Abstract
Background and Objectives
Most studies of attention deficit hyperactivity disorder (ADHD) in the substance dependence literature have assessed ADHD as a single, categorical entity. This approach limits characterization across the spectrum of ADHD symptomatology and may mask differences across the two core domains of ADHD symptoms—hyperactive-impulsive (HI) and inattention (IN). Further, it is unclear whether relations of HI and IN symptoms to substance dependence extend across drug classes and to the general population.
Methods
This cross-sectional study investigated associations of lifetime ADHD HI and IN symptom levels to individual classes of lifetime substance dependence (alcohol, nicotine, depressants, opioids, stimulants, cannabis, hallucinogens, polysubstance) in a population-based sample of 34,653 American adults.
Results
HI and IN were associated with the majority of dependence diagnoses in a linear pattern, such that each additional symptom was associated with a proportional increase in odds of dependence. After adjusting for the overlap between symptom domains, both HI and IN uniquely associated with alcohol, nicotine, and polysubstance dependence, but only HI uniquely associated with dependence on illicit substances.
Conclusions and Scientific Significance
These findings suggest that individuals in the general population with elevated levels of ADHD (particularly HI) symptoms are at risk for various forms of substance dependence and could benefit from preventive interventions.
Attention deficit hyperactivity disorder (ADHD)—a disruptive behavior disorder that onsets in childhood and is characterized by chronic and impairing levels of inattention, hyperactivity, or their combination1—has a well-documented relationship with substance dependence. This relationship encompasses a variety of substances, including alcohol,2,3 nicotine,4,5 illicit substances,3,6,7 and polysubstance dependence (ie, a concomitant dependence on multiple substances).8–10 The ADHD-substance dependence relation has been demonstrated in both retrospective cross-sectional and prospective longitudinal studies, illustrating that ADHD predicts risk of substance dependence onset.11–14 Further, this association is evident in clinical,9,15–18 population, and community-based19,20 samples. Because substance dependence results in substantial burden and cost to the individual and society,21–23 a comprehensive knowledge of the ADHD-substance dependence relationship is theoretically and clinically important for: (a) enhancing understanding of underlying mechanisms responsible for the association; and (b) informing interventions that target substance dependence for people with comorbid ADHD. However, despite evidence that a relation between ADHD and substance dependence likely exists, several important aspects regarding the nature of this relationship are not entirely clear.
First, most studies have examined the association between ADHD and substance dependence from a categorical perspective of ADHD symptomatology (eg, presence vs. absence of ADHD clinical diagnosis). Nonetheless, the underlying structure of variation in ADHD symptoms may be best characterized as a continuum, with important clinical variation above and below diagnostic thresholds.24,25 Indeed, studies have found that gradations in ADHD symptoms are associated with substance dependence indices.26–28 Moreover, one study showed that ADHD significantly predicted nicotine dependence and cannabis abuse/dependence when measured continuously but not when measured categorically,29 indicating that ADHD symptom levels, but not necessarily ADHD clinical status, may be an important risk factor for substance dependence. However, prior studies of gradations in ADHD symptoms and their relation to substance dependence utilized restricted community-based or clinical samples, which leaves unclear whether these findings will extend to the general population.
Second, the majority of studies have examined ADHD as a single entity, though the fundamental symptoms of ADHD represent two distinct constructs: inattention (IN; eg, does not seem to listen when spoken to directly, difficulty organizing tasks and activities) and hyperactive-impulsive (HI; eg, fidgets with hands or feet or squirms in seat, interrupts or intrudes others).1 Though HI and IN are generally correlated with one another,30–32 these constructs are not entirely redundant. In fact, these two dimensions are psychometrically distinct,33,34 differ in clinical correlates,35 and have distinct genetic influences.36 Thus, distinguishing HI and IN may reveal differential patterns of associations with substances that are masked when examining ADHD as a single construct. The few studies of substance dependence that have differentiated dimensional measures of HI and IN have produced mixed results, with some showing that both HI and IN are associated with substance dependence29,37 but others showing no association.38 These studies have utilized restricted community or clinical samples and have applied differing analytic approaches, which could account for the mixed findings. Thus, a comprehensive analytic approach utilizing a nationally representative sample is needed to clarify the unique roles of HI and IN in substance dependence.
Finally, studies examining the relationship between gradations in ADHD symptom dimensions and substance dependence have been limited to a small number of dependence outcomes (ie, nicotine, alcohol, cannabis, and a combined “hard drug” category consisting of various illicit drug dependence diagnoses). However, HI and IN may exhibit differential relationships with certain types of substance dependence (eg, stimulants vs. depressants). These specific relationships are important to clarify because they may provide insight into potential mechanisms underlying the ADHD-substance dependence relationship and may have clinical implications for targeting specific populations of drug-dependent individuals.
The purpose of this report is to clarify fundamental questions regarding the association between gradations in ADHD symptomatology and substance dependence. To that end, we examined cross-sectional associations of retrospectively assessed ADHD HI and IN symptom levels to individual lifetime Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV)1 substance dependence diagnoses (ie, alcohol, nicotine, depressants, opioids, stimulants, cannabis, hallucinogens, polysubstance) in a population-based sample of American adults. Though limited by a cross-sectional design, this study leverages off of a large representative sample that provides adequate numbers of dependence cases to examine relations with discrete dependence categories that have received limited investigation (eg, opioid, hallucinogen, sedative, stimulant). Additionally, this study includes careful diagnostic assessments of psychiatric disorders, which permits examination of whether ADHD symptoms associate with substance dependence over and above other psychiatric disorders.
METHODS
Participants
Participants were respondents in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), which assessed the prevalence of substance use, psychiatric disorders, and other characteristics in the U.S. adult population at two time points. At Wave 1 (2001–2002), 43,093 individuals completed the in-person interview, and 34,653 of these individuals subsequently completed the Wave 2 (2004–2005) survey. Participants were all non-institutionalized civilians, aged 18 and older, residing in the United States. Young adults aged 18–24, were over-sampled by a 2.25:1 ratio. African-Americans and Hispanic-Americans were also over-sampled and each group accounted for approximately 20% of the sample. To account for over-sampling, the data had accompanying weights so adjustment was representative of the 2000 U.S. Census results. Additional details of the sampling, purpose of the survey, and weighting procedures have been published elsewhere.39,40 ADHD was assessed only at Wave 2, thus only those individuals who completed the Wave 2 survey (N = 34,653) were included for analyses.
Procedure
The U.S. Census Bureau selected one adult from each participating household. The response rate for Wave 1 was 81%. After efforts were made to re-interview all participants excluding individuals who were not eligible (eg, those who were deceased), the response rate for Wave 2 was 86.7%. The cumulative response rate across both waves was 70.2%. After obtaining informed consent, interviewers conducted face-to-face interviews of the Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS-IV).41 The protocol was approved by the U.S. Census Bureau and the U.S. Office of Management.
Measures
The AUDADIS-IV gathers information on demographic characteristics, substance use patterns, DSM-IV criteria for psychiatric and substance use disorders, and other clinical characteristics. Previous analyses have shown adequate psychometric properties for mental disorder diagnoses, including ADHD, and other information gathered from the AUDADIS-IV.41–43
ADHD Symptoms
The ADHD module of the AUDADIS-IV was administered only at Wave 2. All participants were asked whether they experienced any of a list of 20 ADHD symptoms before they were 18 years old (Yes/No). Though the presence of symptoms prior to the age of seven is required for DSM-IV diagnostic criteria, research suggests that symptoms that onset during the entire childhood years (<18 years old) are clinically meaningful,44,45 and because we are examining the influence of childhood ADHD symptoms independent of clinical diagnosis, this age cutoff is adequate for the purpose of our analyses.
The list of 20 symptoms included dichotomous (present vs. absent) measures of nine IN items each reflecting a different symptom (eg, “did you frequently lose things like assignments or books or other things you needed?” and, “did you dislike, avoid, or put off doing things that required a lot of concentration?”) and eleven HI items reflecting 9 HI symptoms (eg, “did you usually fidget or squirm a lot when you were sitting down?” and, “did you often blurt out answers to other people’s questions ever before they were finished talking?”), based on DSM-IV criteria. The AUDADIS-IV divided two of the DSM-IV HI criteria each into two separate questions. To remain consistent with the DSM-IV we collapsed these items, such that a person was considered positive if they endorsed either AUDADIS-IV symptom question for these two DSM-IV criteria. Participants received two separate scores based on symptom count: (1) number of IN symptoms endorsed, and (2) number of HI symptoms endorsed. Those who had missing values for either IN or HI symptoms (N = 309) were eliminated, for a final sample of 34,344 used in analyses.
Substance Dependence
Lifetime DSM-IV substance dependence was diagnosed for alcohol, nicotine, sedatives, tranquilizers, opioid, heroin, cocaine, amphetamine, cannabis, and hallucinogens, among those who endorsed lifetime use of the index drug, regardless of whether abuse criteria were met. To reduce the number of tests performed and to increase the number of individuals in each dependence category, sedative and tranquilizer were collapsed into a “depressants” category, all licit (eg, oxyco-done) and illicit (eg, heroin) opioids were collapsed into an “opioids” category, and cocaine and amphetamine were collapsed into a “stimulant” category. This created a total of seven dependence outcomes: alcohol, nicotine, depressant, stimulant, opioid, cannabis, and hallucinogens. Additionally, we created two other dependence categories: an “any illicit” substance dependence category (representing those who met dependence criteria for one of the following categories: depressants, opioids, stimulants, cannabis, or hallucinogens), as in previous research,46 and a “polysubstance” dependence category, representing those who had two or more lifetime substance dependence diagnoses. Although investigating associations between ADHD symptoms and substance abuse are also of interest, we decided to focus on substance dependence as the main outcome for the following reasons: (1) to reduce the number of statistical tests performed and associated risk for Type 1 error, (2) because dependence is generally accepted as the more severe form of substance use disorder and associated with worse mental health, social functioning, and emotional functioning compared to substance abuse,47–49 and (3) because prior research has indicated issues with the reliability and the clinical significance of the DSM-IV substance abuse diagnosis.50–52
Demographic Characteristics
Demographic covariates include age, gender, education, income, and ethnicity/race.
Psychiatric Characteristics
Conduct disorder symptoms reflect the total number of lifetime DSM-IV conduct disorder symptoms (out of 14 symptoms) endorsed. Mood disorder reflects a lifetime DSM-IV diagnosis of any of several mood disorders (ie, major depressive disorder, [hypo]mania, dysthymia) and anxiety disorder reflects a lifetime DSM-IV diagnosis of any of several anxiety disorders (ie, panic disorder, agoraphobia, social phobia, specific phobia, generalized anxiety disorder, posttraumatic stress disorder).
Statistical Analysis
For preliminary analyses, we calculated descriptive statistics for demographic and psychiatric correlates, ADHD symptoms, and substance dependence diagnoses, as well as correlations between HI and IN symptom scales and Cronbach alpha coefficients for HI and IN. To reduce positive skewness, HI and IN symptom counts were square root transformed and then standardized (M = 0, SD = 1) to aid interpretation of the odds ratios (ORs).
For primary analyses we used logistic regression models to test the associations of total IN and HI symptoms to the presence (vs. absence) of each substance dependence diagnosis. For each substance dependence diagnosis two types of models were calculated: (1) a univariate model that included total IN symptoms as the sole predictor and (2) a univariate model that included total HI symptoms as the sole predictor. Following the unadjusted models, all models were recalculated twice, once after adjusting for demographic characteristics (sex, age, ethnicity/race, education, income) and psychiatric disorders (conduct disorder symptoms, mood disorder, anxiety disorder) and then after additionally adjusting for the other ADHD symptom domain.
We also conducted supplemental analyses to examine whether relationships between ADHD and classes of substance dependence were specific to particular substances or reflective of a general propensity toward dependence on any type of substance. Accordingly, we recalculated each of the models after controlling for number of comorbid substance dependence diagnoses. To do this, we created eight continuous variables (each variable was applied for each analysis with the exception of polysubstance dependence) representing the number of other lifetime dependence diagnoses, independent of the index dependence. For example, if a person had nicotine dependence, alcohol dependence, and cannabis dependence, they received a “2” for the number of non-nicotine dependence diagnoses covariate, which was included in previously unadjusted models predicting nicotine dependence as the outcome variable.
All analyses were performed in SAS using PROC SURVEY procedures53 to account for the complex sampling methodology of the NESARC and to use the recommended Wave 2 sampling weights to approximate the U.S. population.39 Primary results of logistic regressions are reported as ORs, and significance was set at p < .05 (two-tailed).
RESULTS
Preliminary Analyses
ADHD Symptoms
The M ± SD of ADHD symptom indexes were .88 ± 1.80 for IN symptoms and 1.31 ± 1.94 for HI symptoms. The two scales were significantly but moderately correlated with one another (r = .56, p < .0001), indicating that HI and IN are likely assessing related but non-redundant constructs, and both scales evidenced adequate internal consistency (IN Cronbach α = .87; HI Cronbach α = .82).
Substance Dependence
The weighted prevalence rates (% ± SE) for lifetime substance dependence outcomes were as follows: alcohol (15.2% ± .24), nicotine (14.6% ± .24), depressants (.5% ± .05), opioids (.7% ± .06), stimulants (1.6% ± .09), cannabis (1.7% ± .09), hallucinogens (.3% ± .04), any illicit drug (3.4% ± .13), and polysubstance (6.6% ± .17).
Psychiatric Characteristics
Weighted mean number of conduct disorder symptoms was .35 (± .88), Cronbach α = .65, and the weighted prevalence for DSM-IV mood and anxiety disorders were 25.7% (± .29) and 25.5% (± .28), respectively.
Primary Analyses
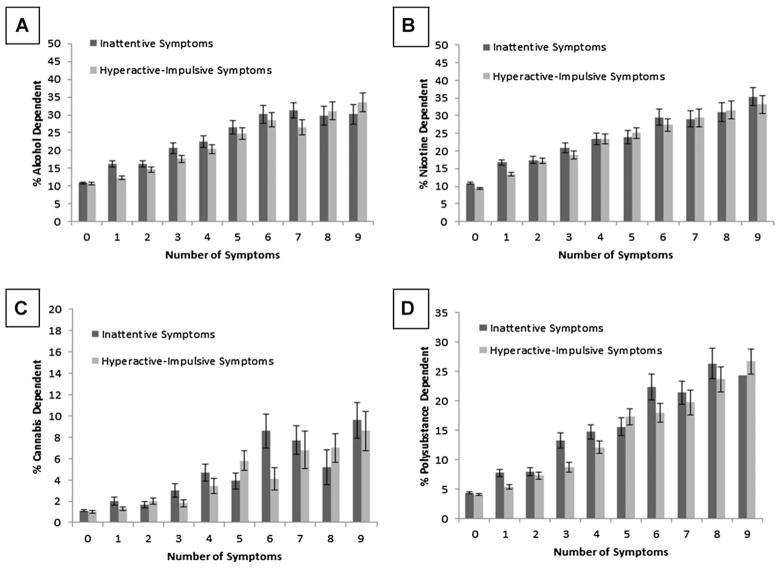
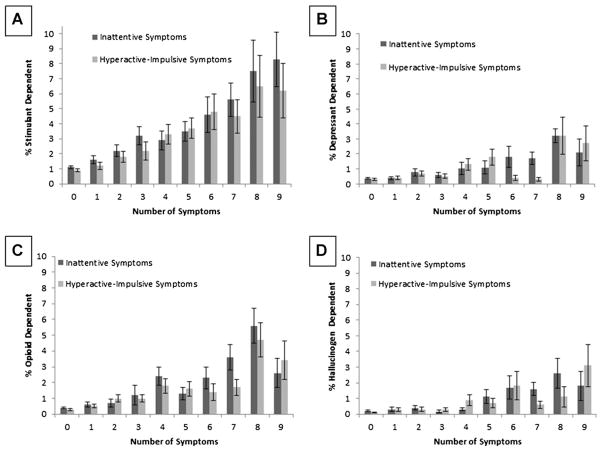
In unadjusted models, increases in symptom levels for both IN and HI were significantly associated with each substance dependence outcome (p’s < .0001) (see Table 1). The pattern of associations appeared to represent a linear pattern, such that each additional ADHD symptom was generally associated with a proportional increase in odds of substance dependence (see Figs. 1 and 2). After controlling for demographic (sex, ethnicity/race, income, education, age) and psychiatric (conduct disorder, anxiety disorder, mood disorder) covariates, ORs were reduced in size and a few relations fell below significance. In adjusted models that included both IN and HI as simultaneous predictors (and demographic and psychiatric covariates), IN only retained a significant association with alcohol, nicotine, and polysubstance dependence, whereas HI remained significantly associated with alcohol, nicotine, stimulants, cannabis, hallucinogens, any illicit substance, and polysubstance dependence.
TABLE 1.
Odds ratios (and 95% confidence intervals) for associations between ADHD symptom domains and classes of DSM-IV lifetime substance dependence (N = 34,344)
| Dependence class | OR (95% CI)‡ | OR (95% CI)§ | OR (95% CI)|| |
|---|---|---|---|
| Inattentive symptoms | |||
| Alcohol | 1.46 (1.42–1.51)† | 1.14 (1.09–1.18)† | 1.07 (1.02–1.12)** |
| Nicotine | 1.48 (1.43–1.53)† | 1.19 (1.15–1.24)† | 1.05 (1.01–1.10)* |
| Depressants | 1.62 (1.37–1.91)† | .99 (.84–1.17) | .92 (.74–1.13) |
| Opioids | 1.85 (1.62–2.11)† | 1.18 (1.02–1.38)* | 1.08 (.87–1.33) |
| Stimulants | 1.66 (1.52–1.82)† | 1.10 (.99–1.22) | 1.01 (.89–1.15) |
| Cannabis | 1.80 (1.65–1.95)† | 1.14 (1.04–1.25)** | 1.05 (.93–1.20) |
| Hallucinogens | 1.72 (1.38–2.15)† | 1.01 (.81–1.26) | .82 (.59–1.14) |
| Any illicit substance | 1.77 (1.67–1.88)† | 1.18 (1.10–1.26)† | 1.08 (.98–1.18) |
| Polysubstance | 1.72 (1.64–1.80)† | 1.22 (1.16–1.29)† | 1.11 (1.04–1.18)** |
| Hyperactive-impulsive symptoms | |||
| Alcohol | 1.47 (1.42–1.53)† | 1.16 (1.12–1.21)† | 1.12 (1.07–1.18)† |
| Nicotine | 1.59 (1.53–1.64)† | 1.32 (1.27–1.37)† | 1.29 (1.23–1.35)† |
| Depressants | 1.74 (1.44–2.11)† | 1.11 (.93–1.33) | 1.17 (.93–1.48) |
| Opioids | 1.94 (1.64–2.30)† | 1.26 (1.08–1.47)** | 1.20 (.97–1.49) |
| Stimulants | 1.76 (1.59–1.94)† | 1.19 (1.07–1.33)** | 1.18 (1.03–1.36)* |
| Cannabis | 1.86 (1.68–2.06)† | 1.21 (1.09–1.35)*** | 1.17 (1.01–1.35)* |
| Hallucinogens | 2.15 (1.68–2.75)† | 1.33 (1.03–1.70)* | 1.51 (1.04–2.18)* |
| Any illicit substance | 1.85 (1.72–1.99)† | 1.25 (1.16–1.35)† | 1.20 (1.08–1.33)*** |
| Polysubstance | 1.79 (1.69–1.88)† | 1.29 (1.22–1.37)† | 1.22 (1.13–1.31)† |
OR = odds ratio; CI = confidence interval. Depressants = sedatives and tranquilizers; opioids = opioid and heroin; stimulants = amphetamine and cocaine. Illicit drug = depressants, opioids, stimulants, cannabis, and hallucinogens. Polysubstance = greater than one substance dependence versus one or zero substance dependences. Significant findings are bolded.
Results reflect increase in odds of respective substance dependence diagnosis that is associated with each increase in 1 SD in the index symptom domain.
p < .05;
p < .01;
p < .001;
p < .0001;
Unadjusted model.;
Adjusted for age, sex, ethnicity/race, income, education, conduct disorder symptoms, mood disorder, and anxiety disorder;
Adjusted for age, sex, ethnicity/race, income, education, conduct disorder symptoms, mood disorder, anxiety disorder, and other ADHD symptom domain.
FIGURE 1.
(a) Weighted percentage (± SE) of lifetime DSM-IV alcohol dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms (y-axis ranges from 0% to 50%). (b) Weighted percentage (± SE) of lifetime DSM-IV nicotine dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms (y-axis ranges from 0% to 50%). (c) Weighted percentage (± SE) of lifetime DSM-IV cannabis dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms (y-axis ranges from 0% to 20%). (d) Weighted percentage (± SE) of lifetime DSM-IV polysubstance dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms (y-axis ranges from 0% to 35%). N = 34,344.
FIGURE 2.
(a) Weighted percentage (±SE) of lifetime DSM-IV stimulant dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms. (b) Weighted percentage (±SE) of lifetime DSM-IV depressant dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms. (c) Weighted percentage (±SE) of lifetime DSM-IV opioid dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms. (d) Weighted percentage (±SE) of lifetime DSM-IV hallucinogen dependence by increasing number of inattentive symptoms and hyperactive-impulsive symptoms. All y-axes range from 0% to 10%; N = 34,344.
Supplemental Analyses
We recalculated each of the unadjusted models after controlling for the number of comorbid substance dependences. Results indicated that HI and IN retained significant associations to all classes of substances (p’s range from <.0001 to .0194) over and above comorbid dependence diagnoses, with the exception of both HI and IN’s relations to depressants and IN with hallucinogens, which fell below significance.
DISCUSSION
In this study of associations between gradations in ADHD symptomatology and substance dependence in a nationally representative sample, results indicated that levels of HI and IN ADHD symptoms were both associated with increased rates of most classes of substance dependence. These relations appeared to reflect a linear pattern whereby each additional ADHD symptom was generally associated with a proportional increase in odds of substance dependence. Further, many of these relationships remained significant in supplemental analyses controlling for number of comorbid substance dependence diagnoses. Thus, ADHD’s relation with individual classes of substance dependence may not be entirely accounted for by a nonspecific risk towards dependence on any type of substance.
Examination of the relative roles of HI and IN in dependence revealed several notable patterns. HI and IN both exhibited unique associations with nicotine dependence even after accounting for their covariance with each other and demographics and psychiatric disorders; however, effects appeared to be stronger for HI. A 1-SD increase in HI symptom levels was associated with a nearly 30% increase in odds for lifetime nicotine dependence; whereas IN was associated with only a 5% increase in odds of nicotine dependence (see Table 1). While both IN and HI have been linked with nicotine dependence in prior studies of circumscribed samples27,29; the current findings suggest that these results extend to the general U.S. population and may be more robust for the HI subtype of ADHD symptoms.
Previous studies have not found that HI and IN are associated with alcohol dependence.28,29,38 However, the current population-based sample may have afforded increased statistical power to detect effects that may not have been present in prior studies with comparatively smaller samples. Indeed, we found that both IN and HI were uniquely associated with alcohol dependence over and above their co-variation with demographics, psychiatric characteristics, and each other.
HI generally showed more robust and consistent relations with dependence on various illicit substances than IN. Our results are consistent with Elkins et al.’s29 finding that both HI and IN individually associated with cannabis dependence, but IN did not have an incremental relation above and beyond its covariance with HI. We extend these results by illustrating a unique relationship of HI to illicit drug dependence that generalizes to other illicit substances in addition to cannabis (ie, stimulants, hallucinogens, and “any illicit substance”).
To the best of our knowledge, no previous investigations have examined the relationship between gradations in ADHD symptoms and polysubstance dependence. Studies utilizing categorical diagnoses of ADHD have similarly found significantly higher rates of comorbid drug and alcohol disorder together among adults with ADHD (vs. no ADHD),9 and that among those with alcohol disorders ADHD significantly increased the risk for subsequent comorbid drug dependence.2 Our findings extend these results by showing that gradations in HI and IN were both associated with polysubstance dependence, independent of demographic and psychiatric covariates.
Though this study does not allow for conclusions on mechanisms underlying the ADHD-substance dependence relationship, which likely involve complex interactions of biological, psychological, and social factors, by parsing out specific relations between HI and IN across discrete classes of substances, these findings may provide insight into proposed theoretical perspectives on the relationship between ADHD and substance use disorders. First, the association of both HI and IN symptoms with nicotine and alcohol dependence may reflect a self-medication hypothesis54,55—in this case a desire to self-medicate HI and IN symptoms and related distress. Individuals with increasing levels of HI and IN symptoms may have higher rates of nicotine dependence due to the ability of nicotine to provide both stimulating and sedating effects,56–58 such as improving attention59–63 and behavioral inhibition64,65 and decreasing arousal and hyperactivity.56,66 Likewise, individuals with a greater prevalence of HI and IN symptoms may be more likely to turn to alcohol as a coping mechanism to deal with emotional, social, and occupational distress67 resulting from their HI and IN symptoms.68,69
Second, the greater generalizability and strength of relationships for HI (vs. IN) across substance dependence types, including illicit drugs, may be reflective of HI-related developmental processes. HI symptoms predominantly decline at an earlier age and a faster rate compared to IN symptoms,70,71 thus the finding that childhood HI symptoms still presented significant associations with lifetime substance dependence indicates that childhood HI symptoms may give rise in protracted personality characteristics in adulthood (eg, impulsivity) that may not reflect ADHD per se, but may continue to increase risk for substance dependence.72 Interestingly, Miller et al.73 found that HI and IN associated with different facets of impulsivity—lack of premeditation (ie, the inability to think through possible consequences of one’s behavior before acting) associated with only HI symptoms whereas lack of perseverance only associated with IN symptoms. In turn, only lack of premeditation associated with substance use behaviors. Thus, HI symptoms may contribute to an impulsive personality trait in which individuals are less likely to consider the negative medical and legal consequences of substance use, which could in turn increase their vulnerability to dependence across multiple licit and illicit substances.74,75 A second potential influence of childhood HI on lifetime substance dependence may be that HI is a precursor or marker of a general progression toward youth aggression76,77 and antisocial tendencies,78 which may eventually develop into adult substance related problems.79–81 Though it is important to note that we adjusted for conduct disorder symptoms, which suggests that the relations demonstrated herein are not entirely accounted for by a general propensity toward externalizing or defiant behavior. Last, symptoms of HI may take on different forms in adulthood82 such as mental/ internal restlessness (associated with childhood hyperactivity symptoms)83–86 and emotional lability/low frustration tolerance (associated with childhood impulsivity symptoms)68,86 which may influence substance use problems.87–89
Another possible reason for the greater strength and generalizability of HI-substance dependence relations is that persistent substance use dysregulates the brain and causes ADHD symptoms, particularly those of the HI variety.90,91 Indeed, we cannot rule out the possibility that dependence onset prior to ADHD symptoms. Longitudinal research is needed to determine the precedence of onset (symptoms or substance dependence) to better understand the risk ADHD symptoms pose for development of substance dependence throughout the lifespan. Lastly, unmeasured confounds may perhaps explain the relations presented herein, although we rigorously controlled for a host of covariates, which makes this unlikely. Regardless of the putative mechanism, the current findings suggest that HI-related processes may be a more important driving force that explains ADHD-dependence comorbidity than IN symptoms.
The present findings should be interpreted with respect to its limitations. First, ADHD symptoms and substance dependence symptom criteria were measured via retrospective self-report; therefore, these measurements were subject to recall bias and are not as accurate or reliable as would be if measured in longitudinal studies and with multiple measures of ADHD symptoms. However, previous studies have found that retrospective self-report measures of ADHD have shown adequate psychometric properties,92,93 and specifically in the AUDADIS-IV.43 Second, due to the cross-sectional nature of this study, temporality cannot be determined, which leaves unclear whether HI may increase risk for dependence or vice versa. Because there is a developmental influence on ADHD symptoms70,94 and because substance dependence may result in ADHD-like symptoms,90,91,95 future longitudinal research that maps the trajectory of ADHD symptoms from childhood through adolescence and into adulthood, and the associations of these trajectories with different types and onset of substance dependence, is needed to better understand the nature of these relationships. Third, because we examined presence vs. absence of ADHD symptoms, it is unknown how the severity of each symptom may influence substance dependence relations. Fourth, because only those who completed Wave 2 were included, those who dropped out between Wave 1 and Wave 2 were not taken into account. However, sampling weights were applied to the Wave 2 dataset to approximate the demographic profile 2000 U.S. Census, which helps to limit any differences in sample composition across the two waves. Last, because we set significance at p < .05 for the purposes of broadly examining relationship patterns across substances, these findings are subject to Type 1 error due to analyses of multiple outcomes. However, this concern is offset given that the findings were robust in many analyses (ps < .001) and generally consistent across multiple dependence outcomes.
Limitations notwithstanding, these results suggest that increasing levels of ADHD symptoms, and HI in particular, are associated with corresponding increases in the odds of a variety of substance dependence diagnoses. As such, these findings have preventive implications, such that individuals with elevated HI symptoms (though not necessarily reaching a diagnostic cutoff for ADHD) may benefit from preventive interventions designed to reduce substance use risk. Further, interventions designed to broadly improve hyperactivity and impulsivity for all children (in addition to targeted efforts for those children diagnosed with ADHD) may potentially reduce risk for substance dependence.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Assocation; 1994. [Google Scholar]
- 2.Biederman J, Wilens TE, Mick E, et al. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44:269–273. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- 3.Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 4.Biederman J, Monuteaux MC, Mick E, et al. Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychol Med. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- 5.Pomerleau OF, Downey KK, Stelson FW, et al. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 6.Carroll KM, Rounsaville BJ. History and significance of childhood attention deficit disorder in treatment-seeking cocaine abusers. Compr Psychiatry. 1993;34:75–82. doi: 10.1016/0010-440x(93)90050-e. [DOI] [PubMed] [Google Scholar]
- 7.Dennis M, Titus JC, Diamond G, et al. The Cannabis Youth Treatment (CYT) experiment: Rationale, study design and analysis plans. Addiction. 2002;97:16–34. doi: 10.1046/j.1360-0443.97.s01.2.x. [DOI] [PubMed] [Google Scholar]
- 8.Arias AJ, Gelernter J, Chan G, et al. Correlates of co-occurring ADHD in drug-dependent subjects: Prevalence and features of substance dependence and psychiatric disorders. Addict Behav. 2008;33:1199–1207. doi: 10.1016/j.addbeh.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biederman J, Wilens T, Mick E, et al. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier PJ, van Gogh MT, Knapen LJ, et al. Influence of attention deficit hyperactivity disorder and conduct disorder on opioid dependence severity and psychiatric comorbidity in chronic methadone-maintained patients. Eur Addict Res. 2011;17:10–20. doi: 10.1159/000321259. [DOI] [PubMed] [Google Scholar]
- 11.Biederman J, Petty CR, Wilens TE, et al. Familial risk analyses of attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2008;165:107–115. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- 12.Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:1792–1798. doi: 10.1176/ajp.150.12.1792. [DOI] [PubMed] [Google Scholar]
- 13.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 14.Mannuzza S, Klein RG, Bonagura N, et al. Hyperactive boys almost grown up. V. Replication of psychiatric status. Arch Gen Psychiatry. 1991;48:77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- 15.Milberger S, Biederman J, Faraone SV, et al. Associations between ADHD and psychoactive substance use disorders. Findings from a longitudinal study of high-risk siblings of ADHD children. Am J Addict. 1997;6:318–329. [PubMed] [Google Scholar]
- 16.King VL, Brooner RK, Kidorf MS, et al. Attention deficit hyperactivity disorder and treatment outcome in opioid abusers entering treatment. J Nerv Ment Dis. 1999;187:487–495. doi: 10.1097/00005053-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Schubiner H, Tzelepis A, Milberger S, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61:244–251. doi: 10.4088/jcp.v61n0402. [DOI] [PubMed] [Google Scholar]
- 18.Wood D, Wender PH, Reimherr FW. The prevalence of attention deficit disorder, residual type, or minimal brain dysfunction, in a population of male alcoholic patients. Am J Psychiatry. 1983;140:95–98. doi: 10.1176/ajp.140.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Adler LA, Barkley R, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: Results from the national comorbidity survey replication. Biol Psychiatry. 2005;57:1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szobot CM, Rohde LA, Bukstein O, et al. Is attention-deficit/hyperactivity disorder associated with illicit substance use disorders in male adolescents? A community-based case-control study. Addiction. 2007;102:1122–1130. doi: 10.1111/j.1360-0443.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2007. Atlanta: U.S. Department of Health and Human Services Centers for Disease Control and; Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Oct, 2007. [Google Scholar]
- 22.Office of National Drug Control Policy. The Economic Costs of Drug Abuse in the United States, 1992–2002. Washington, D.C: Executive Office of the President (Publication No. 207303); 2004. [Google Scholar]
- 23.Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 24.Frazier TW, Youngstrom EA, Naugle RI. The latent structure of attention-deficit/hyperactivity disorder in a clinic-referred sample. Neuropsychology. 2007;21:45–64. doi: 10.1037/0894-4105.21.1.45. [DOI] [PubMed] [Google Scholar]
- 25.Haslam N, Williams B, Prior M, et al. The latent structure of attention-deficit/hyperactivity disorder: A taxometric analysis. Aust N Z J Psychiatry. 2006;40:639–647. doi: 10.1080/j.1440-1614.2006.01863.x. [DOI] [PubMed] [Google Scholar]
- 26.Heffner JL, Johnson CS, Blom TJ, et al. Relationship between cigarette smoking and childhood symptoms of inattention and hyperactivity/ impulsivity in alcohol-dependent adults without attention-deficit hyperactivity disorder. Nicotine Tob Res. 2010;12:243–250. doi: 10.1093/ntr/ntp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilens TE, Vitulano M, Upadhyaya H, et al. Cigarette smoking associated with attention deficit hyperactivity disorder. J Pediatr. 2008;153:414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flory K, Milich R, Lynam DR, et al. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: Individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychol Addict Behav. 2003;17:151–158. doi: 10.1037/0893-164x.17.2.151. [DOI] [PubMed] [Google Scholar]
- 29.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 30.Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? J Child Psychol Psychiatry. 2001;42:493–502. [PubMed] [Google Scholar]
- 31.Lahey BB, Willcutt EG. Predictive validity of a continuous alternative to nominal subtypes of attention-deficit/hyperactivity disorder for DSM-V. J Clin Child Adolesc Psychol. 2010;39:761–775. doi: 10.1080/15374416.2010.517173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez D, Tercyak KP, Audrain-McGovern J. Effects of inattention and hyperactivity/impulsivity symptoms on development of nicotine dependence from mid adolescence to young adulthood. J Pediatr Psychol. 2008;33:563–575. doi: 10.1093/jpepsy/jsm100. [DOI] [PubMed] [Google Scholar]
- 33.Spencer TJ, Adler LA, Meihua Q, et al. Validation of the adult ADHD investigator symptom rating scale (AISRS) J Atten Disord. 2010;14:57–68. doi: 10.1177/1087054709347435. [DOI] [PubMed] [Google Scholar]
- 34.Toplak ME, Pitch A, Flora DB, et al. The unity and diversity of inattention and hyperactivity/impulsivity in ADHD: Evidence for a general factor with separable dimensions. J Abnorm Child Psychol. 2009;37:1137–1150. doi: 10.1007/s10802-009-9336-y. [DOI] [PubMed] [Google Scholar]
- 35.Sprafkin J, Gadow KD, Weiss MD, et al. Psychiatric comorbidity in ADHD symptom subtypes in clinic and community adults. J Atten Disord. 2007;11:114–124. doi: 10.1177/1087054707299402. [DOI] [PubMed] [Google Scholar]
- 36.Larsson H, Lichtenstein P, Larsson JO. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2006;45:973–981. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- 37.Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol. 2007;32:1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- 38.Abrantes AM, Strong DR, Ramsey SE, et al. Substance use disorder characteristics and externalizing problems among inpatient adolescent smokers. J Psychoactive Drugs. 2005;37:391–399. doi: 10.1080/02791072.2005.10399812. [DOI] [PubMed] [Google Scholar]
- 39.Grant BF, Kaplan K, Shepard J, et al. Source and Accuracy Statement for Wave 1 of the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
- 40.Grant BF, Kaplan KD. Source and Accuracy Statement for the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 2005. [Google Scholar]
- 41.Grant BF, Dawson DA, Stinson FS, et al. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): Reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 42.Chatterji S, Saunders J, Vrasti MRS, et al. Reliability of the alcohol and drug modules of the Alcohol Use Disorder and Associated Disabilities Interview Schedule—Alcohol/Drug-Revised (AUDADIS-ADR): An international comparison. Drug Alcohol Depend. 1997;47:171–185. doi: 10.1016/s0376-8716(97)00088-4. [DOI] [PubMed] [Google Scholar]
- 43.Ruan WJ, Goldstein RB, Chou SP, et al. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): Reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92:27–36. doi: 10.1016/j.drugalcdep.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:1204–1210. doi: 10.1097/00004583-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Kieling C, Kieling RR, Rohde LA, et al. The age at onset of attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:14–16. doi: 10.1176/appi.ajp.2009.09060796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 47.Compton WM, Thomas YF, Stinson FS, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States—Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 48.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders—Results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 49.Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States—Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 50.Hasin DS, Schuckit MA, Martin CS, et al. The validity of DSM-IV alcohol dependence: What do we know and what do we need to know? Alcohol Clin Exp Res. 2003;27:244–252. doi: 10.1097/01.ALC.0000060878.61384.ED. [DOI] [PubMed] [Google Scholar]
- 51.Hasin D, Carpenter KM, McCloud S, et al. The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): Reliability of alcohol and drug modules in a clinical sample. Drug Alcohol Depend. 1997;44:133–141. doi: 10.1016/s0376-8716(97)01332-x. [DOI] [PubMed] [Google Scholar]
- 52.Hasin DS, Trautman KD, Miele GM, et al. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): Reliability for substance abusers. Am J Psychiatry. 1996;153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 53.SAS Institute, Inc. The SAS System for Windows. Version 9.2. Cary, NC: SAS Institute, Inc; 2009. [Google Scholar]
- 54.Khantzian EJ. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- 55.Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 56.Gilbert DG. Paradoxical tranquilizing and emotion-reducing effects of nicotine. Psychol Bull. 1979;86:643–661. [PubMed] [Google Scholar]
- 57.Jarvik ME. Nicotine as a psychoactive drug—Panel summary. Psychopharmacol Bull. 1986;22:882–883. [Google Scholar]
- 58.Pomerleau OF, Jarvik ME. Nicotine as a psychoactive drug—Anxiety and pain reduction, arousal, and appetite regulation—Introduction. Psychopharmacol Bull. 1986;22:863–864. [PubMed] [Google Scholar]
- 59.Gehricke JG, Whalen CK, Jamner LD, et al. The reinforcing effects of nicotine and stimulant medication in the everyday lives of adult smokers with ADHD: A preliminary examination. Nicotine Tob Res. 2006;8:37–47. doi: 10.1080/14622200500431619. [DOI] [PubMed] [Google Scholar]
- 60.Conners CK, Levin ED, Sparrow E, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- 61.Levin ED, Conners CK, Silva D, et al. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharm. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- 62.Levin ED, Conners CK, Silva D, et al. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- 63.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 64.Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- 65.Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Cinciripini PM, Benedict CE, Van Vunakis H, et al. The effects of smoking on the mood, cardiovascular and adrenergic reactivity of heavy and light smokers in a non-stressful environment. Biol Psychol. 1989;29:273–289. doi: 10.1016/0301-0511(89)90023-9. [DOI] [PubMed] [Google Scholar]
- 67.Wilens TE. AOD use and attention deficit hyperactivity disorder. Alcohol Health Res World. 1998;22:127–130. [PMC free article] [PubMed] [Google Scholar]
- 68.Faraone SV, Biederman J, Spencer T, et al. Attention-deficit/hyperactivity disorder in adults: An overview. Biol Psychiatry. 2000;48:9–20. doi: 10.1016/s0006-3223(00)00889-1. [DOI] [PubMed] [Google Scholar]
- 69.Murphy K, Barkley RA. Attention deficit hyperactivity disorder adults: Comorbidities and adaptive impairments. Compr Psychiatry. 1996;37:393–401. doi: 10.1016/s0010-440x(96)90022-x. [DOI] [PubMed] [Google Scholar]
- 70.Hart EL, Lahey BB, Loeber R, et al. Developmental change in attention-deficit hyperactivity disorder in boys: A four-year longitudinal study. J Abnorm Child Psychol. 1995;23:729–749. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- 71.Murphy K, Barkley RA. Prevalence of DSM-IV symptoms of ADHD in adult licensed drivers: Implications for clinical diagnosis. J Attention Disord. 1996;1:147–161. [Google Scholar]
- 72.Nigg JT, John OP, Blaskey LG, et al. Big five dimensions and ADHD symptoms: Links between personality traits and clinical symptoms. J Pers Soc Psychol. 2002;83:451–469. doi: 10.1037/0022-3514.83.2.451. [DOI] [PubMed] [Google Scholar]
- 73.Miller J, Flory K, Lynam D, et al. A test of the four-factor model of impulsivity-related traits. Pers Indiv Differ. 2003;34:1403–1418. [Google Scholar]
- 74.Bubier JL, Drabick DA. Affective decision-making and externalizing behaviors: The role of autonomic activity. J Abnorm Child Psychol. 2008;36:941–953. doi: 10.1007/s10802-008-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toplak ME, Jain U, Tannock R. Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD) Behav Brain Funct. 2005;1:8. doi: 10.1186/1744-9081-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annu Rev Psychol. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- 77.Gagnon C, Craig WM, Tremblay RE, et al. Kindergarten predictors of boys’ stable behavior problems at the end of elementary school. J Abnorm Child Psychol. 1995;23:751–766. doi: 10.1007/BF01447475. [DOI] [PubMed] [Google Scholar]
- 78.Colledge E, Blair RJR. The relationship in children between the inattention and impulsivity components of attention deficit and hyperactivity disorder and psychopathic tendencies. Pers Indiv Differ. 2001;30:1175–1187. [Google Scholar]
- 79.Clark DB, Parker AM, Lynch KG. Psychopathology and substance-related problems during early adolescence: A survival analysis. J Clin Child Psychol. 1999;28:333–341. doi: 10.1207/S15374424jccp280305. [DOI] [PubMed] [Google Scholar]
- 80.Taylor J, Malone S, Iacono WG, et al. Development of substance dependence in two delinquency subgroups and nondelinquents from a male twin sample. J Am Acad Child Adolesc Psychiatry. 2002;41:386–393. doi: 10.1097/00004583-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 81.Tarter RE, Blackson T, Brigham J, et al. The association between childhood irritability and liability to substance use in early adolescence: A 2-year follow-up study of boys at risk for substance abuse. Drug Alcohol Depend. 1995;39:253–261. doi: 10.1016/0376-8716(95)01175-6. [DOI] [PubMed] [Google Scholar]
- 82.Barkley RA. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis And Treatment. 2. New York: Guilford Press; 1998. [Google Scholar]
- 83.Resnick RJ. Attention deficit hyperactivity disorder in teens and adults: They don’t all outgrow it. J Clin Psychol. 2005;61:529–533. doi: 10.1002/jclp.20117. [DOI] [PubMed] [Google Scholar]
- 84.Weyandt LL, Iwaszuk W, Fulton K, et al. The internal restlessness scale: Performance of college students with and without ADHD. J Learn Disabil. 2003;36:382–389. doi: 10.1177/00222194030360040801. [DOI] [PubMed] [Google Scholar]
- 85.Downey KK, Stelson FW, Pomerleau OF, et al. Adult attention deficit hyperactivity disorder: Psychological test profiles in a clinical population. J Nerv Ment Dis. 1997;185:32–38. doi: 10.1097/00005053-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Conners CK, Erhardt D, Epstein JN, et al. Self-ratings of ADHD symptoms in adults I: Factor structure and normative data. J Attention Disord. 1999;3:141–151. [Google Scholar]
- 87.Chalmers DK, Bowyer CA, Olenick NL. Problem drinking and obesity: A comparison in personality patterns and life-style. Int J Addict. 1990;25:803–817. doi: 10.3109/10826089009056219. [DOI] [PubMed] [Google Scholar]
- 88.Segal BM, Stewart JC. Substance use and abuse in adolescence: An overview. Child Psychiatry Hum Dev. 1996;26:193–210. doi: 10.1007/BF02353237. [DOI] [PubMed] [Google Scholar]
- 89.Simons JS, Carey KB. Risk and vulnerability for marijuana use problems: The role of affect dysregulation. Psychol Addict Behav. 2002;16:72–75. [PubMed] [Google Scholar]
- 90.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 91.Volkow ND, Fowler JS, Wang GJ, et al. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- 93.Murphy P, Schacher R. Use of self-ratins in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry. 2000;157:1156–1159. doi: 10.1176/appi.ajp.157.7.1156. [DOI] [PubMed] [Google Scholar]
- 94.Achenbach TM, Howell CT, Mcconaughy SH, et al. 6-Year predictors of problems in a national sample of children and youth 1. Cross-informant syndromes. J Am Acad Child Psychiatry. 1995;34:336–347. doi: 10.1097/00004583-199503000-00020. [DOI] [PubMed] [Google Scholar]
- 95.Fergusson DM, Boden JM. Cannabis use and adult ADHD symptoms. Drug Alcohol Depend. 2008;95:90–96. doi: 10.1016/j.drugalcdep.2007.12.012. [DOI] [PubMed] [Google Scholar]