor patterns underlying successive steps of the reproductive behavior are inefficient and fragmented in nematocin mutant males, reducing their mating success.12 Upon hermaphrodite encounter, these worms often don’t initiate mating and if so, have difficulties to localize and maintain contact with the vulva, execute turns and complete mating in a reasonable amount of time. Based on these findings, vasopressin/oxytocin-related signaling is suggested to prime various neuronal circuits to stimulate an overall coordinated mating drive.12 The influence of nematocin on male reproductive behavior is most likely a combination of both central and peripheral actions. Nematocin receptors are expressed in male-specific muscles of the copulatory organ and some of the observed effects can be rescued by reintroducing the receptor in male-specific sensory neurons.12 Nematocin secretion from the DVA tail neuron is important for the response on initial contact with the hermaphrodite and localization of the vulva, though not for increasing the efficiency of other mating steps.12 This indicates that different sub-sets of mating behavior are governed by vasopressin/oxytocin-like signaling between different sets of cells including non-sexual dimorphic neurons.
Table 2. Expression of the nematocin precursor (italic) and receptors at sites involved in different sub-steps of C. elegans male mating behavior.12,42-45.
| Mating step | Involved site(s) expressing nematocin precursor/receptorsa |
|---|---|
| Response to hermaphrodite contact |
ray sensory neurons, DVA sensory neuron |
| Turning |
ray sensory neurons |
| Vulva location |
HOB sensory neuron, DVA sensory neuron |
| Spicule prodding/insertion |
spicule protractor muscles, oblique muscles, SPC sensory-motor neurons |
| Spicule protraction | spicule protractor muscles, SPC sensory-motor neurons |
Sexual behavior of animals is often composed of patterned behavioral motifs—of which some are known to be regulated by vasopressin/oxytocin-like peptides.36,37 These motifs, however, require refined coordination to ensure successful reproduction. Particularly under natural conditions where the timeframe for mating with a free-moving partner is mostly limited, an efficient and coherent mating behavior determines reproductive success. The overall picture that emerges from findings in C. elegans, points out vasopressin/oxytocin-related signaling as a neuromodulatory input for orchestrating the coherence of diverse sub-sets of reproductive behavior.12 In correspondence to this, nematocin receptors are expressed in male-specific neurons and muscles implicated in successive mating steps (Table 2). In addition, the context-dependent regulation of mating motifs requires the integration of nematocin with other neuropeptidergic signals.46,47 In combination with studies in vertebrates and lophotrochozoans,36-38 C. elegans findings indicate that—despite the phenotypic diversity of species- and sex-specific sexual behaviors—the vasopressin/oxytocin family has an ancient role in the regulation of animal reproductive behavior through both central and peripheral effects.
Ancient Roots for the Neuromodulation of Associative Learning
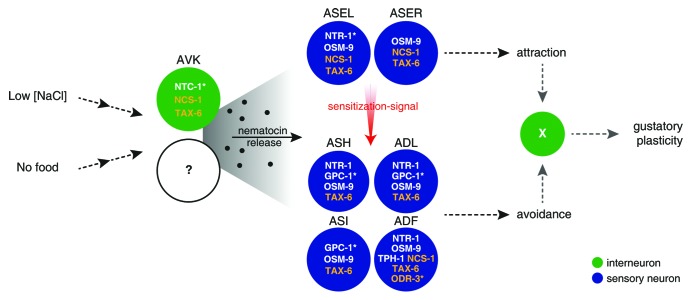
Besides male mating, nematocin is involved in C. elegans’ ability to modify its behavior in light of recent experience.13 Worms are normally attracted to low salt concentrations but show gustatory plasticity when shortly pre-exposed to it in the absence of food, resulting in salt avoidance.48 This behavioral change represents a type of associative learning using taste as the conditioned stimulus.49 Animals lacking nematocin or the NTR-1 receptor are inefficient in gustatory plasticity, matching the expression of NTR-1 in several chemosensory neurons involved in this type of learning (Fig. 2). The release of nematocin from AVK interneurons regulates gustatory plasticity, although other neurons might also be implicated.13 Though AVK cells do not match the proposed ancient sensory-neurosecretory cell type of vasopressin-/oxytocinergic cells, they receive input from various sensory neurons and interneurons, likely influencing nematocin release. Potential candidates to mediate nematocin secretion could be the AVK-expressed neuronal calcium sensor NCS-1 and the calcineurin homolog TAX-6,50,51 which are both also implicated in gustatory plasticity (Fig. 2).52
Figure 2. Putative model for nematocin-dependent regulation of gustatory plasticity in C. elegans (based on Hukema et al.).52 Cells and selected genes implicated in gustatory plasticity are depicted. Genetic evidence supports for genes depicted in white to be active in the same genetic pathway, and effects of asterisk-marked genes were assigned to gene functions in the indicated neuron(s). Chemoattraction to low salt concentrations is primarily mediated by the ASE neurons; while ASH, ADL, ASI and ADF neurons are thought to promote salt avoidance in gustatory plasticity. Integration of attraction and avoidance signals determines the worm’s chemotaxis behavior. In this model, gustatory plasticity following pre-exposure to “low salt” and “no food” cues could result from the sensitization of avoidance-promoting neurons, most likely by an ASE-derived signal and/or the desensitization of attraction-promoting cells. Sensory information is also indirectly received by the nematocin (NTC-1)-producing AVK interneurons and potentially other nematocinergic cells. Nematocin secretion from these neurons activates the NTR-1 receptor in the ASEL neuron among others, which in turn may contribute to the production of the ASE-derived sensitization-signal. In addition, nematocin could act on avoidance-promoting neurons to shift the balance toward salt avoidance.
Gustatory responses and plasticity in C. elegans are governed by neuronal activities balancing attraction and avoidance behaviors (Fig. 2). The gustatory ASE neurons are the main sensory neurons that promote chemoattraction toward low amounts of salt.53 Attraction is antagonized by avoidance, mediated by the ASH neurons that become activated only at high salt concentrations.53,54 Following the model proposed by Hukema et al.,52 gustatory plasticity probably results from the sensitization of avoidance-promoting neurons, including ASH but also ADF, ADL and ASI cells, whether or not combined with the desensitization of attraction-promoting neurons. This is supported by the observed change in neuronal activity of ASE neurons following the short pre-exposure of worms to salt in the absence of food.55 For now, it remains unclear where the salt and no-food signals are integrated during gustatory plasticity. As the ASE neurons become activated at low salt concentrations, it is hypothesized that these cells produce a signal for sensitizing avoidance-promoting neurons (Fig. 2).52 NTR-1 signaling in the left ASE neuron restores gustatory plasticity in receptor mutants; therefore, nematocin might modulate the production of this sensitizing signal or function in the potential desensitization of ASEL.13 In addition, the NTR-1 receptor is expressed in several neurons that are thought to stimulate avoidance in the gustatory plasticity paradigm (Fig. 2).12,13,52 Here, nematocin may be important for priming the neuronal circuit to generate avoidance, in a similar manner to the way in which it increases the efficiency of male mating circuits.12
The vasopressin/oxytocin family is a known regulator of vertebrate learning and memory processes in both a social and non-social context10,11,40 that often translate to the experience-reflected association of different stimuli or behaviors. The role of nematocin in C. elegans’ gustatory plasticity highlights ancient roots for the effects of this peptide family on experience-based learning. At the molecular level, nematocin signaling acts in a genetic pathway for gustatory plasticity including the transient receptor potential vanilloid (TRPV) protein OSM-9 (Fig. 2).13,56 Based on the function of vertebrate counterparts in the sensory-dependent regulation of vasopressin and oxytocin secretion,57 sensory transduction through OSM-958 may be used in C. elegans to relay this information to nematocin-producing neurons. In addition, nematocin signaling interacts with serotonergic and dopaminergic neurotransmission in gustatory plasticity,13 both known to modulate C. elegans’ behavior in response to food.59 Dopamine and serotonin signaling are important for learning and memory across metazoans and vertebrate studies show that neuromodulation by vasopressin and oxytocin relies on interactions with these signaling systems in the brain.9,10 Partner-bond formation for example is hypothesized to reflect the association of sexual reward with partner-specific sensory cues through converging dopaminergic and vasopressin-/oxytocinergic pathways within the brain’s reward circuit.60 Besides vasopressin/oxytocin-related actions in learning circuits, the molecular mechanisms underlying these effects may thus be well conserved.
Conclusions
Neuropeptidergic signaling through vasopressin- and oxytocin-related peptides is widely adopted by bilaterian animals for the regulation of physiology and behavior, reflecting the ancient origin of this peptide family. Nevertheless, some invertebrate species also seem to have lost this signaling system, potentially as a consequence of lifestyle adaptation or competition with other hormonal systems. The ancestral vasopressin/oxytocin system might already have been involved in a number of functions. Invertebrate studies underscore the conservation of hormonal effects on peripheral target tissues including the control of myoactivity and reproduction.5,15,16 In addition, genetic analysis of the C. elegans nematocin system provides evidence for neuromodulatory actions on invertebrate neuronal circuits.12,13 Neuromodulation by nematocin occurs in at least two functional systems: in reconfiguring the neuronal circuit for salt chemotaxis in light of previous experience; and in functionally coordinating local sensory-motor circuits for male mating into a coherent reproductive behavior. Nematocin signaling increases the efficiency by which these systems generate behavioral output, which may be achieved by priming or stabilizing the involved neuronal circuits. As vasopressin/oxytocin-related receptors are expressed in the nervous system of many invertebrates,4,29,38 neuromodulation by the vasopressin/oxytocin family most likely occurs in all these species. In this context, it is reasonable to hypothesize that especially from the moment animals started exploring new environments—where they encountered a variety of novel cues related to e.g., mating partners or food availability—the emergence of a neuropeptidergic system that could accordingly direct their behavior and decisions was of great benefit to their survival.
Comparing findings in C. elegans to vertebrates and lophotrochozoans36-38 denotes the ancient regulation of reproductive behavior by the vasopressin/oxytocin peptide family across the bilaterian branches, likely through combined central and peripheral effects. The nematode perspective also highlights ancient roots for the neuromodulation of learning and memory circuits by vasopressin and oxytocin in the mammalian brain. In mammals, vasopressin has general facilitating effects on cognitive performances, while oxytocin acts inhibitory;40 although variations to this rule exist dependent on the specific behavior or brain area being studied. Additional research will need to show whether nematocin influences general or specific experience-based learning.
Like the peripheral hormonal effects, neuromodulation of neuronal circuits that generate social behavior and cognition represents an important, ancient function of the vasopressin/oxytocin family. In addition, molecular mechanisms by which vasopressin and oxytocin modulate central circuits are likely well-conserved in C. elegans. In vertebrates, the study of these mechanisms is blurred by the diversity of effects in different brain regions. Functional conservation enables future exploitations of invertebrate models for extensive characterization of the basic mechanisms of central vasopressin/oxytocin signaling.
Acknowledgments
We thank Gert Jansen for his advice and support throughout our work on the nematocin system. I.B., L.T. and T.J. benefit from financial support of the Fund for Scientific Research-Flanders (FWO).
Glossary
Abbreviations:
- TRPV
transient receptor potential vanilloid
- GPCR
G protein-coupled receptor
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/24246
References
- 1.Du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–57. [PubMed] [Google Scholar]
- 2.Acher R, Chauvet J. La structure de la vasopressine de boeuf. Biochim Biophys Acta. 1953;12:487–8. doi: 10.1016/0006-3002(53)90173-5. [DOI] [PubMed] [Google Scholar]
- 3.Hoyle CH. Neuropeptide families and their receptors: evolutionary perspectives. Brain Res. 1999;848:1–25. doi: 10.1016/S0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- 4.Stafflinger E, Hansen KK, Hauser F, Schneider M, Cazzamali G, Williamson M, et al. Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects. Proc Natl Acad Sci U S A. 2008;105:3262–7. doi: 10.1073/pnas.0710897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elphick MR, Rowe ML. NGFFFamide and echinotocin: structurally unrelated myoactive neuropeptides derived from neurophysin-containing precursors in sea urchins. J Exp Biol. 2009;212:1067–77. doi: 10.1242/jeb.027599. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BB. General introduction to vasopressin and oxytocin: structure/metabolism, evolutionary aspects, neural pathway/receptor distribution, and functional aspects relevant to memory processing. Adv Pharmacol. 2004;50:1–50, 655-708. doi: 10.1016/S1054-3589(04)50001-7. [DOI] [PubMed] [Google Scholar]
- 7.McCormick SD, Bradshaw D. Hormonal control of salt and water balance in vertebrates. Gen Comp Endocrinol. 2006;147:3–8. doi: 10.1016/j.ygcen.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 10.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–8. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- 11.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–59. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 2012;338:540–3. doi: 10.1126/science.1226201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beets I, Janssen T, Meelkop E, Temmerman L, Suetens N, Rademakers S, et al. Vasopressin/oxytocin-related signaling regulates gustatory associative learning in C. elegans. Science. 2012;338:543–5. doi: 10.1126/science.1226860. [DOI] [PubMed] [Google Scholar]
- 14.Acher R. Neurohypophysial peptide systems: processing machinery, hydroosmotic regulation, adaptation and evolution. Regul Pept. 1993;45:1–13. doi: 10.1016/0167-0115(93)90174-7. [DOI] [PubMed] [Google Scholar]
- 15.Oumi T, Ukena K, Matsushima O, Ikeda T, Fujita T, Minakata H, et al. Annetocin, an annelid oxytocin-related peptide, induces egg-laying behavior in the earthworm, Eisenia foetida. J Exp Zool. 1996;276:151–6. doi: 10.1002/(SICI)1097-010X(19961001)276:2<151::AID-JEZ8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Van Kesteren RE, Smit AB, De Lange RP, Kits KS, Van Golen FA, Van Der Schors RC, et al. Structural and functional evolution of the vasopressin/oxytocin superfamily: vasopressin-related conopressin is the only member present in Lymnaea, and is involved in the control of sexual behavior. J Neurosci. 1995;15:5989–98. doi: 10.1523/JNEUROSCI.15-09-05989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ukena K, Iwakoshi-Ukena E, Hikosaka A. Unique form and osmoregulatory function of a neurohypophysial hormone in a urochordate. Endocrinology. 2008;149:5254–61. doi: 10.1210/en.2008-0607. [DOI] [PubMed] [Google Scholar]
- 18.Kawada T, Sekiguchi T, Itoh Y, Ogasawara M, Satake H. Characterization of a novel vasopressin/oxytocin superfamily peptide and its receptor from an ascidian, Ciona intestinalis. Peptides. 2008;29:1672–8. doi: 10.1016/j.peptides.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Ocampo Daza D, Lewicka M, Larhammar D. The oxytocin/vasopressin receptor family has at least five members in the gnathostome lineage, inclucing two distinct V2 subtypes. Gen Comp Endocrinol. 2012;175:135–43. doi: 10.1016/j.ygcen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Kaiya H, Konno N, Iwata E, Miyazato M, Uchiyama M, et al. The fifth neurohypophysial hormone receptor is structurally related to the V2-type receptor but functionally similar to V1-type receptors. Gen Comp Endocrinol. 2012;178:519–28. doi: 10.1016/j.ygcen.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Roch GJ, Busby ER, Sherwood NM. Evolution of GnRH: diving deeper. Gen Comp Endocrinol. 2011;171:1–16. doi: 10.1016/j.ygcen.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Lindemans M, Liu F, Janssen T, Husson SJ, Mertens I, Gäde G, et al. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:1642–7. doi: 10.1073/pnas.0809881106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caers J, Verlinden H, Zels S, Vandersmissen HP, Vuerinckx K, Schoofs L. More than two decades of research on insect neuropeptide GPCRs: an overview. Front Endocrinol (Lausanne) 2012;3:151. doi: 10.3389/fendo.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frooninckx L, Van Rompay L, Temmerman L, Van Sinay E, Beets I, Janssen T, et al. Neuropeptide GPCRs in C. elegans. Front Endocrinol (Lausanne) 2012;3:167. doi: 10.3389/fendo.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen T, Meelkop E, Lindemans M, Verstraelen K, Husson SJ, Temmerman L, et al. Discovery of a cholecystokinin-gastrin-like signaling system in nematodes. Endocrinology. 2008;149:2826–39. doi: 10.1210/en.2007-1772. [DOI] [PubMed] [Google Scholar]
- 26.Janssen T, Husson SJ, Lindemans M, Mertens I, Rademakers S, Ver Donck K, et al. Functional characterization of three G protein-coupled receptors for pigment dispersing factors in Caenorhabditis elegans. J Biol Chem. 2008;283:15241–9. doi: 10.1074/jbc.M709060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertens I, Husson SJ, Janssen T, Lindemans M, Schoofs L. PACAP and PDF signaling in the regulation of mammalian and insect circadian rhythms. Peptides. 2007;28:1775–83. doi: 10.1016/j.peptides.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Lindemans M, Janssen T, Husson SJ, Meelkop E, Temmerman L, Clynen E, et al. A neuromedin-pyrokinin-like neuropeptide signaling system in Caenorhabditis elegans. Biochem Biophys Res Commun. 2009;379:760–4. doi: 10.1016/j.bbrc.2008.12.121. [DOI] [PubMed] [Google Scholar]
- 29.Kanda A, Satake H, Kawada T, Minakata H. Novel evolutionary lineages of the invertebrate oxytocin/vasopressin superfamily peptides and their receptors in the common octopus (Octopus vulgaris) Biochem J. 2005;387:85–91. doi: 10.1042/BJ20041230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aikins MJ, Schooley DA, Begum K, Detheux M, Beeman RW, Park Y. Vasopressin-like peptide and its receptor function in an indirect diuretic signaling pathway in the red flour beetle. Insect Biochem Mol Biol. 2008;38:740–8. doi: 10.1016/j.ibmb.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Takuwa-Kuroda K, Iwakoshi-Ukena E, Kanda A, Minakata H. Octopus, which owns the most advanced brain in invertebrates, has two members of vasopressin/oxytocin superfamily as in vertebrates. Regul Pept. 2003;115:139–49. doi: 10.1016/S0167-0115(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 32.Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, et al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res. 1998;119:351–64. doi: 10.1016/S0079-6123(08)61580-0. [DOI] [PubMed] [Google Scholar]
- 34.Fujino Y, Nagahama T, Oumi T, Ukena K, Morishita F, Furukawa Y, et al. Possible functions of oxytocin/vasopressin-superfamily peptides in annelids with special reference to reproduction and osmoregulation. J Exp Zool. 1999;284:401–6. doi: 10.1002/(SICI)1097-010X(19990901)284:4<401::AID-JEZ6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Kawada T, Kanda A, Minakata H, Matsushima O, Satake H. Identification of a novel receptor for an invertebrate oxytocin/vasopressin superfamily peptide: molecular and functional evolution of the oxytocin/vasopressin superfamily. Biochem J. 2004;382:231–7. doi: 10.1042/BJ20040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagenaar DA, Hamilton MS, Huang T, Kristan WB, French KA. A hormone-activated central pattern generator for courtship. Curr Biol. 2010;20:487–95. doi: 10.1016/j.cub.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm Behav. 2012;61:266–76. doi: 10.1016/j.yhbeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.van Kesteren RE, Tensen CP, Smit AB, van Minnen J, van Soest PF, Kits KS, et al. A novel G protein-coupled receptor mediating both vasopressin- and oxytocin-like functions of Lys-conopressin in Lymnaea stagnalis. Neuron. 1995;15:897–908. doi: 10.1016/0896-6273(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 39.Gupta J, Russell R, Wayman C, Hurley D, Jackson V. Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br J Pharmacol. 2008;155:118–26. doi: 10.1038/bjp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- 41.Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- 42.Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, et al. The connectome of a decision-making neural network. Science. 2012;337:437–44. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- 43.Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 44.Garcia LR, Mehta P, Sternberg PW. Regulation of distinct muscle behaviors controls the C. elegans male’s copulatory spicules during mating. Cell. 2001;107:777–88. doi: 10.1016/S0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, LeBeouf B, Guo X, Correa PA, Gualberto DG, Lints R, et al. A cholinergic-regulated circuit coordinates the maintenance and bi-stable states of a sensory-motor behavior during Caenorhabditis elegans male copulation. PLoS Genet. 2011;7:e1001326. doi: 10.1371/journal.pgen.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrios A, Ghosh R, Fang C, Emmons SW, Barr MM. PDF-1 neuropeptide signaling modulates a neural circuit for mate-searching behavior in C. elegans. Nat Neurosci. 2012;15:1675–82. doi: 10.1038/nn.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Kim K, Li C, Barr MM. FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J Neurosci. 2007;27:7174–82. doi: 10.1523/JNEUROSCI.1405-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen G, Weinkove D, Plasterk RH. The G-protein gamma subunit gpc-1 of the nematode C.elegans is involved in taste adaptation. EMBO J. 2002;21:986–94. doi: 10.1093/emboj/21.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ardiel EL, Rankin CH. An elegant mind: learning and memory in Caenorhabditis elegans. Learn Mem. 2010;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- 50.Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, et al. Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron. 2001;30:241–8. doi: 10.1016/S0896-6273(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 51.Kuhara A, Inada H, Katsura I, Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;33:751–63. doi: 10.1016/S0896-6273(02)00607-4. [DOI] [PubMed] [Google Scholar]
- 52.Hukema RK, Rademakers S, Dekkers MP, Burghoorn J, Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 2006;25:312–22. doi: 10.1038/sj.emboj.7600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–42. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 54.Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oda S, Tomioka M, Iino Y. Neuronal plasticity regulated by the insulin-like signaling pathway underlies salt chemotaxis learning in Caenorhabditis elegans. J Neurophysiol. 2011;106:301–8. doi: 10.1152/jn.01029.2010. [DOI] [PubMed] [Google Scholar]
- 56.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–69. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudbury JR, Ciura S, Sharif-Naeini R, Bourque CW. Osmotic and thermal control of magnocellular neurosecretory neurons--role of an N-terminal variant of trpv1. Eur J Neurosci. 2010;32:2022–30. doi: 10.1111/j.1460-9568.2010.07512.x. [DOI] [PubMed] [Google Scholar]
- 58.Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–18. doi: 10.1016/S0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 59.Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans In: Wormbook, ed. The C. elegans research community. Wormbook, 2007:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]