Abstract
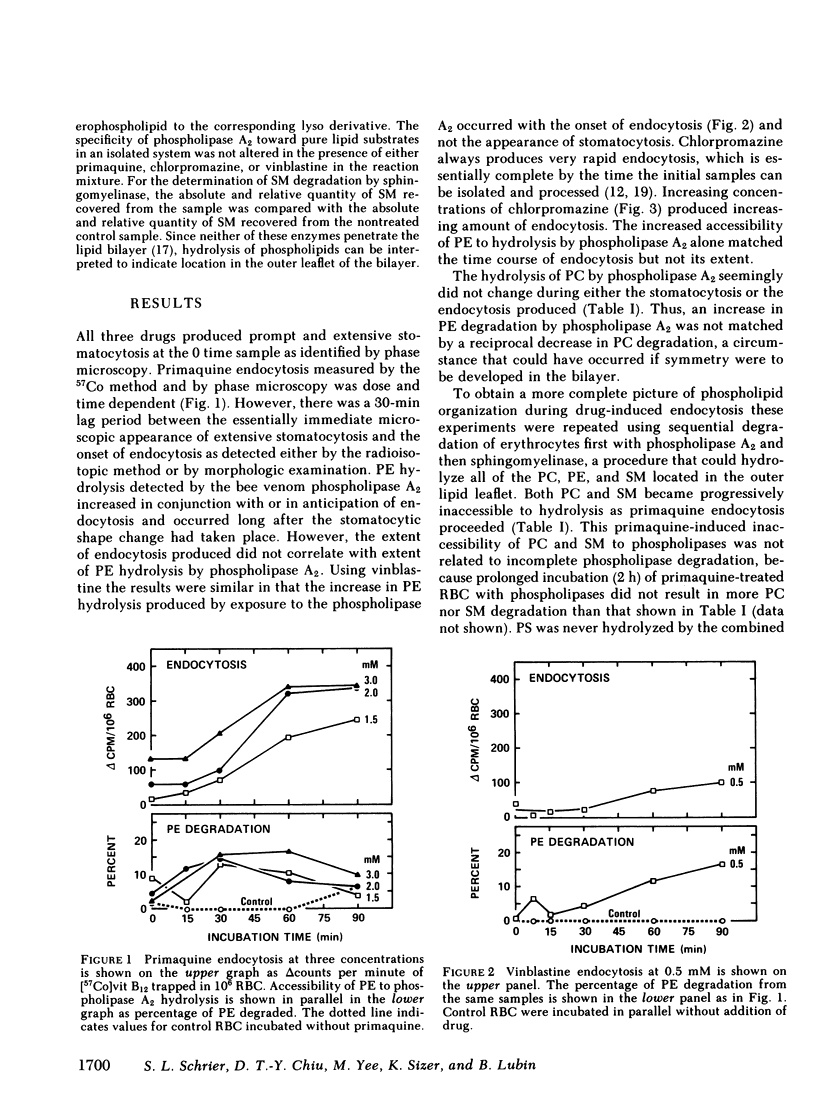
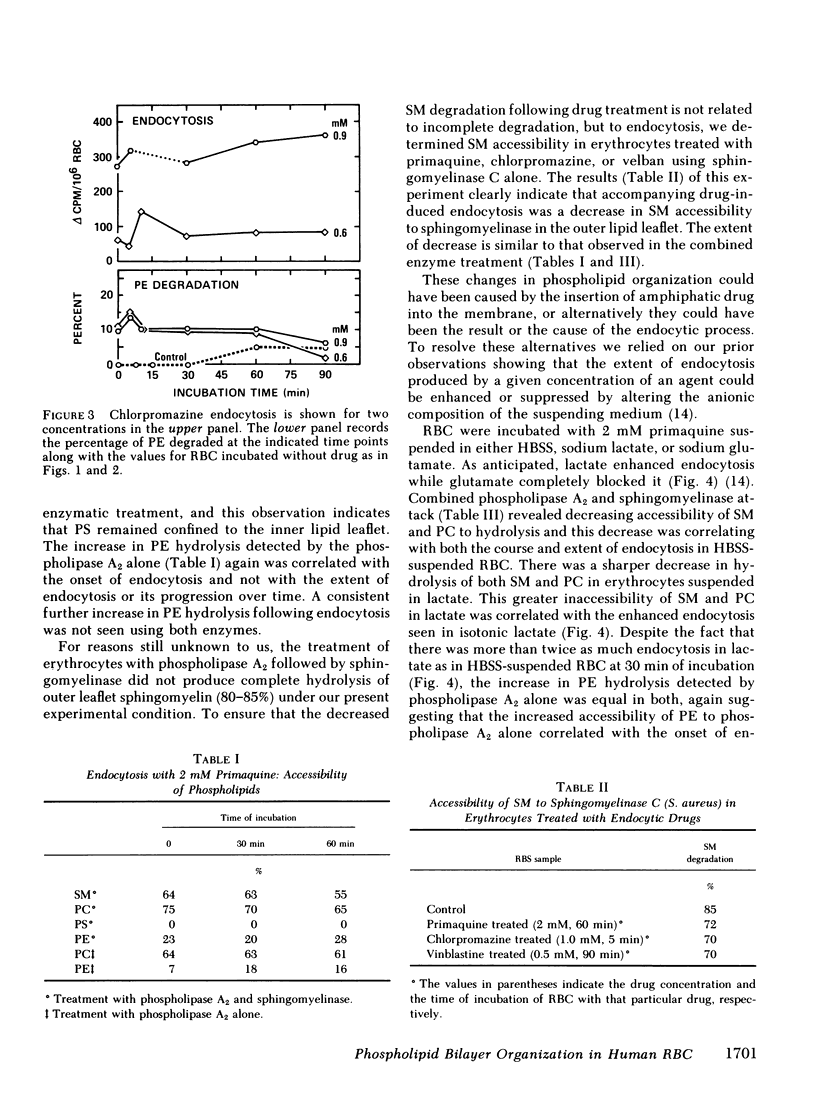
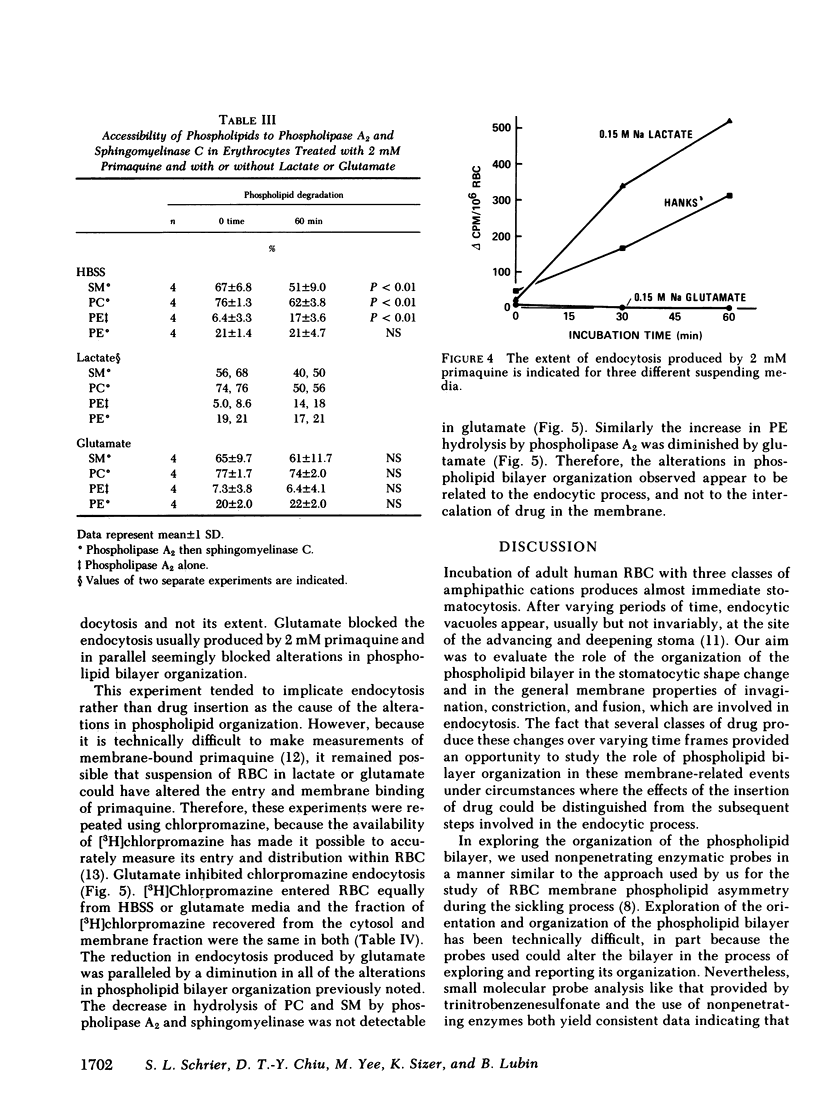
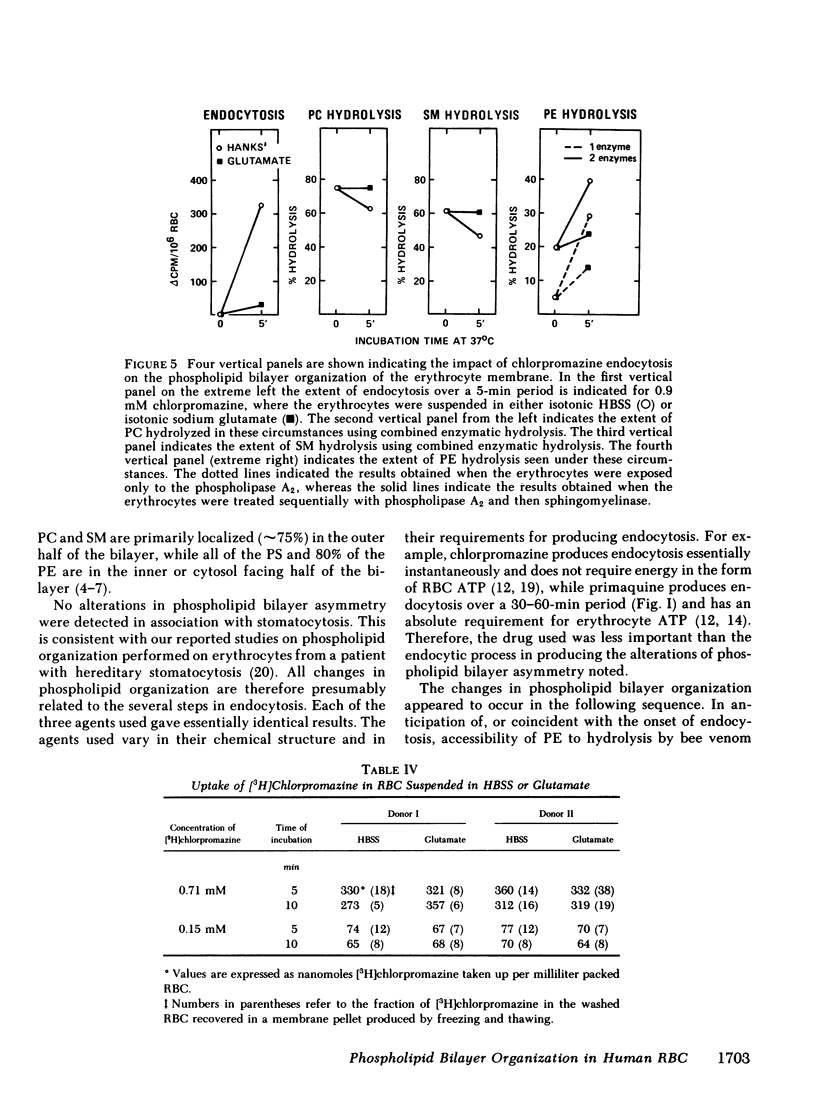
Our plan was to evaluate the potentially important role of phospholipids in erythrocyte shape alterations by determining if their orientation was altered during endocytosis. Stomatocytosis and endocytosis were induced in normal intact human erythrocytes by incubation with three agents: primaquine, vinblastine, and chlorpromazine, each of which has its own requirements and time course for producing endocytosis. The organization of the phospholipid bilayer was assessed by measuring the extent of degradation of phophatidylcholine (PC), phophatidylethanolamine (PE), phosphatidylserine (PS), and sphingomyelin (SM) produced by exposure of erythrocytes to a nonpenetrating protease-free phospholipase A2 alone or in combination with a purified sphingomyelinase as well. The induction of stomatocytosis did not change this orientation. However, correlating with the onset of endocytosis but not its extent, there was an increase in PE degradation, which could be detected regularly only by use of phospholipase A2 alone. Use of the combination of phospholipase A2 and sphingomyelinase showed that the extent and course of endocytosis was paralleled by an apparent movement of PC and SM from the outer to the inner half of the lipid bilayer. Since no further PE was hydrolyzed and because no PS was ever degraded, this inward movement of PC and SM did not represent the establishment of complete symmetry in the membrane. By adjusting the experimental design it was possible to implicate the endocytic process, and not insertion of drug in the membrane, as the cause of the alterations in phospholipid organization seen. Our findings indicate that the phospholipid orientation is very closely involved in the endocytosis process and that specific states of phospholipid asymmetry may be related to identifiable membrane events.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Bassat I., Bensch K. G., Schrier S. L. Drug-induced erythrocyte membrane internalization. J Clin Invest. 1972 Jul;51(7):1833–1844. doi: 10.1172/JCI106985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. The molecular basis for membrane - cytoskeleton association in human erythrocytes. J Cell Biochem. 1982;18(1):49–65. doi: 10.1002/jcb.1982.240180106. [DOI] [PubMed] [Google Scholar]
- Chiu D., Lubin B., Shohet S. B. Erythrocyte membrane lipid reorganization during the sickling process. Br J Haematol. 1979 Feb;41(2):223–234. doi: 10.1111/j.1365-2141.1979.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature. 1978 Feb 16;271(5646):672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim Biophys Acta. 1968 Dec 10;163(4):494–500. doi: 10.1016/0005-2736(68)90078-3. [DOI] [PubMed] [Google Scholar]
- Feo C., Mohandas N. Clarification of role of ATP in red-cell morphology and function. Nature. 1977 Jan 13;265(5590):166–168. doi: 10.1038/265166a0. [DOI] [PubMed] [Google Scholar]
- Gordesky S. E., Marinetti G. V., Love R. The reaction of chemical probes with the erythrocyte membrane. J Membr Biol. 1975;20(1-2):111–132. doi: 10.1007/BF01870631. [DOI] [PubMed] [Google Scholar]
- Gupta C. M., Mishra G. C. Transbilayer phospholipid asymmetry in Plasmodium knowlesi-infected host cell membrane. Science. 1981 May 29;212(4498):1047–1049. doi: 10.1126/science.7233198. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Deuticke B. Possible relationship between membrane proteins and phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1976 Jun 17;436(2):353–365. doi: 10.1016/0005-2736(76)90199-1. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Plasa G., Kamp D., Deuticke B. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1978 May 4;509(1):21–32. doi: 10.1016/0005-2736(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Lubin B., Chiu D., Bastacky J., Roelofsen B., Van Deenen L. L. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981 Jun;67(6):1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin B., Chiu D. Membrane phospholipid organization in pathologic human erythrocytes. Prog Clin Biol Res. 1982;97:137–150. [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Marinetti G. V., Cattieu K. Asymmetric metabolism of phosphatidylethanolamine in the human red cell membrane. J Biol Chem. 1982 Jan 10;257(1):245–248. [PubMed] [Google Scholar]
- Martin J. K., Luthra M. G., Wells M. A., Watts R. P., Hanahan D. J. Phospholipase A2 as a probe of phospholipid distribution in erythrocyte membranes. Factors influencing the apparent specificity of the reaction. Biochemistry. 1975 Dec 16;14(25):5400–5408. doi: 10.1021/bi00696a003. [DOI] [PubMed] [Google Scholar]
- Miller L. H., McAuliffe F. M., Johnson J. G. Invasion of erythrocytes by malaria merozoites. Prog Clin Biol Res. 1979;30:497–502. [PubMed] [Google Scholar]
- Palek J., Liu S. C. Dependence of spectrin organization in red blood cell membranes on cell metabolism: implications for control of red cell shape, deformability, and surface area. Semin Hematol. 1979 Jan;16(1):75–93. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Schrier S. L., Hardy B., Junga I., Ma L. Actin-activated ATPase in human red cell membranes. Blood. 1981 Nov;58(5):953–962. [PubMed] [Google Scholar]
- Schrier S. L., Junga I. Entry and distribution of chlorpromazine and vinblastine into human erythrocytes during endocytosis. Proc Soc Exp Biol Med. 1981 Nov;168(2):159–167. doi: 10.3181/00379727-168-41252. [DOI] [PubMed] [Google Scholar]
- Schrier S. L., Junga I., Krueger J., Johnson M. Requirements of drug-induced endocytosis by intact human erythrocytes. Blood Cells. 1978;4(1-2):339–359. [PubMed] [Google Scholar]
- Schrier S. L., Junga I., Seeger M. The mechanism of drug-induced erythrocyte vacuole formation. J Lab Clin Med. 1974 Feb;83(2):215–227. [PubMed] [Google Scholar]
- Shukla S. D., Hanahan D. J. Identification of domains of phosphatidylcholine in human erythrocyte plasma membranes. Differential action of acidic and basic phospholipases A2 from Agkistrodon halys blomhoffii. J Biol Chem. 1982 Mar 25;257(6):2908–2911. [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Organization of phospholipids in human red cell membranes as detected by the action of various purified phospholipases. Biochim Biophys Acta. 1975 Sep 16;406(1):83–96. doi: 10.1016/0005-2736(75)90044-9. [DOI] [PubMed] [Google Scholar]



