Abstract
The nematode (worm) C. elegans is a leading multicellular animal model to study neuronal-basis of behavior. Worms respond to a wide range of stimuli and exhibit characteristic movement patterns. Here we describe the use of a microfluidics setup to probe neuronal activity that relies on the innate response of C. elegans to swim toward the cathode in the presence of a DC electric field (termed “electrotaxis”). Using this setup, we examined mutants affecting sensory and dopaminergic neurons and found that their electrotactic responses were defective. Such animals moved with reduced speed (35–80% slower than controls) with intermittent pauses, abnormal turning and slower body bends. A similar phenotype was observed in worms treated with neurotoxins 6-OHDA (6- hydroxy dopamine), MPTP (1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine) and rotenone (20–60% slower). We also found that neurotoxin effects could be suppressed by pre-exposing worms to a known neuroprotective compound acetaminophen. Collectively, these results show that microfluidic electrotaxis can identify alterations in dopamine and amphid neuronal signaling based on swimming responses of C. elegans. Further characterization has revealed that the electrotactic swimming response is highly sensitive and reliable in detecting neuronal abnormalities. Thus, our microfluidics setup could be used to dissect neuronal function and toxin-induced neurodegeneration. Among other applications, the setup promises to facilitate genetic and chemical screenings to identify factors that mediate neuronal signaling and neuroprotection.
Keywords: C. elegans, nematode, microfluidics, electrotaxis, neuronal signaling, dopamine signaling, neurodegeneration, neurotoxin, 6-OHDA, MPTP, Rotenone
Introduction
The survival and functioning of living organisms depend on their ability to continuously monitor their surrounding environment. This process is mediated by the nervous system that detects environmental stimuli and depending upon the context and the experience of the animal generates an appropriate response. Understanding how neurons respond to stimuli and initiate a specific behavioral outcome is complicated in vertebrates due to the presence of a very large number of neurons and complex interconnections. Invertebrate animal models, such as the nematode (worm) C. elegans, offer a simpler nervous system and a wide range of experimental techniques to dissect the neuronal basis of behavior. The adult C. elegans hermaphrodite contains 302 neurons, whose morphology and inter-connections are well established.1,2 The animal has a short life cycle (2.5–3 d) making it possible to study developmental processes relatively quickly. In addition, it can be manipulated in the laboratory using a number of powerful genetic and genomic tools. These features have greatly facilitated the study of neuronal cell fate specification and cell signaling.
The sensory neurons in C. elegans that mediate odor detection (chemosensory) and physical contacts (mechanosensory) have been studied in significant detail. Many of these have ciliated dendrites that are directly or indirectly exposed to the external environment and facilitate detection of environmental stimuli by activating signal transduction pathways. The signaling process is mediated by several proteins including G-protein coupled receptors (GPCRs) and transient receptor potential (TRP) channels.3-5 In hermaphrodites eight of the mechanosensory neurons (two pairs of CEPs and one pair of ADE in the head region and one pair of PDE in the posterior region) produce dopamine (DA) neurotransmitter.6 All of these neurons have ciliated endings that are embedded in the cuticle and their processes extend to the nose tip (CEPs) and along the anterior and posterior lateral midlines (ADE and PDE, respectively).5 The dopaminergic neurons modulate different behavioral responses of the animal such as foraging, locomotion rate, egg laying, defecation and swimming in a liquid environment.7-11
The traditional assays to measure behavioral activities of C. elegans involve qualitative and quantitative analyses of phenotypes using manual and semi-automated approaches. These methods are slow, labor-intensive and prone to errors. Consequently, they represent a significant bottleneck in analyzing a large number of animals in a rapid and unbiased manner. Recent advances in microfluidics research has made it possible to perform many of the routine and laborious experiments in C. elegans automatically, precisely and in a high-throughput manner.12-15 Microfluidics offers unparalleled control over manipulation of worms because of its ability to precisely control microscopic volumes of fluids inside minute chambers and channels that are comparable to the size of worms. This allows efficient handling of worms and confers greater control over confinement, treatment and observation at a high resolution. Furthermore, microfluidics deals with tiny volumes of liquid resulting in reduced cost of media and chemical usage. Microfluidics devices have a wide variety of applications in research involving C. elegans. These include growth studies, mutant screening, nanosurgery, neuronal imaging and movement analysis.16-19
We recently demonstrated that low voltage direct current (DC) and pulsed DC electric fields inside a microfluidic channel cause C. elegans to swim toward cathode (termed “electrotaxis”).20,21 The electrotactic responses of some of the nematodes, including C. elegans, were examined earlier using an open gel surface Petri plate setup.22-24 It was noted that although animals generally were attracted toward the cathode, they did not travel directly toward it along the imaginary axis from anode to the cathode. Instead, they were found to travel at an angle with respect to this line. The angle of motion was proportional to the strength of the electric field making the response more complex to analyze.24 Unlike the open gel surface setup, the microchannel environment streamlines the electric field along its axis and stimulates worms to swim in a straight line with a characteristic sinusoidal pattern. This simplifies the movement analysis of animals by allowing precise quantification of parameters such as speed, body bend frequency and reversals. Furthermore, the electrotaxis response is instant, reversible and highly robust. As a result, abnormalities in neuronal signaling can be identified fairly quickly and reliably. Finally, microfluidics is amenable to parallelization and automation thereby accelerating the pace of discovery.
The precise role of electrotaxis behavior in worms is currently unknown but research involving plant and insect parasitic nematodes has suggested that it could facilitate host finding.23,25 Studies in C. elegans have revealed that such a response in an open gel surface environment is mediated by amphid neurons.24 In this study, we examined the electrotactic swimming behavior of worms in a microfluidic channel device using neuronal mutants and found that in addition to amphid sensory neurons dopaminergic neurons are also involved in this process. The role of dopaminergic neurons was further investigated by exposing worms to three different neurotoxins, 6-OHDA (6-hydroxy dopamine), MPTP (1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine) and rotenone (a pesticide). These toxins cause degeneration of DA neurons similar to that shown in mammalian models.26-28 A common mode of their action appears to be the inhibition of mitochondrial respiratory chain and generation of reactive oxygen species (ROS).29 We found that toxin-treated worms had abnormal swimming behavior. A comparison with plate-based behavioral assays revealed that the microfluidics assay is highly sensitive in detecting movement defects. The phenotypic analyses of worms were performed by quantifying parameters that eliminated subjectivity and bias. Lastly, we investigated the role of a neuroprotective compound acetaminophen and found that it suppresses the effect of toxins in the channel assay. Together, these results demonstrate that the microfluidic electrotaxis is a sensitive assay to study neuronal signaling and toxin-induced neuronal damage, as well as to identify neuroprotective drugs in C. elegans.
Results
The microfluidic device used in our electrotaxis assay (Fig. 1) provides an environment in which the electric field streamlines are confined in the axial direction of the channel. This results in a uniform stable field that stimulates worms to move along the channel length. Furthermore, the narrow diameter (300 μm) ensures motion in a near straight line fashion. We have earlier shown that this setup allows worms to swim toward the cathode when exposed to a low voltage DC electric field.20 The electrotactic swimming response of C. elegans is robust, continuous and occurs at a characteristic speed. Changing the direction of the electric field causes the worm to stop transiently, turn toward the cathode and resume motion (Fig. 1D and E). Therefore, any deviation from these characteristics can be considered as an abnormality. As described in Materials and Methods, we characterized the electrotaxis behavior of worms using four parameters, namely electrotaxis speed, turn time, body bend frequency and electrotaxis time index (ETI).
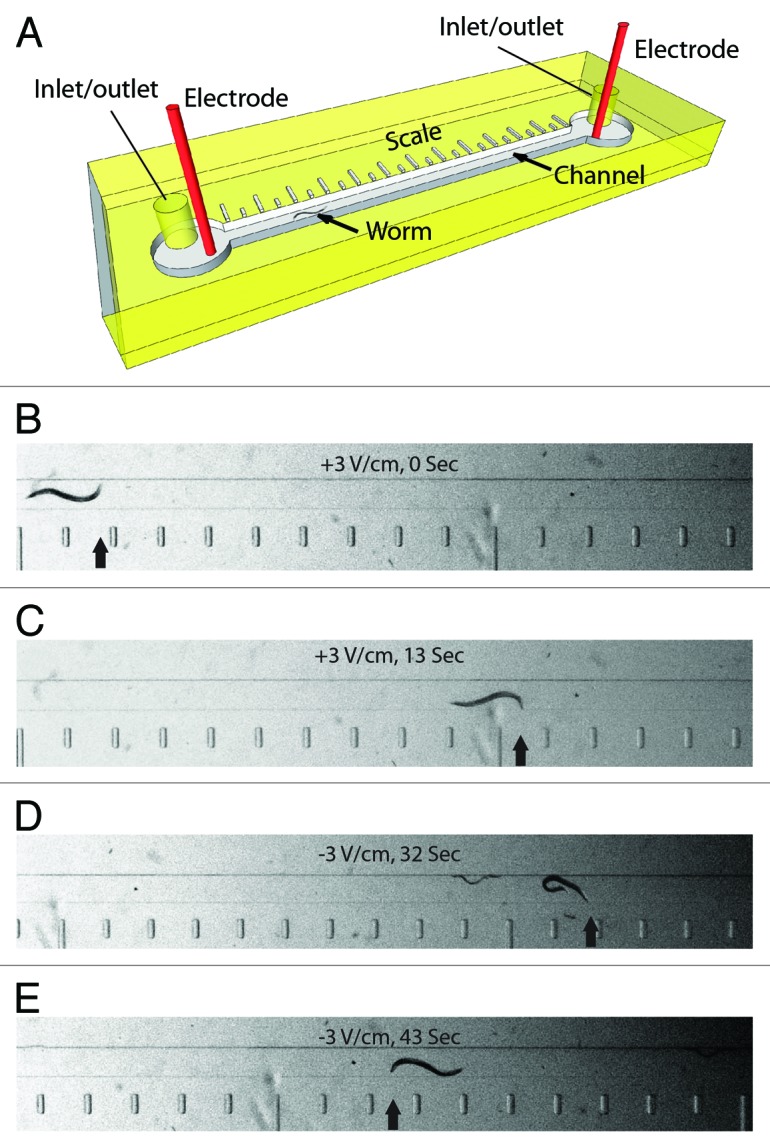
Figure 1. Microfluidic electrotaxis setup. (A) A detailed view of the microfluidic device. Worms are loaded and removed through inlet/outlet tubes. Electrotaxis is performed in the channel (a worm is shown). The scale along the length of the channel is used to determine the speed. (B‒E) Snapshots of a worm in the channel during electrotaxis. Scale bar is visible on the bottom. The electric field voltage and time are shown in each panel. The head of the worm is marked by an arrow. Reversal of the electric field polarity (D and E) causes the worm to switch its direction of motion.
Initially, we investigated the behavior of wild-type N2 worms in the channel in the absence of an electric field. The animals showed random swimming and turning activities with a mean body bend frequency of 1.3 (n = 6) (Video 1). Due to multiple reversals, they failed to cover long distance in any one direction. Thus, the electrical stimulus is required to propel worms in a directed manner.
The electrotactic swimming of C. elegans in the channel depends on its intact neuronal and muscular systems.20 Consequently, defects in any of these components could alter the swimming behavior. While the electrosensory defect may result from the inability of sensory neurons to receive and process electrical signals, general locomotion defects could arise due to problems in the neuromuscular system that controls motor responses such as speed and amplitude of motion. Hence, electrosensory mutants will have difficulty in sensing the direction of the electric field. Such animals are expected to swim toward either pole while reversing direction frequently. However, the locomotory mutants should recognize the electric field polarity and move specifically toward cathode, albeit at a reduced pace. Our analysis of the neuronal mutants in following sections agrees with these possibilities and demonstrates that the microfluidics setup can be used to identify and characterize new genes mediating electrotaxis behavior.
Amphid sensory neurons mediate electrosensory responses in a microfluidic channel device
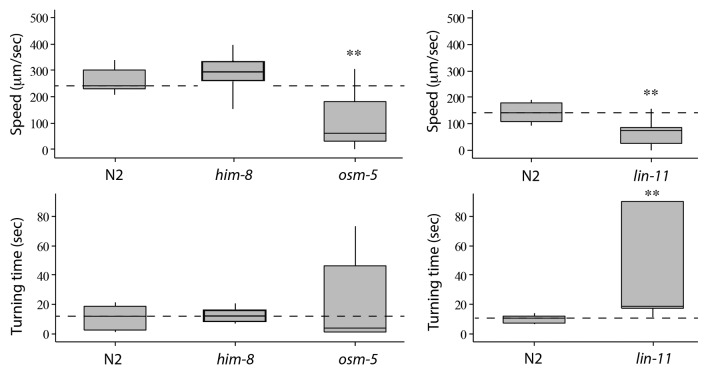
Although the biological basis and the mechanism of electrotaxis is poorly understood, a subset of neurons in the anterior ganglion were found to respond to the electric field stimulus in an open gel surface Petri dish setup.24 We used osm-5 mutants to examine the role of sensory neurons in our channel assay. The osm-5 gene encodes an intraflagellar transport protein that is homologous to human IFT88 and is required for cilia formation in sensory neurons including the amphids.30 The osm-5 animals appeared very active and in the absence of the electric field exhibited swimming behavior similar to wild-type N2 (body bend frequency of 1.8 Hz, n = 6; see above for N2 data) (Fig. S1). However, in the presence of the electric field, the animals showed severely defective electrotactic response. The average speed of osm-5 was nearly 60% lower than the wild-type N2 (n = 11) (Fig. 2). Whereas N2 worms swam straight toward cathode without pausing (Video 2), the osm-5 worms stopped and reversed direction many times (number of reversals for each animal ranged between 3‒20, n = 11) (Video 3). Few of them became immobile after slight initial swimming and did not recover (18%, n = 11) (Video 4). In addition to spontaneous turning and lack of motion, osm-5 worms also exhibited intermittent pauses, abnormal body postures and swimming in wrong direction (i.e., toward anode) indicating that they lacked a sense of direction. Consequently, the turn time of animals was highly variable (Fig. 2). To further demonstrate the electrotaxis defect in osm-5 animals, we computed the time of all cathode-directed swimming events and determined ETI (see Materials and Methods). As expected, the ETI of osm-5 was greatly reduced compared to N2 (p < 0.0001) (Fig. 3). As a control, we also tested a non-neuronal mutant him-8(e1479) that produces increased frequency of males due to defects in X-chromosome segregation.31 The speed, turn time and ETI of him-8 were comparable to N2 (Figs. 2 and 3).
Figure 2. Electrotaxis speed and turn time responses of wild-type N2 and mutant animals. The lower and upper lines of each box represent the 25th and 75th quartile of data sample, respectively. The middle line inside the box marks the median. The end points of the vertical line (both top and botom) are the maximum and minimum data points of the sample, respectively. The dotted horizontal line corresponds to the median of the control. The him-8 worms are comparable to wild-type, whereas osm-5 and lin-11 move slower than N2 and take much longer time to turn. Statistically significant responses are marked with stars (*: p < 0.05, **: p < 0.01, ***: p < 0.0001).
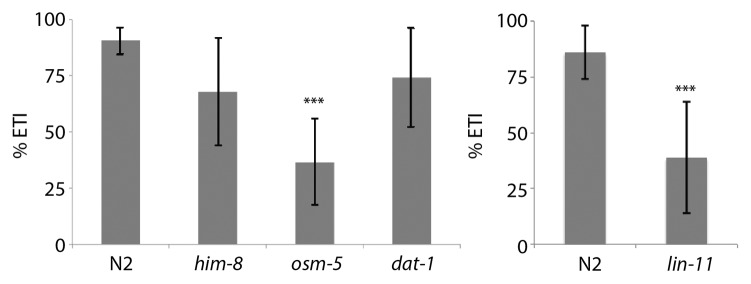
Figure 3. Electrotaxis time index (ETI) of N2 control and mutant animals. The him-8 and dat-1 are normal whereas osm-5 and lin-11 show a defective response (***: p < 0.0001).
We examined another neuronal mutant lin-11 that is weakly uncoordinated and exhibits chemosensory and thermosensory defects.32-34 lin-11 is a founding member of the LIM Homeobox family of transcription factors.35 It is expressed in a subset of neurons in the head ganglion (including sensory neurons ADF and ADL, interneurons AIZ, RIC, AVG, AVA and AVE, and chemosensory neuron ASG) and is necessary for their differentiation.32,33 We found that lin-11(n389)-null mutants35 had severely defective electrotactic response. Some animals showed no reaction to the stimulus (17%, n = 23) (Videos 5 and 6) whereas others moved in the channel but with multiple pauses and significantly reduced speed (76 μm/sec, n = 19, compared with N2 control: 141 μm/sec, n = 12) (Fig. 2). Additionally, lin-11 worms were also defective in sensing the direction of the electric field. Upon switching the field polarity, the animals either took a very long time to turn (average turn time 49.2 sec, n = 16, compared with 10.3 sec, n = 12 for control) (Fig. 2) or failed to turn at all (16%, n = 19). In agreement with these findings, the ETI of lin-11 was greatly reduced (p < 0.0001) (Fig. 3). Taken together, these results demonstrate that osm-5 and lin-11 are required for the electrosensory response of C. elegans in the microfluidic channel assay.
Defects in dopamine signaling affect electrotaxis of animals
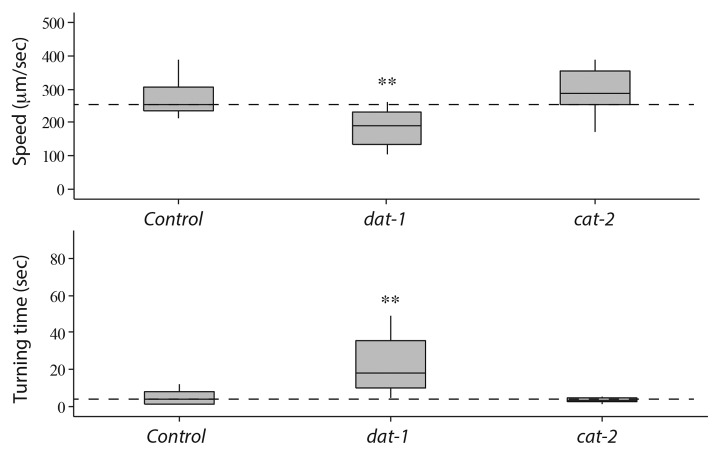
DA signaling is involved in modulating the locomotion of C. elegans in response to environmental stimuli.7 Therefore, we examined the electrotaxis swimming response of DA pathway mutants in our microfluidic channel setup. Animals having mutation in the cat-2 gene, the tyrosine hydroxylase required for DA biosynthesis,36 showed normal response (average speed 296 μm/sec and turn time 4.1 sec, n = 10 for the e1121 hypomorph); however, dopamine transporter mutants (dat-1(ok157)) showed a defective phenotype (average speed 189 μm/sec and turn time 26.1 sec, n = 10) compared with control animals (Fig. 4). Since DAT-1 mediates the reuptake of DA from the synaptic cleft back into the presynaptic terminal,37 it is possible that in the absence of DAT-1 function, extracellular DA alters the activity of certain post-synaptic neurons, thereby causing reduced electrotaxis speed in the microfluidic channel. Additionally, DA could stimulate certain dopamine receptors on motor neurons resulting in a slower speed of animals. The lack of electrotaxis phenotype in cat-2(e1121) animals may be attributed to the fact that DA level is not completely abolished (40% residual DA compared with wild type38). Because dat-1 mutants show reduced speed but no impact on sensing the electric field polarity, it suggests that DA signaling modulates locomotion without affecting the electrosensory response of animals. This conclusion is supported by the normal ETI response of dat-1 animals (p = 0.1253) (Fig. 3).
Figure 4. Electrotaxis speed and turn time responses of dat-1 and cat-2 mutants. Refer to Figure 2 for an explanation of the plot structure and statistical significance of data. dat-1 mutants move slower and have higher turn time compared with controls. However, cat-2 animals show no such defect.
In addition to mutants, we also used three different chemical compounds 6-OHDA, MPTP and rotenone that are toxic to DA neurons. Previous work in vertebrates has shown that these chemicals cause degeneration of DA neurons in the substantia nigra region of the brain.39,40 6-OHDA is preferentially taken up by DA neurons via the DAT transporter. Once inside the DA neuron, it causes multiple reactions including inactivation of the mitochondrial respiratory chain leading to an increase in ROS level.29,41,42 In the case of MPTP, it is metabolized into an active toxic product MPP+ that enters DA neurons through the DAT-1 transporter. MPP+ induces inhibition of mitochondrial respiratory enzyme complex I and an increase in ROS production.43,44 Exposure to rotenone in rat and Drosophila melanogaster models has shown to cause apoptosis and oxidative damage of DA neurons.39,45 C. elegans DA neurons are equally sensitive to the above three neurotoxins and undergo degeneration upon exposure.26,27
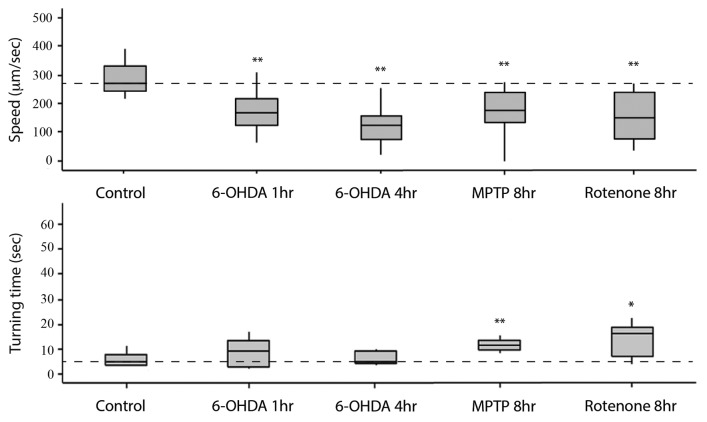
To carry out the electrotaxis assay on worms exposed to 6-OHDA, MPTP and rotenone, we first optimized chemical exposure conditions (see Materials and Methods). Toxin-treated worms were placed inside the channel without any pre-selection and their electrotactic responses were analyzed. We found that animals had defects in swimming behavior. The phenotypes included slower speed, intermittent pauses and reduced sensitivity. Exposure to 6-OHDA (either 1 h or 4 h at 100 µM concentration) caused a significant reduction in the speed (40–60% slower, 126–174 μm/sec average speed) of animals (n = 12 for 1 h condition and 15 for 4 h condition) without altering the turn time (Fig. 5). Besides reduced speed, we also observed other defects in movement. Frequently, animals showed incoherent electrotaxis characterized by active sinusoidal motion followed by periods of slow responses or a lack of activity (Videos 7 and 8). Partial paralysis was also observed where the posterior half of the body was rigid such that the worm appeared to drag itself while moving (Video 9). In addition, we also detected phenotypes such as sudden freeze (Video 10), tremor (Video 11) and a lack of motion (Video 12). Not all phenotypes were observed in every animal and, furthermore, these were specific to microfluidic channel environment. Animals grown on Petri plates did not show any such phenotype. In few cases, we also measured body bend frequency and found it to be lower in exposed worms compared with controls (average frequency 0.2 Hz for 6-OHDA 4 h exposure, n = 11 animals and 1.8 Hz for N2, n = 10 animals).
Figure 5. Electrotaxis speed and turn time responses of toxin-treated worms inside the channel. Refer to Figure 2 for an explanation of the plot structure and other details. Control refers to unexposed wild-type N2 animals. In all cases, the speed of exposed worms is significantly different from the control. A similar phenotype is also observed for turn time of animals.
Treatments of MPTP and rotenone for 8 h caused similar defects (roughly 40% slower speed in each case, MPTP: 178 μm/sec, rotenone: 160 μm/sec; n = 12 and 13 worms, respectively), albeit the reduction in speed was somewhat less compared with 6-OHDA (see above, Fig. 5). This is different from the 6-OHDA exposure that caused maximum defect in as little as 1 h (see above). The turn time was also affected (up to 2-fold slower for each toxin; MPTP: 19 sec; rotenone: 15 sec) (Fig. 5). Consistent with slow electrotaxis swimming speed, the body bend frequency of animals was also reduced (MPTP 8 h: 1.0 Hz, n = 3, compared with 1.6 Hz, n = 4 for N2 control; rotenone, data not shown).
In summary, our experiments involving DA pathway mutants and neurotoxin-treated worms demonstrate the sensitivity of the microfluidic electrotaxis assay in detecting abnormalities in DA signaling. Quantification of swimming defects allowed us to compare phenotypes in different conditions as well as between different sets of animals. Together, these results showed that a reduction in DA signaling contributes to abnormalities in electrotaxis swimming behavior in the channel assay. We conclude that our microfluidics setup can be used to identify and study factors affecting DA signaling and neurodegeneration in worms.
Plate-based phenotypic analysis of mutants and toxin-treated animals
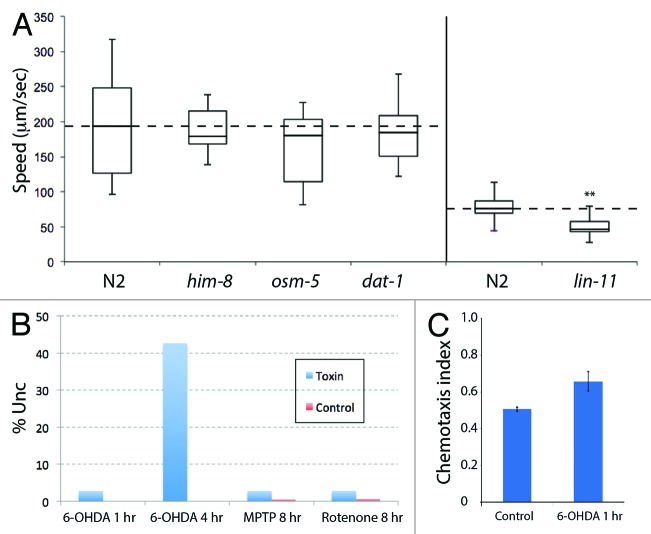
In addition to the electrotaxis phenotype, we also examined gross morphology and behavior of animals on Petri plates. This was done to investigate whether movement defects are specific to the microfluidic device or observed on open agar gel surface as well. For this, we measured the speed of osm-5, dat-1 and lin-11 mutants by following their tracks on bacterial lawns (see Materials and Methods). The average speed of dat-1 and osm-5 was 162 µm/sec (n = 23) and 183 µm/sec (n = 18), respectively, which is not significantly different from the wild-type N2 (190 µm/sec, n = 19) and him-8 (188 µm/sec, n = 9) (Fig. 6A). However, lin-11 animals showed a lower speed compared with N2 (40 h stage, average speed 53 μm/sec, n = 16, N2: 77 μm/sec, n = 14) (Fig. 6A). This agrees well with earlier studies showing that lin-11 animals are weakly uncoordinated.34 Overall, our results provide support to the conclusion that the microfluidic electrotaxis is a sensitive method to detect abnormalities in the swimming response of animals. Furthermore, they reveal that the lack of electrotaxis in some lin-11 animals and spontaneous reversals in osm-5 are unique to the channel environment and not observed in plate-based assay.
Figure 6. Plate-level phenotypes of mutants and toxin-treated animals. (A) The results are plotted similar to Figure 2. The him-8, osm-5 and dat-1 animals are comparable to the N2 control but lin-11 animals show reduced speed (**: p < 0.01). (B) Exposure to 6-OHDA, MPTP and rotenone causes weak Unc phenotype. Except for 6-OHDA 4 h cases that caused roughly 40% of animals to become Unc, all other treatments affected less than 3% of animals. The numbers of animals examined for each condition are as follows: 6-OHDA - 605 for 1 h and 420 for 4 h, and N2 control - 220; MPTP - 502 and control - 192; Rotenone - 353 and control - 163. (C) The chemotaxis responses of N2 and 6-OHDA 1 h-exposed animals toward NaCl chemoattractant. The toxin-treated worms show normal behavior.
Next, we examined plate-level responses and cellular defects in toxin-treated worms. In general, worms were healthy and fertile with no obvious morphological defect at any stage. Except for 4 h 6-OHDA-exposure condition that caused weak uncoordinated movement (Unc) in roughly 40% of the population, in all other cases, animals moved well and were fairly active (Fig. 6B). In one case, 6-OHDA 1 h, we also determined the chemotaxis response to NaCl and found it to be similar to untreated N2 control animals (Fig. 6C). For chemotaxis assays the numbers are: 6-OHDA 1 h - 169 (NaCI) and 34 (water); control - 101 (NaCI) and 33 (water).
To correlate microfluidic behavioral defects in toxin-treated worms with DA neuronal function, the morphology of neurons was also investigated. For this, we used a dat-1p::YFP transgenic strain (bhEx120) in which YFP expression is observed in DA neuronal cell bodies as well as their projections (Fig. 7A).26,37 The synchronized L1 stage bhEx120 animals were exposed to 100 μM 6-OHDA for 4 h and subsequently grown on a standard NG agar plate seeded with OP50 bacteria. The DA neurons were examined in adults. We found an age-dependent increase in neurodegeneration in toxin-treated animals (18%, n = 81 on day 3 and 26%, n = 42 on day 6) (Fig. 7B‒D). The dendritic processes of CEPs showed variable degeneration such that YFP fluorescence had spotty appearance (Fig. 7B). In some cases, the entire dendritic processes were missing (Fig. 7C). These phenotypes are similar to that reported earlier26 and are consistent with the electrotaxis defect in worms that we observed.
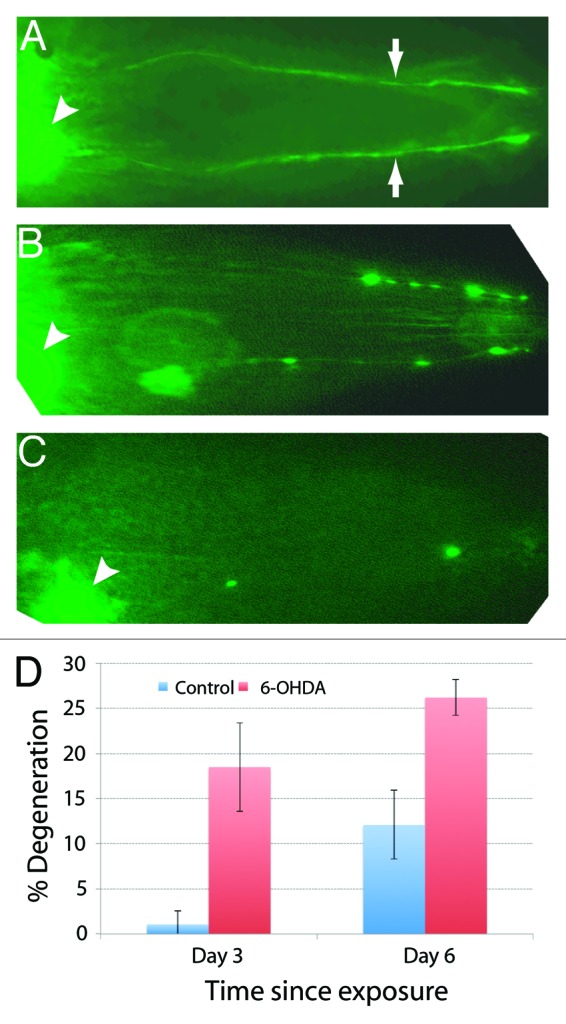
Figure 7. Degeneration of DA neurons in 6-OHDA-exposed worms visualized by dat-1p::YFP expression. In all cases anterior is toward the right. (A) In wild-type bhEx120 animals, YFP fluorescence can be observed in CEP cell bodies (arrowhead) and their processes (arrows). (B) Exposure to 6-OHDA causes spotty appearance of CEP neuronal processes indicating degeneration of neurons. (C) Another 6-OHDA-treated animal. The neuronal processes are almost completely missing. (D) Quantification of neuronal defects in 3-d- and 6-d-old control (untreated) and 6-OHDA-treated animals (sample size: 81 6-OHDA and 88 controls for day 3 set; 42 6-OHDA and 33 controls for day 6 set).
Acetaminophen confers neuroprotection against toxins in the microfluidics assay
If toxin-induced neuronal damage affects the electrotaxis behavior of worms, then the phenotype could be suppressed by protecting DA neurons. To examine this possibility, we tested a known neuroprotective compound acetaminophen. Studies in rats and C. elegans have shown that acetaminophen has a protective effect against MPP+, 6-OHDA and glutamate toxicity in DA neurons.46-48 We found that pre-treatment of acetaminophen conferred significant protection on DA neurons against all three neurotoxins. The pre-exposed worms were phenotypically normal and had significantly faster speed compared with neurotoxin-treated worms (Fig. 8). However, the turn time of animals showed no improvement (data not shown). Because acetaminophen protects DA neurons,46,48 these results further support the involvement of DA signaling in controlling the electrotaxis behavior of animals. Additionally, our findings show that the microfluidic channel-based assay can be used as a screening tool to identify new chemicals with neuroprotective properties.
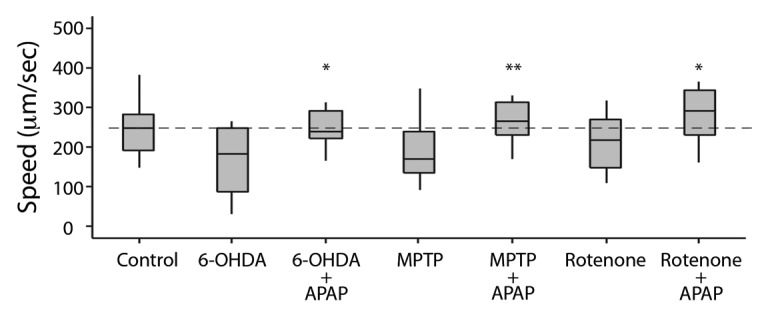
Figure 8. Electrotactic responses of worms treated with toxins and acetaminophen (para-acetylaminophenol, APAP). Control refers to untreated wild-type N2 animals. Refer to Figure 2 for an explanation of the plot structure and other details. The speed of APAP-treated group is comparable to the control and higher than the corresponding toxin-treated group.
Discussion
Microfluidic electrotaxis as a sensitive and reliable assay to detect neuronal activity
We have used a new microfluidics electrotaxis assay to examine neuronal signaling in worms. This unique method allows us to indirectly control the motion inside the channel. In the absence of an electric field, worms show random swimming behavior and cannot be guided in a desired direction. However, when exposed to the field, they move toward cathode in a stereotypic manner and with a constant speed.20 Previous findings20,24 and results presented in this paper demonstrate that this process depends on the function of neurons and muscles. The electrotaxis response is highly stereotypic, reproducible and can be quantified.20,21 This makes it possible to assess precisely the impact of alterations on worm’s behavior by observing its swimming characteristics.
We have shown that the normal electrotaxis response relies on the intact electrosensory system. In the case of osm-5 (human IFT88 homolog) and lin-11 (LIM Homeobox family) mutants that affect amphid neurons, animals showed significantly reduced speed and frequently failed to detect the electric field polarity. While the involvement of amphid neurons and osm-5 in mediating electrosensory behavior on open gel surface was previously reported,24 our work has revealed novel turning and paralysis phenotypes in osm-5 and lin-11 animals in the microfluidic device assay.
We have provided the evidence for the role of DA neuron signaling in modulating electric field-induced swimming responses. This is based on results that DA transporter mutant dat-1 has a reduced activity as judged by slower speed and higher turn time of animals. A similar phenotype was also observed in worms exposed to DA-specific neurotoxins (6-OHDA, MPTP and rotenone). The low doses of toxins (25–700 μM range, for a maximum duration of 8 h) caused electrotaxis defects in the channel without seriously impacting the growth and viability of animals. Thus, the microfluidic platform can be used as an effective and non-invasive tool to detect neuronal abnormalities in worms.
The effect of neurotoxins on C. elegans electrotaxis
Previous studies and our own work have shown that neurotoxins 6-OHDA, MPTP and rotenone cause movement and other abnormalities in C. elegans27,28 (see Materials and Methods). Some of the phenotypes, such as lethality, could be non-specific and may result from exposure to high doses of toxins. Because DA neurons are not required for survival, the viability defect could result from disruption of other cellular processes. Consistent with this, we found that reducing toxin doses eliminated lethality.
Because worms exposed to low doses of toxins appear generally healthy and active on plates, a sensitive assay is needed to monitor DA signaling defects. While one could directly visualize neurons using a GFP reporter, such an approach is slow and subjective. Therefore, it is unlikely to be a sensitive measure of DA neuronal activity. Our work establishes microfluidic electrotaxis as a rapid and sensitive assay to monitor movement and its neuronal basis in worms. The quantification of movement parameters allows comparison between different groups of animals in a reliable manner and eliminates any bias associated with manual counting and judgment.
Aside from the reliable quantitative analysis, our assay provides a unique opportunity to investigate some of the phenotypes caused by DA neuronal loss. During electrotaxis experiments, we observed that toxin-treated worm often failed to coordinate the swimming of different parts of their body relative to one another. This lack of synchrony translates into an overall gait abnormality that is characterized by short, staggered movements. We did not observe such a phenotype on the plate level. 6-OHDA-induced gait problems have been observed previously in the rat model but most importantly, this phenomenon relates closely with the shuffling gait that is a classical symptom of human Parkinson disease.49 The execution of synchronized voluntary movement requires not only proprioceptive feedback from peripheral receptors but also higher-level supraspinal processing that allows for kinesthesia.50 Defects in kinesthetic ability in toxin-treated worms could explain the etiology behind the short, staggered movements observed during electrotaxis. The gait abnormality observed in toxin-treated worms may result from a loss of proprioceptive ability due to the loss of dopaminergic neurons. Studies have shown that TRPN mechanosensitive ion channels are implicated in nematode proprioception.51,52 Therefore, in the future it will be useful to examine TRPN function in the electrotaxis behavior.
The sudden freeze, tremor and partial paralysis phenotypes of toxin-treated worms in the channel assay may be reminiscent of bradykinesia in Parkinsonian subjects. In C. elegans, sinusoidal movement requires the out-of-phase contraction of dorsal and ventral musculature.53 In Parkinson patients, D1 and D2 class of DA receptors are proposed as major contributing factors to the development of bradykinesia.54 The corresponding receptor family members in C. elegans, dop-1 (D1) and dop-3 (D2), are co-expressed on the cholinergic motor neurons of the ventral and dorsal cord and control locomotion.55 Therefore, it is conceivable that reduced dopamine in toxin-treated worms might affect DOP receptor function resulting in abnormal biphasic activity of muscles. Analysis of mutants affecting these and two other DA receptors (dop-4, D1 class; dop-2, D2 class) in the microfluidics setup should reveal their function in mediating electrotaxis.
Electrotaxis-based behavioral assay as a tool to screen for neuroprotective compounds
Traditional chemical screening using C. elegans involves feeding worms with compounds of interest present in food (e.g., in a 96-well microtiter plate). The subsequent analysis of animals involves monitoring their growth, viability and specific cellular and molecular markers. The existing methods of phenotypic analyses are slow, tedious and prone to human errors. The microfluidic system described here allows for objective and quantitative analysis of worm behavior following drug exposure. With some modifications, including development of automated video capturing and data analysis tools, the system can be used to screen for chemicals affecting neuronal function in a high-throughput manner. As an example, we tested the effect of acetaminophen, a compound with known neuroprotective response in C. elegans.48 The results showed that acetaminophen protects worms from the toxic effect of 6-OHDA as judged by the normal electrotaxis response in the channel assay. Therefore, our microfluidic electrotaxis system holds promise in chemical screening and identification of potential therapeutic compounds with neuroprotective properties.
Materials and Methods
C. elegans cultures
Worms were grown at 20°C on standard NG-agar plates containing E. coli OP50 culture as previously described.56 The strains used in this study are: N2 (wild-type), DY328 dat-1p::YFP (bhEx120), CB1489 him-8(e1489), PS2821 lin-11(n389), PR813 osm-5(p813), RM2702 dat-1(ok157) and CB1112 cat-2(e1112).
Synchronized worms were used for all the assays and were prepared by bleach treatment.20 Briefly, gravid hermaphrodites were treated with a solution containing commercial bleach and 4N NaOH (3:2 ratio). The dead worms were washed with M9 buffer and incubated at room temperature for 24 h to allow fertilized embryos to hatch into L1 larvae.
Except for lin-11(n389) animals, all other plate-based and electrotaxis assays were done with 69 h young adults. This stage was chosen based on our finding that almost all synchronized wild-type L1s, when placed on NG-agar plates, reach to adulthood by 69 h at 20°C (97% adult and remaining younger stages, n = 1,002). The lin-11 mutants were tested at 40 h stage because of their egg laying-defective (Egl) phenotype.57
Molecular biology and transgenics
The dat-1p::YFP plasmid pGLC72 was made by amplifying a 710 bp fragment of dat-1 5′ genomic region using primers GL563 (5′-AGGAAGCTTCCAGTTTTCACTAAAACGACCTCATACACTTCTC-3′) and GL564 (5′-ATGGGTACCGGCACCAACTGCATGGCTAAAAATTGTTGAG-3′). The resulting PCR product was digested with HindIII and KpnI and subcloned into pPD136.64 (Fire lab vector, www.addgene.com). pGLC72 was injected into unc-119(ed4) animals to generate stable transgenic lines.
Toxin treatments and optimizations
All toxin treatments were done with synchronized L1 populations. Worms were exposed to toxins for different time periods with mild shaking on a rocking platform. Following exposure, tubes were briefly centrifuged and worm pellets were washed once with M9 buffer. Worms were transferred to NG-agar culture plates. Desired concentrations of 6-OHDA (100 μM) (Sigma Aldrich, 162957), MPTP (700 μM) (Toronto Research Chemicals, M325913) and rotenone (25 μM) (Sigma Aldrich, R8875) were prepared in M9 1 d before the assay and stored at -20°C. The 6-OHDA solution is sensitive to light therefore it was kept in the dark.
We modified toxin exposure protocols by lowering the concentration and exposure time. This was necessary because plate-based assays in the past used high doses of chemicals resulting in pleotropic defects such as delayed growth and lethality.26,27,58 This precluded us from carrying out electrotaxis experiments. Additionally, we were concerned about non-specific effects due to prolonged exposures to high doses of chemicals that could affect neurons other than those involved in DA signaling. For example, L1 animals treated with 5 mM 6-OHDA for 30 min (one of the lowest concentrations reported) showed extreme sluggishness, uncoordinated (Unc) movement, protruding vulva, growth arrest and early larval lethality58 (data not shown). We were unable to examine such worms in the channel because they were practically immobile and unresponsive to the electric field stimulus. Lowering the 6-OHDA concentration by 50-fold (100 μM) improved the overall health of worms, thereby allowing us to carry out electrotaxis assays.
We also optimized the MPTP and rotenone treatment protocols. It was reported earlierthat worms grown in the presence of 1.4 mM MPTP for 3 d (starting L1 larval stage) were uncoordinated and extremely slow growing.27 In the case of rotenone, a dose of 25 μM for 4 d caused lethality.28 We found that reducing the rotenone exposure (25 μM rotenone for 12 h) allowed animals to survive but affected their growth and movement. However, none of the above toxin conditions could be used in the channel assay since worms were too sick to move and did not respond to the electric field stimulus. So, we further modified the toxin treatment conditions and found that animals exposed to 700 μM MPTP or 25 μM rotenone up to 8 h were generally healthy on plates and could be used to perform electrotaxis experiments.
Neuroprotection assay
The 10 mM stock solution of acetaminophen (Sigma Aldrich, A7085) was prepared fresh and diluted to a final concentration of 100 μM at the time of the assay. Synchronized L1 stage animals were incubated for 24 h in 100 μM drug containing M9 buffer. They were washed once with M9 and placed in toxin-containing solution for 1 h (100 μM 6-OHDA) or 8 h (700 μM MPTP and 25 μM rotenone). After an additional wash, the animals were transferred to NG-agar plates. The electrotaxis assays were performed on 69 h adults.
Microscopy
Worms were mounted on glass slides containing agar pads and observed using Zeiss AxioImager D1 and Nikon Eclipse Nomarski fluorescence microscopes. Epifluorescence was visualized using a GFP filter (HQ485LP, Chroma Technology USA). Degeneration of DA neurons was examined in 6-OHDA-treated dat-1p::YFP transgenic worms. For this, L1 worms were exposed to 6-OHDA for 4 h, washed once with M9 and then plated on NG agar plates. Adults were examined for YFP fluorescence in dopaminergic neurons and their processes.
Plate-based assays
Well-fed synchronized worms (described above) were used to quantify their movement responses on agar plates. The stages were 69 h (post-L1) young adult for osm-5, dat-1 and him-8 and 40 h (post-L1) larvae for lin-11. The corresponding stages of N2 worms were used as controls. Worms were placed, one at a time, on a fresh 1-d-old thin bacterial lawn. Following acclimatization for 5 min. the animal’s movement was observed for 30 sec. The beginning and end positions of tracks were marked and the image was captured using a digital camera (Point Grey, FL3-GE-13S2C) attached to Leica S8APO microscope. The movement speed was calculated by NIH ImageJ software (rsbweb.nih.gov/ij/).
Chemotaxis assay was performed as described earlier.59,60 NaCl 100 mM solution was used as a chemo-attractant. 6-OHDA-treated animals were washed thoroughly and placed at the center of the agar plate. The plate contained a drop of NaCl at one end and water control at the other end. The assay was run for 1 h. After this, animals toward water and NaCl spots were counted and chemotaxis index (CI) was calculated.
Electrotaxis assays and data analysis
The experimental setup of microfluidic electrotaxis and fabrication of channels have been described earlier20 (Fig. 1). Briefly, soft lithography technique was used to fabricate the microchannel (5 cm long and 300 µm wide) in polydimethylsiloxane (PDMS) (Fig. 1A). The DC electric field strength was set at 3 V/cm. Worms were loaded into the channel using a syringe pump and brought in the field of view. The electric field was activated and each animal was allowed to travel a minimum distance of 5,000 μm in one direction (toward cathode) (Fig. 1B and C). The electric field was reversed when the worm reached the end of the channel thereby causing it to turn and swim in the opposite direction (Fig. 1D and E). It should be mentioned here that the movement of the worm inside the microfluidic channel during electrotaxis assay is not affected by the electrokinetic flow.20
Electrotaxis responses of worms were recorded using a Nikon Coolpix digital camera (P5100) attached to a Leica stereomicroscope. Each data set typically consisted of 10–25 animals. For each animal, the response was monitored for 2–9 min duration and up to 45,000 μm total distance (in both directions). NIH ImageJ was used to analyze the captured videos.
Four swimming parameters, electrotaxis speed, turn time, body bend frequency and electrotaxis time index (ETI), were quantified manually. The electrotaxis speed is an average response of animals that was obtained by dividing the total swimming distance by elapsed time. In this analysis, only movement toward cathode was used to calculate the distance. Any motion toward anode was ignored. The elapsed time is the duration of the assay. The turn time is the period to complete a U-turn following a switch in the electric field polarity. Up to three turning events were used to determine the average response of each animal. The body bend frequency is the average number of sine waves per second. This was obtained by dividing the total number of sine waves produced by an animal in an experiment by the duration of the assay (in seconds). Only those sine waves were counted that spanned at least half of the channel diameter. Finally, ETI is the measure of electrotaxis response. The ETI of an animal was calculated by dividing the sum of all cathode-directed swimming time by total time of assay. For a given genotype, a lower ETI indicates less cathode-directed motion of animals whereas higher ETI indicates greater amount of time spent in moving toward cathode.
The statistical significance of data was evaluated using unpaired Student’s t-test and non-parametric Mann Whitney test. The p values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Devika Sharanya for generating dat-1p::YFP transgenic worms and members of BPG, PRS and RM laboratories for feedbacks. Some of the strains used in this study were obtained by Caenorhabditis Genetics Center that is supported by National Institutes of Health - National Center for Research Resources.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/24558
References
- 1.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–33. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–52. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargmann CI. Chemosensation in C. elegans WormBook 2006; ed. The C. elegansResearch Community, WormBook, / 10.1895/wormbook.1.123.1, http://www.wormbook.org [DOI]
- 6.Sulston J, Dew M, Brenner S. Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 1975;163:215–26. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- 7.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–31. doi: 10.1016/S0896-6273(00)81199-X. [DOI] [PubMed] [Google Scholar]
- 8.Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24:1217–25. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;375:73–8. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- 10.Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–85. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald PW, Jessen T, Field JR, Blakely RD. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol. 2006;26:593–618. doi: 10.1007/s10571-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulme SE, Shevkoplyas SS, Samuel A. Microfluidics: streamlining discovery in worm biology. Nat Methods. 2008;5:589–90. doi: 10.1038/nmeth0708-589. [DOI] [PubMed] [Google Scholar]
- 13.Crane MM, Chung K, Stirman J, Lu H. Microfluidics-enabled phenotyping, imaging, and screening of multicellular organisms. Lab Chip. 2010;10:1509–17. doi: 10.1039/b927258e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezai P, Salam S, Selvaganapathy PR, Gupta BP. Microfluidic systems to study the biology of human diseases and identify potential therapeutic targets in Caenorhabditis elegans. In: Iniewski K, ed. Integrated microsystems. Florida: CRC Press, 2012:581-608. [Google Scholar]
- 15.Ben-Yakar A, Chronis N, Lu H. Microfluidics for the analysis of behavior, nerve regeneration, and neural cell biology in C. elegans. Curr Opin Neurobiol. 2009;19:561–7. doi: 10.1016/j.conb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung K, Crane MM, Lu H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat Methods. 2008;5:637–43. doi: 10.1038/nmeth.1227. [DOI] [PubMed] [Google Scholar]
- 17.Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–31. doi: 10.1038/nmeth1075. [DOI] [PubMed] [Google Scholar]
- 18.Rohde CB, Zeng F, Gonzalez-Rubio R, Angel M, Yanik MF. Microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc Natl Acad Sci USA. 2007;104:13891–5. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Hwang H, Nam SW, Martinez F, Austin RH, Ryu WS. Enhanced Caenorhabditis elegans locomotion in a structured microfluidic environment. PLoS One. 2008;3:e2550. doi: 10.1371/journal.pone.0002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezai P, Siddiqui A, Selvaganapathy PR, Gupta BP. Electrotaxis of Caenorhabditis elegans in a microfluidic environment. Lab Chip. 2010;10:220–6. doi: 10.1039/b917486a. [DOI] [PubMed] [Google Scholar]
- 21.Rezai P, Salam S, Selvaganapathy PR, Gupta BP. Effect of pulse direct current signals on electrotactic movement of nematodes Caenorhabditis elegans and Caenorhabditis briggsae. Biomicrofluidics. 2011;5:44116–441169. doi: 10.1063/1.3665224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sukul NC, Croll NA. Influence of potential difference and current on the electrotaxis of Caenorhaditis elegans. J Nematol. 1978;10:314–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Bird AF. The attractiveness of roots to the plant parasitic nematode Meloidogyne javanica and M. hapla. Nematologica. 1959;4:322–35. doi: 10.1163/187529259X00534. [DOI] [Google Scholar]
- 24.Gabel CV, Gabel H, Pavlichin D, Kao A, Clark DA, Samuel AD. Neural circuits mediate electrosensory behavior in Caenorhabditis elegans. J Neurosci. 2007;27:7586–96. doi: 10.1523/JNEUROSCI.0775-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro-Ilan DI, Campbell JF, Lewis EE, Elkon JM, Kim-Shapiro DB. Directional movement of steinernematid nematodes in response to electrical current. J Invertebr Pathol. 2009;100:134–7. doi: 10.1016/j.jip.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:3264–9. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC. Caenorhabditis elegans MPP+ model of Parkinson’s disease for high-throughput drug screenings. Neurodegener Dis. 2004;1:175–83. doi: 10.1159/000080983. [DOI] [PubMed] [Google Scholar]
- 28.Ved R, Saha S, Westlund B, Perier C, Burnam LG, Sluder A, et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280:42655–68. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997;50:55–66. doi: 10.1007/978-3-7091-6842-4_7. [DOI] [PubMed] [Google Scholar]
- 30.Haycraft CJ, Swoboda P, Taulman PD, Thomas JH, Yoder BK. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms. Development. 2001;128:1493–505. doi: 10.1242/dev.128.9.1493. [DOI] [PubMed] [Google Scholar]
- 31.Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarafi-Reinach TR, Melkman T, Hobert O, Sengupta P. The lin-11 LIM homeobox gene specifies olfactory and chemosensory neuron fates in C. elegans. Development. 2001;128:3269–81. doi: 10.1242/dev.128.17.3269. [DOI] [PubMed] [Google Scholar]
- 33.Hobert O, D’Alberti T, Liu Y, Ruvkun G. Control of neural development and function in a thermoregulatory network by the LIM homeobox gene lin-11. J Neurosci. 1998;18:2084–96. doi: 10.1523/JNEUROSCI.18-06-02084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freyd G, Kim SK, Horvitz HR. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature. 1990;344:876–9. doi: 10.1038/344876a0. [DOI] [PubMed] [Google Scholar]
- 36.Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development. 1999;126:5819–31. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
- 37.Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, et al. The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol. 1998;54:601–9. [PubMed] [Google Scholar]
- 38.Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, et al. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 2004;23:473–82. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 40.Bové J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–94. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonsson G, Sachs C. Actions of 6-hydroxydopamine quinones on catecholamine neurons. J Neurochem. 1975;25:509–16. doi: 10.1111/j.1471-4159.1975.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 42.Glinka YY, Youdim MB. Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur J Pharmacol. 1995;292:329–32. doi: 10.1016/0926-6917(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 43.Chiba K, Trevor A, Castagnoli N., Jr. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984;120:574–8. doi: 10.1016/0006-291X(84)91293-2. [DOI] [PubMed] [Google Scholar]
- 44.Ali SF, David SN, Newport GD, Cadet JL, Slikker W., Jr. MPTP-induced oxidative stress and neurotoxicity are age-dependent: evidence from measures of reactive oxygen species and striatal dopamine levels. Synapse. 1994;18:27–34. doi: 10.1002/syn.890180105. [DOI] [PubMed] [Google Scholar]
- 45.Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J Neurosci. 2004;24:10993–8. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maharaj DS, Saravanan KS, Maharaj H, Mohanakumar KP, Daya S. Acetaminophen and aspirin inhibit superoxide anion generation and lipid peroxidation, and protect against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats. Neurochem Int. 2004;44:355–60. doi: 10.1016/S0197-0186(03)00170-0. [DOI] [PubMed] [Google Scholar]
- 47.Casper D, Yaparpalvi U, Rempel N, Werner P. Ibuprofen protects dopaminergic neurons against glutamate toxicity in vitro. Neurosci Lett. 2000;289:201–4. doi: 10.1016/S0304-3940(00)01294-5. [DOI] [PubMed] [Google Scholar]
- 48.Locke CJ, Fox SA, Caldwell GA, Caldwell KA. Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson’s disease. Neurosci Lett. 2008;439:129–33. doi: 10.1016/j.neulet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–9. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 50.Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–22. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- 51.Wilson RI, Corey DP. The force be with you: a mechanoreceptor channel in proprioception and touch. Neuron. 2010;67:349–51. doi: 10.1016/j.neuron.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–91. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Driscoll M, Kaplan J. Mechanotransduction. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, eds. C elegans II. Cold Spring Harbor (NY), 1997. [PubMed] [Google Scholar]
- 54.Korchounov A, Meyer MF, Krasnianski M. Postsynaptic nigrostriatal dopamine receptors and their role in movement regulation. J Neural Transm. 2010;117:1359–69. doi: 10.1007/s00702-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen AT, Maher KN, Wani KA, Betts KE, Chase DL. Coexpressed D1- and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans. Genetics. 2011;188:579–90. doi: 10.1534/genetics.111.128512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–47. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marvanova M, Nichols CD. Identification of neuroprotective compounds of caenorhabditis elegans dopaminergic neurons against 6-OHDA. J Mol Neurosci. 2007;31:127–37. doi: 10.1385/jmn/31:02:127. [DOI] [PubMed] [Google Scholar]
- 59.Rezai P, Salam S, Selvaganapathy PR, Gupta BP. Electrical sorting of Caenorhabditis elegans. Lab Chip. 2012;12:1831–40. doi: 10.1039/c2lc20967e. [DOI] [PubMed] [Google Scholar]
- 60.Hart AC. Behavior. Wormbook 2006; ed. The C. elegans Research Community, WormBook, / 10.1895/wormbook.1.87.1, http://www.wormbook.org [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.