Abstract
Pannexins, a class of membrane channels, bear significant sequence homology with the invertebrate gap junction proteins, innexins, and more distant similarities in their membrane topologies and pharmacological sensitivities with the gap junction proteins, connexins. However, the functional role for the pannexin oligomers or pannexons, is different from connexin oligomers, the connexons. Many pannexin publications have used the term “hemichannels” to describe pannexin oligomers while others use the term “channels” instead. This has led to confusion within the literature about the function of pannexins that promotes the idea that pannexons serve as gap junction hemichannels and thus, have an assembly and functional state as gap junctional intercellular channels. Here, we present the case that unlike the connexin gap junction intercellular channels, so far, pannexin oligomers have repeatedly been shown to be channels that are functional in single membranes, but not as intercellular channels in appositional membranes. Hence, they should be referred to as channels and not hemichannels. Thus, we advocate that in the absence of firm evidence that pannexins form gap junctions, the use of the term “hemichannel” be discontinued within the pannexin literature.
Key words: intercellular communication, ATP signaling, connexin, pannexin, innexin, connexon, pannexon, purinergic receptors
Pannexins are channel proteins expressed in virtually all tissues and abundantly expressed in the vertebrate central -nervous system.1 The human pannexin family consists of three members: pannexin1 (PANX1, GenBank AAQ89382.1, 426 amino acids, 47.6 kDa), pannexin2 (PANX2, GenBank: AAI01024.1, 677 amino acids, 74.4 kDa) and pannexin3 (PANX3, GenBank: AAK95655.1, 392 amino acids, 44.7 kDa) with a sequence similarity of ~50–60%. The N and C terminal sequences are least conserved, with the latter having the highest variability. Based on in situ hybridization and northern blot analysis in rodents, Panx1 is ubiquitously expressed (e.g., brain, skeletal and heart muscle, testis, ovary), while Panx2 is expressed predominantly in the central nervous system. Panx3 is expressed in several embryonic tissues as well as in adult bone, skin and cartilage,1-3 while other published western blots revealed weak labeling for a second higher molecular weight species present in a wide range of tissues.4
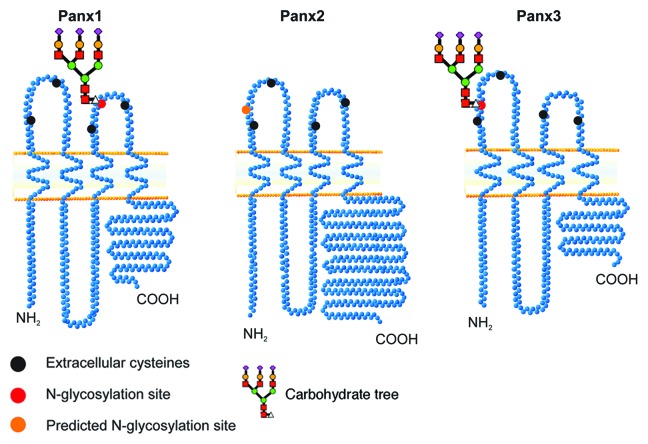
These integral membrane proteins were originally proposed to form gap junction-like structures based on their sequence homology with the invertebrate gap junctions and predicted topology being similar to the gap junction proteins, the connexins (vertebrate form) and innexins (invertebrate form). The “connexin-like topology” consists of four transmembrane segments, two extracellular loops and a cytoplasmic localization of the amino terminus, cytoplasmic loop and carboxy terminus (Fig. 1). Connexins have six conserved cysteine residues in their -extracellular loops that form three disulfide intra-connexin bridges5 while pannexins have four conserved extracellular loop cysteines resulting in the possibility of two intra-pannexin disulfide bonds. It is also notable that the pannexin sequence is moderately similar to the innexins but not to connexins.
Figure 1. Pannexins are predicted to be tetra-spanning membrane proteins with both the amino and carboxy termini exposed to the cytoplasm. Panx1 and Panx3 have N-linked glycosylation sites at N254 and N71, respectively (red circles), while Panx2 has a predicted N-linked glycosylation site at N86 (orange circle). Black circles denote the four conserved cysteines.
The way connexins and pannexins form structures within cells are also different from each other. Gap junctions are localized to specialized cell-cell appositional areas that contain tens to thousands of closely packed intercellular channels that span the two plasma membranes. These intercellular channels each consist of two hexamers formed by one or more connexins, while pannexins primarily form membrane channels, i.e., oligomeric structures embedded in a single plasma membrane that when open, provide a conduction pathway between the cytosol and extracellular space.6 Pannexin membrane channels have been shown to be formed by six (Panx1),7 or eight (Panx2) monomers.6 The oligomeric number of a Panx3 membrane channel8-10 has not yet been determined, but the expectation is that it would be six, based on a greater size and sequence similarity to Panx1 than Panx2. The term “connexon” was first coined by Goodenough and co-workers in the late 1970s to denote a connexin oligomer.11 One of the earliest uses of the term “hemichannel” in the gap junction literature was in the analysis of voltage dependent gating of gap junction channels. Polarity reversal experiments indicated that the gates on each side of the channel (or gates on each hemichannel) were not independent, but rather gating was contingent on the other gate (a hemichannel) being open.12 Within the gap junction field, the term “hemichannel” is often used interchangeably with “connexon”, often with different structural and functional connotations dependent on the authors’ interpretation.13 Recently, the term “pannexon” was coined to reflect pannexin oligomers (a hexamer for Panx1 and an octamer for Panx2).6,7
Early studies suggested that Panx1 had the capability to form functional intercellular channels. Bruzzone et al. used a paired Xenopus oocyte exogenous expression system to show that Panx1 could form intercellular channels.2 However long pairing periods were required to detect low levels of current between two cells, especially when compared to Cx46 channels.2,7 In contrast, robust currents were recorded in unpaired oocytes either exogenously expressing Panx1 channels or Cx46 hemichannels.2,7,14 Also, the gating of Panx1 channels by extracellular ligands such as ATP, as determined by the effects on the macroscopic currents and single channel currents, further reinforce the hypothesis that Panx1 formed functional channels embedded in a single plasma membrane.15 Later, Lai et al. showed dye coupling in glioma cells after transfection with Panx1-GFP,16 however since glioma cells normally express low levels of Cx43, it is not known if Cx43 was recruited into gap junctions at the appositional plasma membrane after high levels of exogenous Panx1 expression. Indeed, any claim for a gap junction channel function of pannexins would require unequivocal evidence for a channel with distinct properties from any connexin-made gap junction. Nonetheless, there is the argument that Panx1 mediates the release of ATP and possibly other signaling molecules from the cytoplasm to the extracellular space. This function is not restricted to exogenously expressed Panx1,14 but also is found under natural conditions in cells endogenously expressing Panx1 such as CNS pyramidal17,35 and Purkinje neurons,18 erythrocytes,19-21 T-cells,22 astrocytes,23 airway epithelial cells,24 taste cells25 and cochlear supporting cells.26 Functional properties previously attributed to connexin hemichannels may be valid, but may need to be re-addressed in light of these pannexin findings.
Gap junctions typically appear in immunolabeled cells and tissues as punctate spots at cell-cell contact points. In polarized epithelial cells, gap junctions are found between basolateral membranes but excluded from apical membranes. In thin cross-sections at EM resolution, gap junctions have a classical pentalaminar appearance (if the outside layers of stain are differentiated, then seven layers are visible, called a septilaminar appearance). A standard feature of a gap junction in these thin section micrographs is the ~2.5 nm stain-filled gap region. In freeze fracture electron micrographs, the intercellular channels appear as clustered membrane particles with an ~10–11 nm center-to-center distance and typically displaying a close packed arrangement.
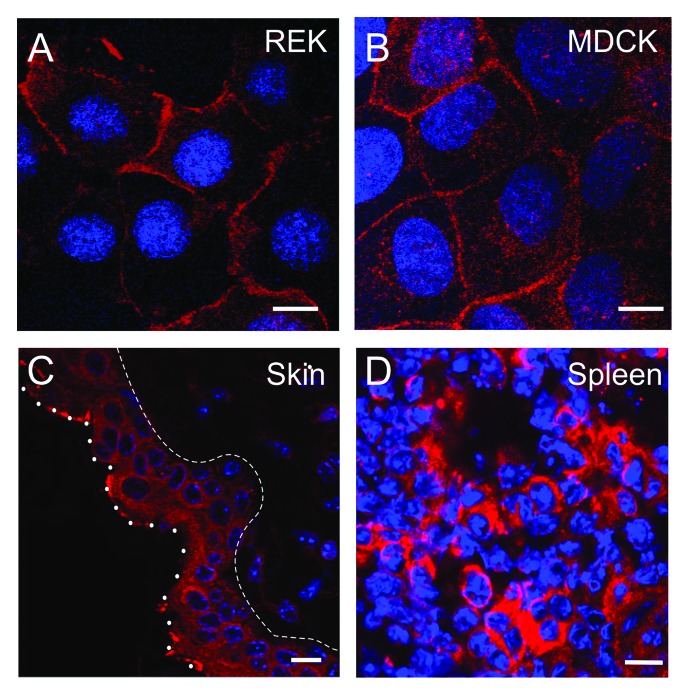
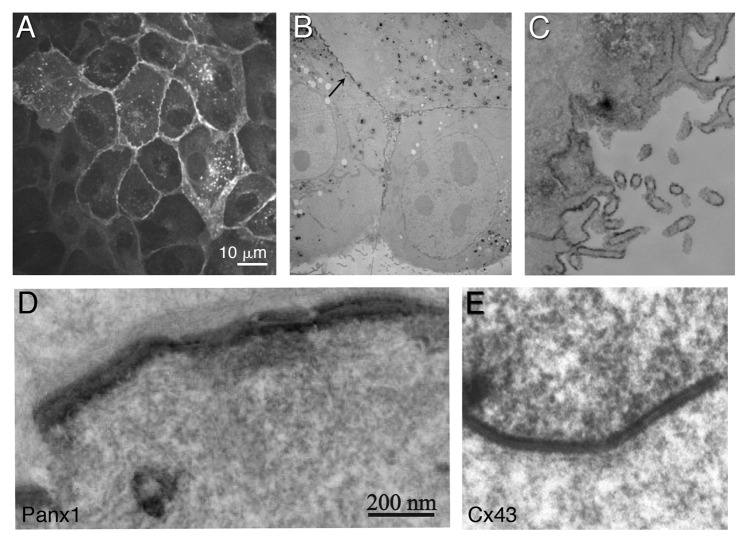
In contrast, plasma membrane-localized pannexin channels have a different appearance in comparison to gap junctions. Fluorescence and EM staining of Panx1 and Panx3 revealed that they are evenly localized throughout the plasma membrane (Figs. 2 and 3) and in particular, at the apical membrane for polarized cells (e.g., airway epithelial cells and MDCK cells). In addition, Panx1 has been shown in some cells and tissues to also be concentrated within intracellular compartments. Whether Panx2 localizes well to the plasma membrane remains an area of intense investigation27-29 as well as whether oligomers of different pannexin isoforms form and function in heterotypic combinations.2,6,9 Vanden Abeele et al. presented evidence for Panx1 forming intercellular channels in HEK293 and human prostate cancer epithelial LNCaP cells,30 but the Panx1 staining in the plasma membrane is more similar to the dispersed pattern observed by several groups than to a canonical gap junction appearance.4,7 In cell appositional areas containing Panx1, higher magnification views such as those afforded by electron microscopy do not show canonical gap junction views (compare Fig. 3D and E). Furthermore, immunocytochemistry and detection by light and electron microscopy revealed Panx1 in rodent hippocampal and cortical principal neurons accumulating exclusively at postsynaptic densities17 consistent with Panx1 localizing within plasma membrane domains that are clearly separated from apposing membranes.
Figure 2. Subcellular localization of Panx1 varies in cells and tissues. Rat epidermal keratinocytes (REK) (A) overexpressing Panx1 show a similar cell surface profile to endogenous Panx1 in Madin-Darby canine kidney cells (MDCK) (B) as revealed via immunolabeling for Panx1 (red). Panx1 exhibits a diffused cellular phenotype in mouse epidermis (between the dashed and dotted lines) (C) not unlike the distribution of Panx1 in thin sections of mouse spleen (D). Nuclei are stained with Hoechst (blue). Bars = 10 μm.
Figure 3. Correlative light and electron microscopy of tagged Panx1 in MDCK cells. Panx1 was tagged with a tetracysteine domain, labeled with ReAsH and photo-oxidized after fluorescence imaging. ReAsH fluorescence (A) Low magnification EM of the same cells (B and C). Higher power EM of area denoted by an arrow in (B), showed staining at appositional areas between cells, where a clear separation of the two plasma membranes are observed (D). Photo-oxidized Cx43-tetracysteine/ReAsH gap junction is shown for comparison (E). (D and E) are displayed with the same magnification. (Adapted from Boassa et al. (2007).7 © The American Society for Biochemistry and Molecular Biology).
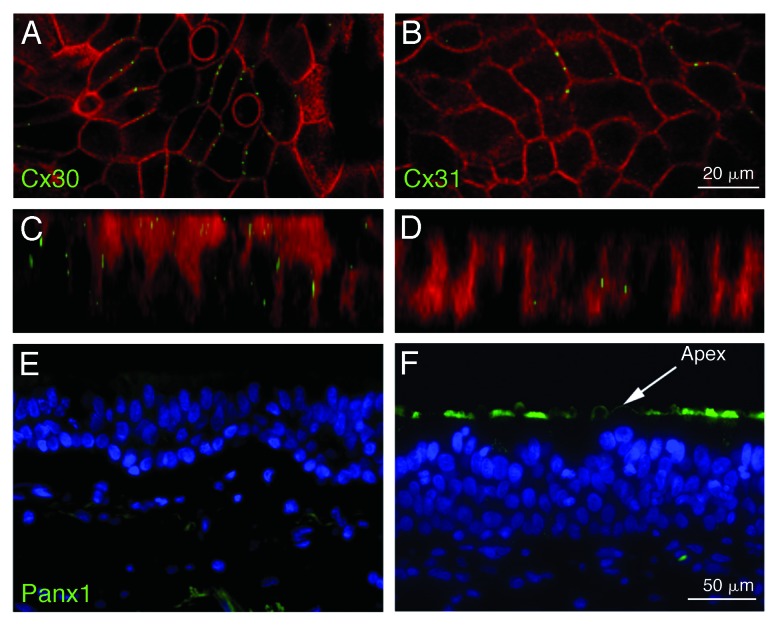
The evidence for Panx1 making single membrane channels is particularly notable in blood where cells classically reside and operate as single cells. Macrophages,31 T cells22 and erythrocytes19 typically express Panx1 that further localizes to both intracellular and plasma membranes. Panx1-mediated release of ATP and UTP from apoptotic single Jurkat cells serves to recruit phagocytes.32 While immune cells are reported to form gap junctions in response to certain stimuli leading to “rosette” formation, erythrocytes in healthy vessels spend their entire life cycle as single cells. Another example of single membrane localized pannexins is at the apical membranes of airway epithelia where these plasma membranes do not abut neighboring cells24 (Fig. 4).
Figure 4. Localization of connexins and Panx1 in airway epithelial cells. These cells express the two connexins, Cx30 and Cx31, which yield the typical punctate staining of gap junctions at the basolateral membranes of contacting cells (A and B). Z-stacks (C and D) do not reveal staining of connexins at the apical membrane. In contrast, staining for Panx1 is restricted to the apical membrane where ATP is released from these cells (E and F). No staining at the basolateral membrane can be detected. (A–D) are from Wiszniewski et al. (2007),34 with permission from Elsevier. (E and F) are adapted from Ransford et al. (2009),24 reprinted with permission of the American Thoracic Society. © Copyright American Thoracic Society.
Another piece of evidence that would argue for pannexins functioning in single membrane environments is based on the fact that pannexins are glycosylated at a single asparagine residue exposed to the extracellular surface.4,7,9 Mutating these amino acids to glutamine significantly reduces cell surface expression. The presence of carbohydrate trees on these glycoproteins would act to repel adjacent plasma membranes from becoming closely apposed that would be necessary for pannexon docking. In contrast, connexins are not glycosylated, thus allowing for connexon-connexon docking.
Other findings on the regulation of pannexin channels set them apart from connexin channels or hemichannels. Examination of the amino acid sequence of the carboxy terminal domain of Panx1 reveals two canonical caspase cleavage sites. This suggests that under conditions in which caspases are activated, such as cellular stress, the Panx1 protein is truncated. Cleavage by caspase 3 at amino acids 376-379 (DVVD) of Panx1 in Jurkat cells under conditions of cellular stress occurs, forming a constitutively active channel.32 Connexins do not contain caspase cleavage sequences and are not cleaved in order to be constitutively activated. Further examinations of domains within the Panx1 channel are likely to provide more information on other- -regulatory mechanisms distinct from connexin-based channels.7
In summary, structural, functional and trafficking studies in many model cell culture systems and in some tissues have indicated that pannexins readily form single membrane channels. Under some exceptional and poorly understood conditions it may be possible that at least Panx1 can form intercellular channels but this does not appear to occur often and has not been shown in vivo. However, the term hemichannel literally means “half channel” which may have some validity in the understanding of connexin -function but it serves no meaningful purpose in the pannexin context as it only leads to -confusion for those not acquainted with the intricacies of gap junction and pannexin biology (see also Dahl and Locovei33 for additional arguments). In the event that at some point in the future pannexins are conclusively shown to have the capacity to function in forming intercellular channels either dispersed in the plasma membrane or within gap junction-like structures, this would not negate the exclusion of referring to pannexons as “hemichannels” and we would instead suggest that the phrase “pannexin cell-cell channels” be used. Collectively, the authors of the present report respectively recommend that the field discontinue the use of the word “hemichannels” in reference to pannexin channels.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/15765
References
- 1.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–16. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–4. doi: 10.1016/S0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 4.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–83. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 5.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, et al. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, et al. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–31. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–43. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 8.Bhalla-Gehi R, Penuela S, Churko JM, Shao Q, Laird DW. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J Biol Chem. 2010;285:9147–60. doi: 10.1074/jbc.M109.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–23. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, et al. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–58. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caspar DLD, Goodenough DA, Makowski L, Phillips WC. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol. 1977;74:605–28. doi: 10.1083/jcb.74.2.605. [PMID.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris AL, Spray DC, Bennett MV. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris A, Locke D. Connexins: A Guide. New York NY: Springer 2009. [Google Scholar]
- 14.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–8. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–5. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007;67:1545–54. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 17.Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, et al. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience. 2007;146:9–16. doi: 10.1016/j.neuroscience.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 18.Ray A, Zoidl G, Wahle P, Dermietzel R. Pannexin expression in the cerebellum. Cerebellum. 2006;5:189–92. doi: 10.1080/14734220500530082. [DOI] [PubMed] [Google Scholar]
- 19.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–9. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–40. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, et al. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–52. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 23.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 2009; PMID: 19213873. [DOI] [PMC free article] [PubMed]
- 25.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–41. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XH, Streeter M, Liu YP, Zhao HB. Identification and characterization of pannexin expression in the mammalian cochlea. J Comp Neurol. 2009;512:336–46. doi: 10.1002/cne.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swayne LA, Sorbara CD, Bennett SA. Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J Biol Chem. 2010;285:24977–86. doi: 10.1074/jbc.M110.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai CP, Bechberger JF, Naus CC. Pannexin2 as a novel growth regulator in C6 glioma cells. Oncogene. 2009;28:4402–8. doi: 10.1038/onc.2009.283. [DOI] [PubMed] [Google Scholar]
- 29.Zappalà A, Li Volti G, Serapide MF, Pellitteri R, Falchi M, La Delia F, et al. Expression of pannexin2 protein in healthy and ischemized brain of adult rats. Neuroscience. 2007;148:653–67. doi: 10.1016/j.neuroscience.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, et al. Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol. 2006;174:535–46. doi: 10.1083/jcb.200601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–7. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–19. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 34.Wiszniewski L, Sanz J, Scerri I, Gasparotto E, Dudez T, Lacroix JS, et al. Functional expression of connexin30 and connexin31 in the polarized human airway epithelium. Differentiation. 2007;75:382–92. doi: 10.1111/j.1432-0436.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 35.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–7. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]