Abstract
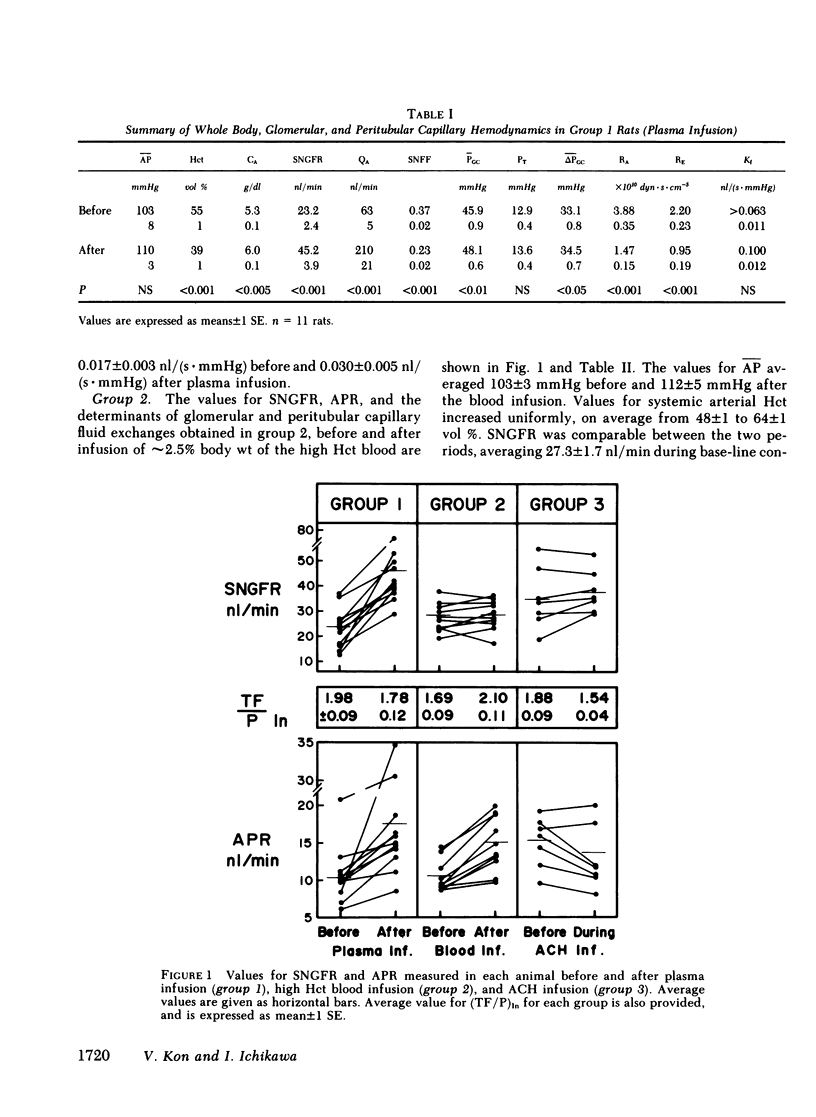
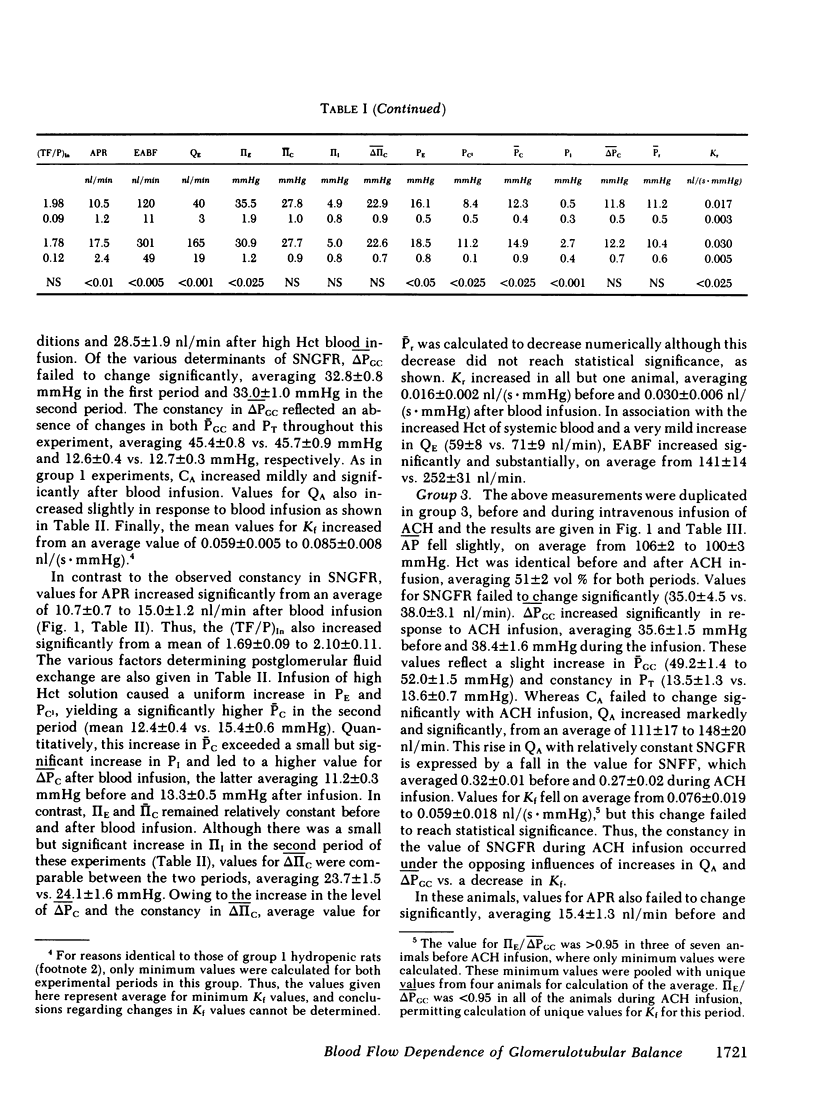
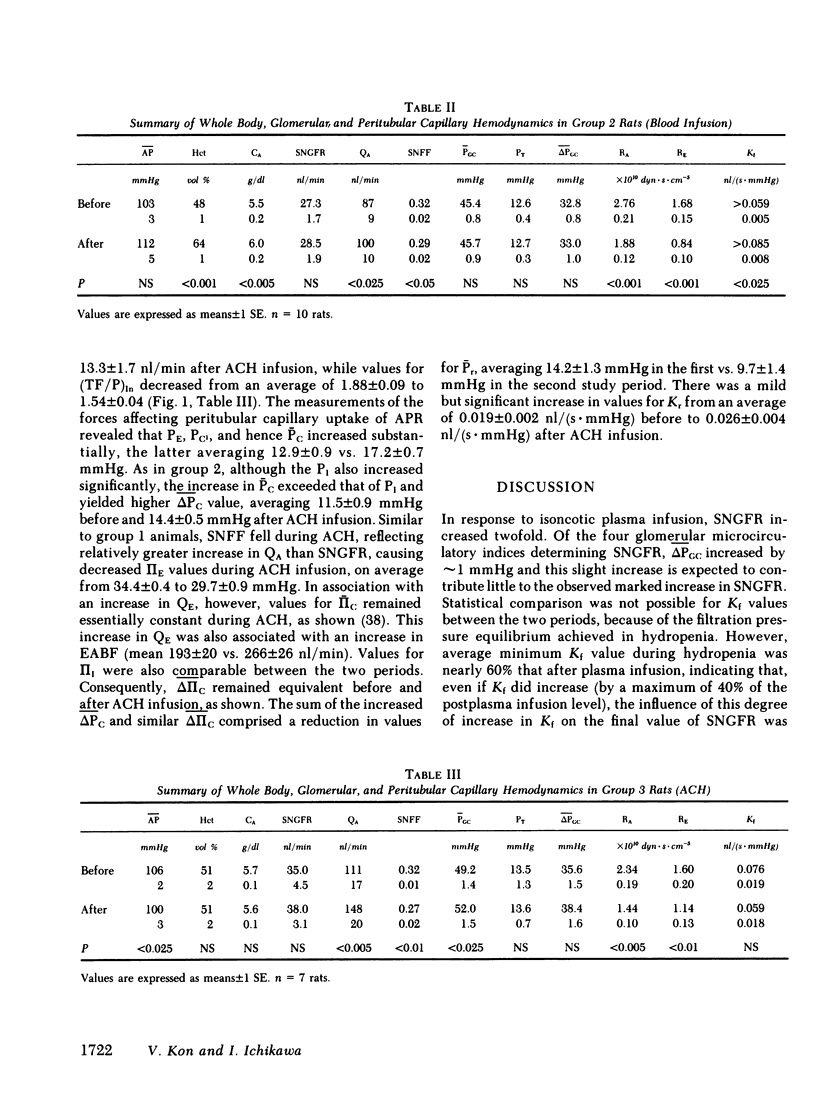
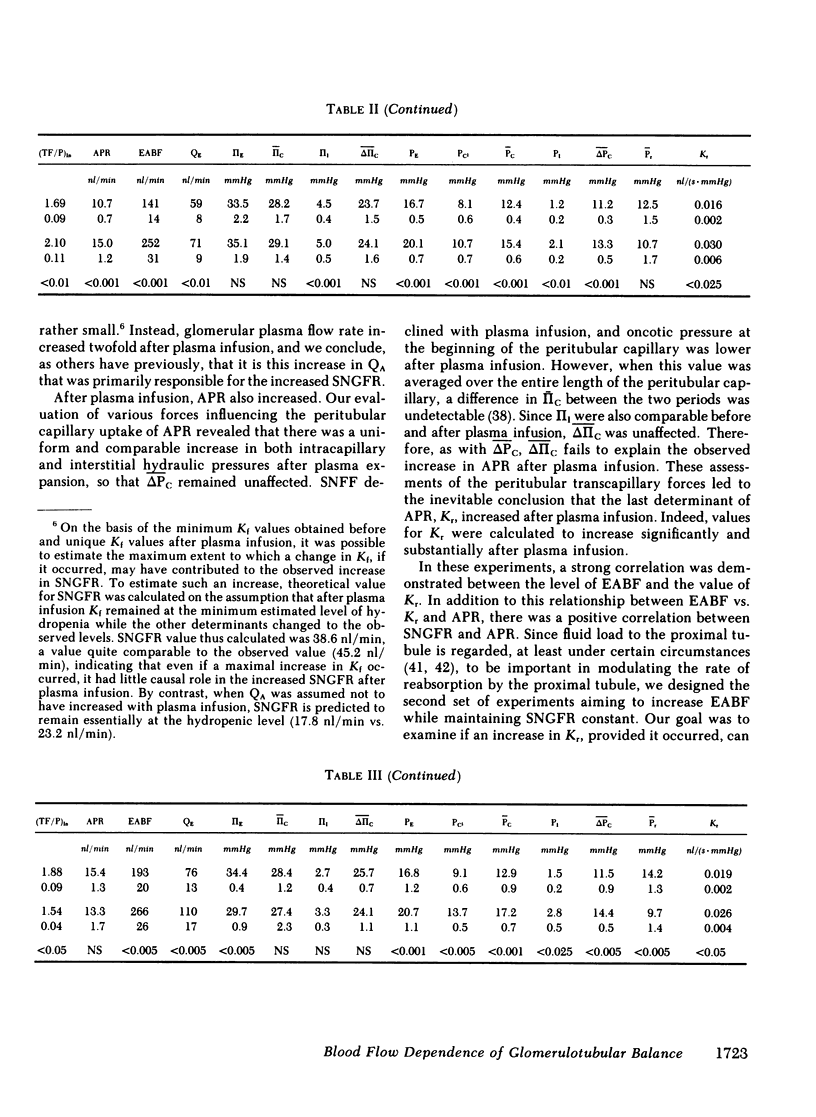
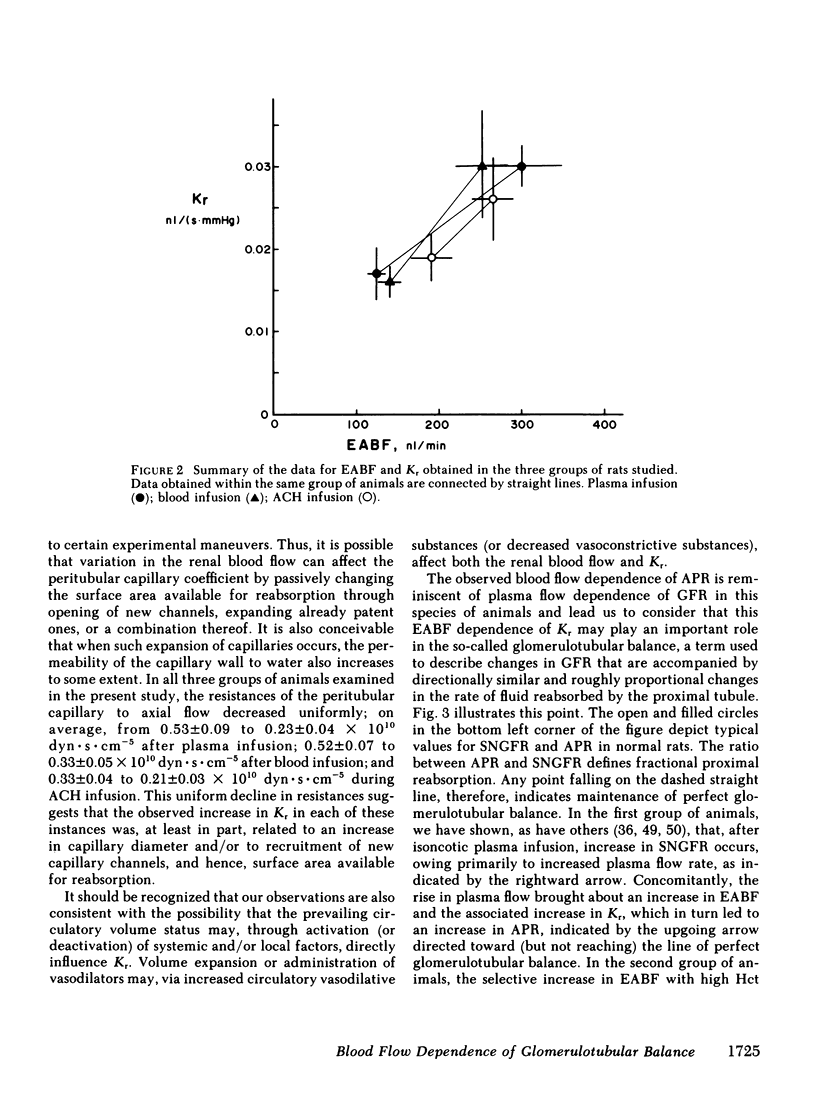
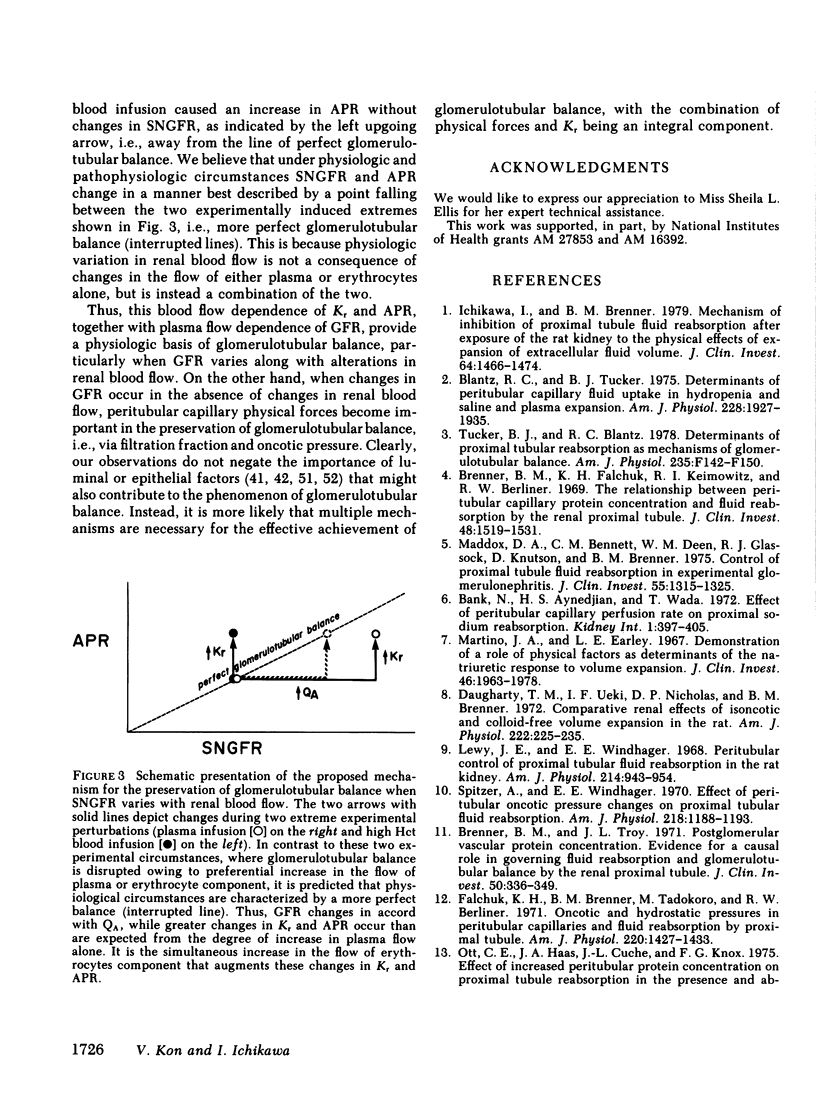
The rate of blood flow entering a capillary network can, in some vascular systems, regulate capillary surface area and the rate of fluid and solute transfer. To determine whether such a mechanism exists in the renal peritubular capillary, we performed micropuncture studies in 28 rats during relatively low and high efferent arteriolar blood flow (EABF). High EABF was achieved by intravenous infusion of isoncotic plasma (group 1: from 120 +/- 11 to 301 +/- 49 nl/min [+/- SE]); whole blood with high hematocrit (approximately 75 vol %) (group 2: from 141 +/- 14 to 252 +/- 31 nl/min); or acetylcholine (group 3: from 193 +/- 20 to 266 +/- 26 nl/min). In group 1 rats, plasma infusion caused an increase in single nephron glomerular filtration rate (SNGFR), on average, from 23.2 +/- 2.4 to 45.2 +/- 3.9 nl/min, owing primarily to increased glomerular plasma flow rate (from 63 +/- 5 to 210 +/- 21 nl/min). The rate of fluid uptake by the peritubular capillary, assessed by the absolute rate of proximal fluid reabsorption (APR), also rose significantly, on average from 10.5 +/- 1.2 to 17.5 +/- 2.4 nl/min. This rise in APR was associated with near constancy in mean transcapillary hydraulic (delta Pc) and oncotic (delta IIc) pressure differences, and was therefore attributed to a significant increase in peritubular capillary reabsorption coefficient (Kr), with the mean from 0.017 +/- 0.003 to 0.030 +/- 0.005 nl/(s . mmHg). In group 2 rats, high hematocrit blood infusion led to a significant rise in APR; on average, from 10.7 +/- 0.7 to 15.0 +/- 1.2 nl/min, without changing SNGFR. This rise in APR occurred despite unfavorable changes in the physical forces, namely a significant increase in delta Pc and constancy in delta IIc. Instead, an increase in EABF was again associated with a significant rise in Kr (on average, from 0.016 +/- 0.002 to 0.030 +/- 0.06 nl/[s . mmHg]), which accounted entirely for the rise in APR, independently of SNGFR. In group 3 rats, in which an increase of EABF was induced pharmacologically with acetylcholine, a rise in EABF was also accompanied by a significant increase in Kr, on average, from 0.019 +/- 0.002 to 0.026 +/- 0.004 nl/(s . mmHg). The results indicate that: (a) Kr is modulated by EABF. (b) In view of plasma flow dependence of GFR, blood flow dependence of Kr and APR provides an important basis for glomerulotubular balance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank N., Aynedjian H. S., Wada T. Effect of peritubular capillary perfusion rate on proximal sodium reabsorption. Kidney Int. 1972 Jun;1(6):397–405. doi: 10.1038/ki.1972.52. [DOI] [PubMed] [Google Scholar]
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Bell R. D., Parry W. L., Grundy W. G. Renal lymph sodium and potassium concentrations following renal vasodilation. Proc Soc Exp Biol Med. 1973 Jun;143(2):499–501. doi: 10.3181/00379727-143-37352. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Tucker B. J. Determinants of peritubular capillary fluid uptake in hydropenia and saline and plasma expansion. Am J Physiol. 1975 Jun;228(6):1927–1935. doi: 10.1152/ajplegacy.1975.228.6.1927. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. On the mechanism of inhibition in fluid reabsorption by the renal proximal tubule of the volume-expanded rat. J Clin Invest. 1971 Aug;50(8):1596–1602. doi: 10.1172/JCI106647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L. Postglomerular vascular protein concentration: evidence for a causal role in governing fluid reabsorption and glomerulotublar balance by the renal proximal tubule. J Clin Invest. 1971 Feb;50(2):336–349. doi: 10.1172/JCI106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Nicholas D. P., Brenner B. M. Comparative renal effects of isoncotic and colloid-free volume expansion in the rat. Am J Physiol. 1972 Jan;222(1):225–235. doi: 10.1152/ajplegacy.1972.222.1.225. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of peritubular capillary control of isotonic fluid reabsorption by the renal proximal tubule. Biophys J. 1973 Apr;13(4):340–358. doi: 10.1016/S0006-3495(73)85989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Ueki I. F., Brenner B. M. Permeability of renal peritubular capillaries to neutral dextrans dextrans and endogenous albumin. Am J Physiol. 1976 Aug;231(2):283–291. doi: 10.1152/ajplegacy.1976.231.2.283. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Brenner B. M., Tadokoro M., Berliner R. W. Oncotic and hydrostatic pressures in peritubular capillaries and fluid reabsorption by proximal tubule. Am J Physiol. 1971 May;220(5):1427–1433. doi: 10.1152/ajplegacy.1971.220.5.1427. [DOI] [PubMed] [Google Scholar]
- Hargens A. R., Tucker B. J., Blantz R. C. Renal lymph protein in the rat. Am J Physiol. 1977 Oct;233(4):F269–F273. doi: 10.1152/ajprenal.1977.233.4.F269. [DOI] [PubMed] [Google Scholar]
- Häberle D. A., Shiigai T. T., Maier G., Schiffl H., Davis J. M. Dependency of proximal tubular fluid transport on the load of glomerular filtrate. Kidney Int. 1981 Jul;20(1):18–28. doi: 10.1038/ki.1981.99. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Brenner B. M. Importance of efferent arteriolar vascular tone in regulation of proximal tubule fluid reabsorption and glomerulotubular balance in the rat. J Clin Invest. 1980 May;65(5):1192–1201. doi: 10.1172/JCI109774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa I., Brenner B. M. Mechanism of inhibition of proximal tubule fluid reabsorption after exposure of the rat kidney to the physical effects of expansion of extracellular fluid volume. J Clin Invest. 1979 Nov;64(5):1466–1474. doi: 10.1172/JCI109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa I., Hoyer J. R., Seiler M. W., Brenner B. M. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982 Jan;69(1):185–198. doi: 10.1172/JCI110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa I., Rennke H. G., Hoyer J. R., Badr K. F., Schor N., Troy J. L., Lechene C. P., Brenner B. M. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983 Jan;71(1):91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. K., Steven K. Angiotensin II induced reduction of peritubular capillary diameter in the rat kidney. Pflugers Arch. 1977 Nov 23;371(3):245–250. doi: 10.1007/BF00586264. [DOI] [PubMed] [Google Scholar]
- Jensen P. K., Steven K. Influence of intratubular pressure on proximal tubular compliance and capillary diameter in the rat kidney. Pflugers Arch. 1979 Nov;382(2):179–187. doi: 10.1007/BF00584220. [DOI] [PubMed] [Google Scholar]
- Kiil F. Mechanism of glomerulotubular balance: the whole kidney approach. Ren Physiol. 1982;5(5):209–221. doi: 10.1159/000172860. [DOI] [PubMed] [Google Scholar]
- Kon V., Ichikawa I. Effector loci for renal nerve control of cortical microcirculation. Am J Physiol. 1983 Nov;245(5 Pt 1):F545–F553. doi: 10.1152/ajprenal.1983.245.5.F545. [DOI] [PubMed] [Google Scholar]
- Källskog O., Wolgast M. Driving forces over the peritubular capillary membrane in the rat kidney during antidiuresis and saline expansion. Acta Physiol Scand. 1973 Sep;89(1):116–125. doi: 10.1111/j.1748-1716.1973.tb05502.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Bennett C. M., Deen W. M., Glassock R. J., Knutson D., Brenner B. M. Control of proximal tubule fluid reabsorption in experimental glomerulonephritis. J Clin Invest. 1975 Jun;55(6):1315–1325. doi: 10.1172/JCI108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J. A., Earley L. E. Demonstraton of a role of physical factors as determinants of the natriuretic response to volume expansion. J Clin Invest. 1967 Dec;46(12):1963–1978. doi: 10.1172/JCI105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. C., Curry F. E., Michel C. C. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res. 1977 Mar;13(2):185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- Mertz J. I., Haas J. A., Berndt T. J., Burnett J. C., Jr, Knox F. G. Effects of secretin on peritubular capillary physical factors and proximal fluid reabsorption in the rat. J Clin Invest. 1983 Aug;72(2):622–625. doi: 10.1172/JCI111011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Brenner B. M. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res. 1975 Jul;37(1):101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- O'morchoe C. C., Omorchoe P. J., Donati E. J. Comparison of hilar and capsular renal lymph. Am J Physiol. 1975 Aug;229(2):416–421. doi: 10.1152/ajplegacy.1975.229.2.416. [DOI] [PubMed] [Google Scholar]
- Ott C. E., Haas J. A., Cuche J. L., Knox F. G. Effect of increased peritubule protein concentration on proximal tubule reabsorption in the presence and absence of extracellular volume expansion. J Clin Invest. 1975 Mar;55(3):612–620. doi: 10.1172/JCI107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelayo J. C., Ziegler M. G., Jose P. A., Blantz R. C. Renal denervation in the rat: analysis of glomerular and proximal tubular function. Am J Physiol. 1983 Jan;244(1):F70–F77. doi: 10.1152/ajprenal.1983.244.1.F70. [DOI] [PubMed] [Google Scholar]
- Quinn M. D., Marsh D. J. Peritubular capillary control of proximal tubule reabsorption in the rat. Am J Physiol. 1979 May;236(5):F478–F487. doi: 10.1152/ajprenal.1979.236.5.F478. [DOI] [PubMed] [Google Scholar]
- Robertson C. R., Deen W. M., Troy J. L., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972 Nov;223(5):1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Berry C. A., Rector F. C., Jr Effect of luminal and peritubular HCO3(-) concentrations and PCO2 on HCO3(-) reabsorption in rabbit proximal convoluted tubules perfused in vitro. J Clin Invest. 1982 Sep;70(3):639–649. doi: 10.1172/JCI110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Watkins M. L., Andreoli T. E. Flow dependence of fluid transport in the isolated superficial pars recta: evidence that osmotic disequilibrium between external solutions drives isotonic fluid absorption. Kidney Int. 1981 Nov;20(5):588–597. doi: 10.1038/ki.1981.181. [DOI] [PubMed] [Google Scholar]
- Spitzer A., Windhager E. E. Effect of peritubular oncotic pressure changes on proximal tubular fluid reabsorption. Am J Physiol. 1970 Apr;218(4):1188–1193. doi: 10.1152/ajplegacy.1970.218.4.1188. [DOI] [PubMed] [Google Scholar]
- Steiner R. W., Tucker B. J., Blantz R. C. Glomerular hemodynamics in rats with chronic sodium depletion. Effect of saralasin. J Clin Invest. 1979 Aug;64(2):503–512. doi: 10.1172/JCI109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Determinants of proximal tubular reabsorption as mechanisms of glomerulotubular balance. Am J Physiol. 1978 Aug;235(2):F142–F150. doi: 10.1152/ajprenal.1978.235.2.F142. [DOI] [PubMed] [Google Scholar]
- Viets J. W., Deen W. M., Troy J. L., Brenner B. M. Determination of serum protein concentration in nanoliter blood samples using fluorescamine or 9-phthalaldehyde. Anal Biochem. 1978 Aug 1;88(2):513–521. doi: 10.1016/0003-2697(78)90451-7. [DOI] [PubMed] [Google Scholar]
- Wolgast M., Persson E., Schnermann J., Ulfendahl H., Wunderlich P. Colloid osmotic pressure of the subcapsular interstitial fluid of rat kidneys during hydropenia and volume expansion. Pflugers Arch. 1973 May 18;340(2):123–131. doi: 10.1007/BF00588171. [DOI] [PubMed] [Google Scholar]


