Abstract
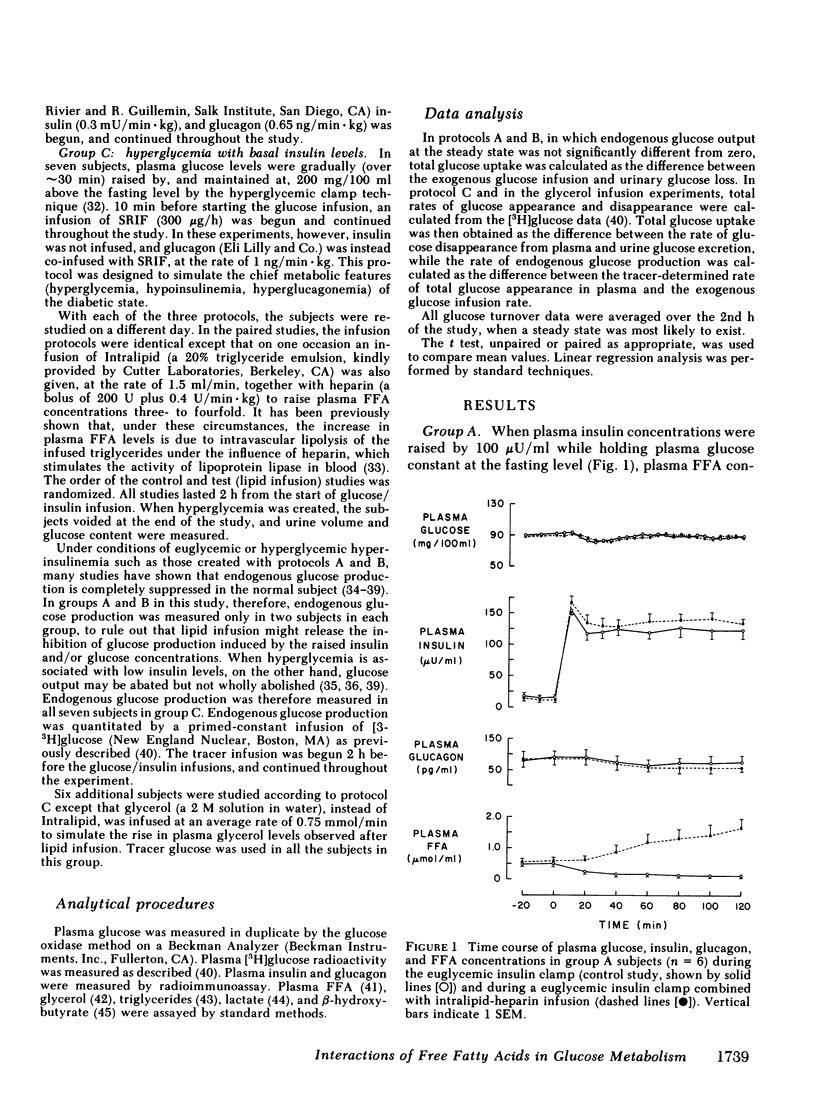
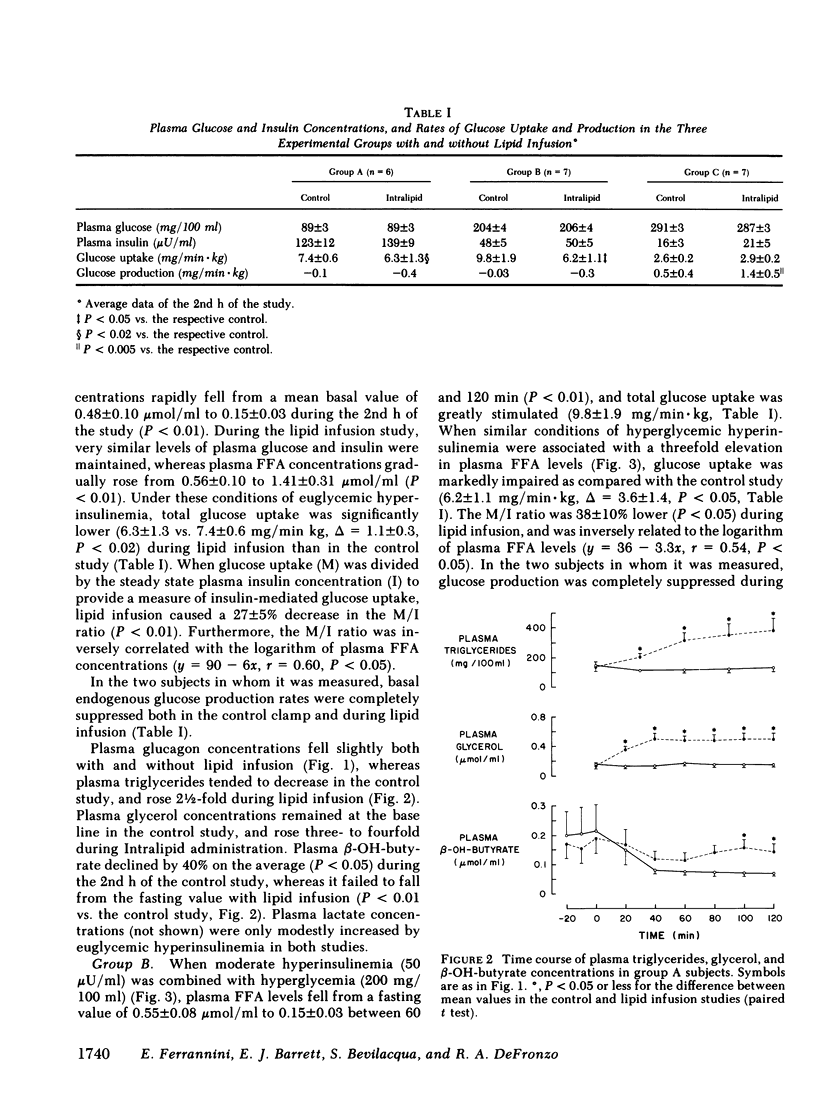
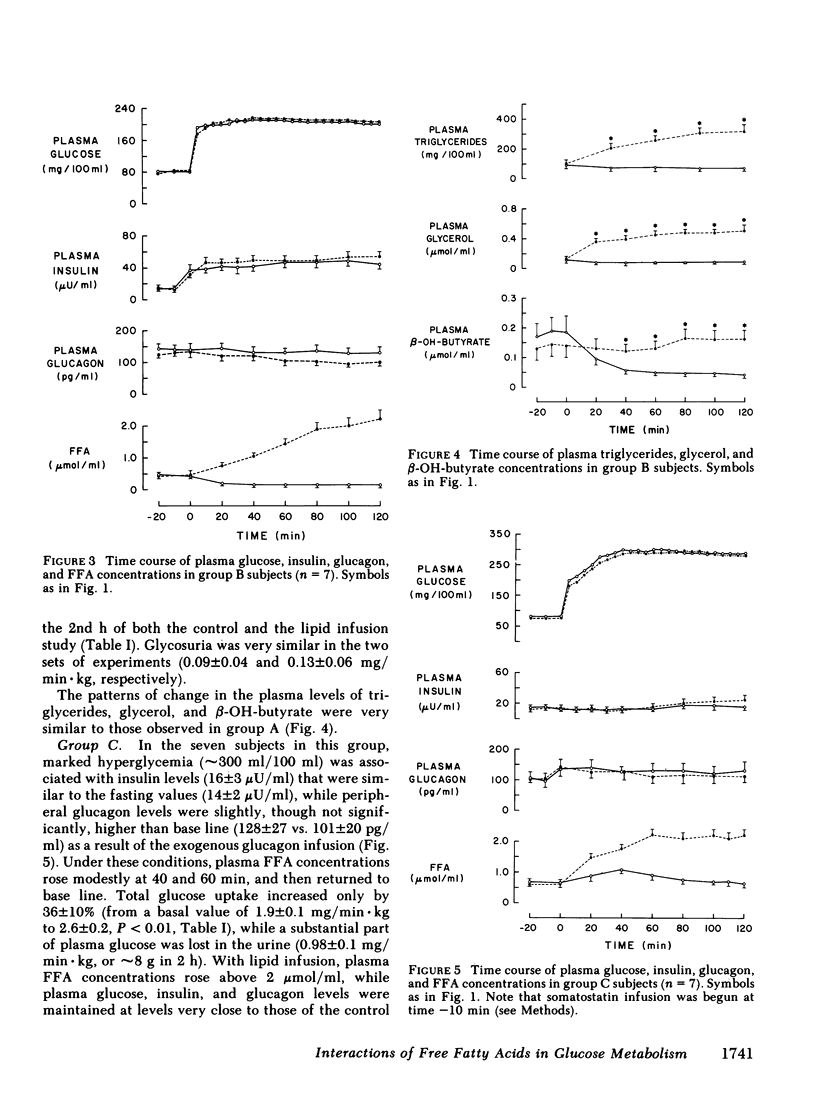
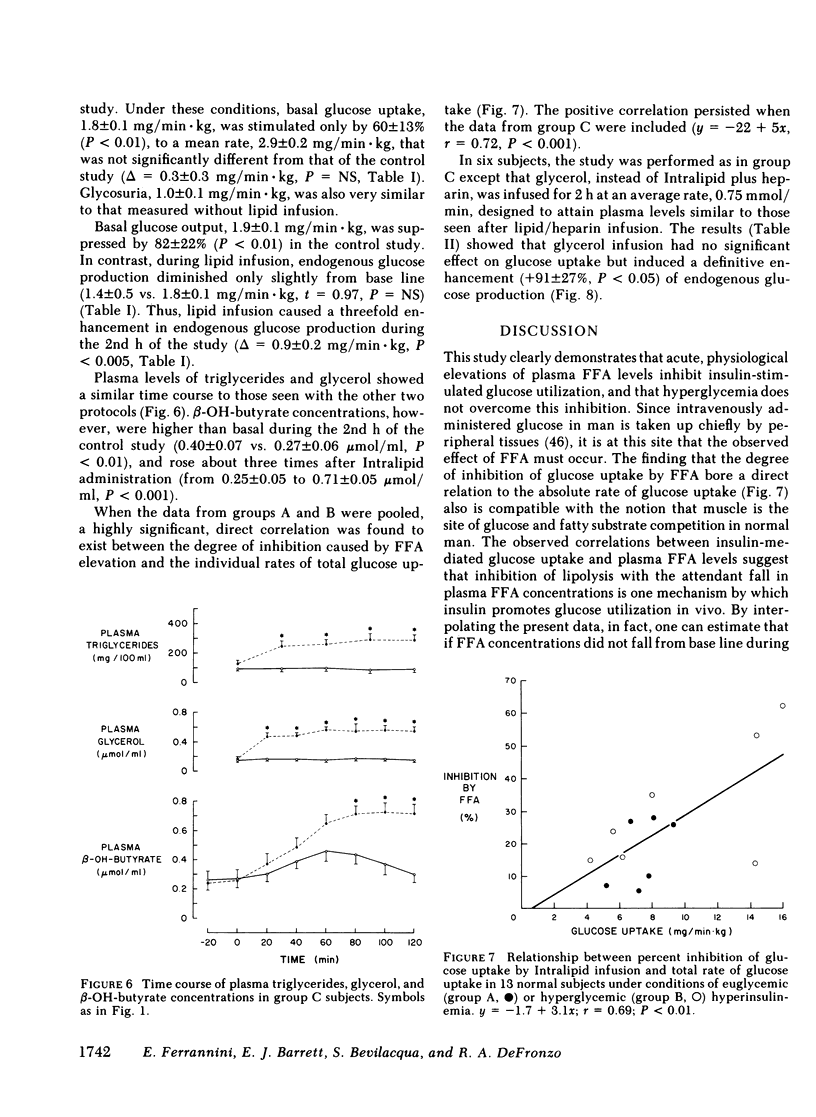
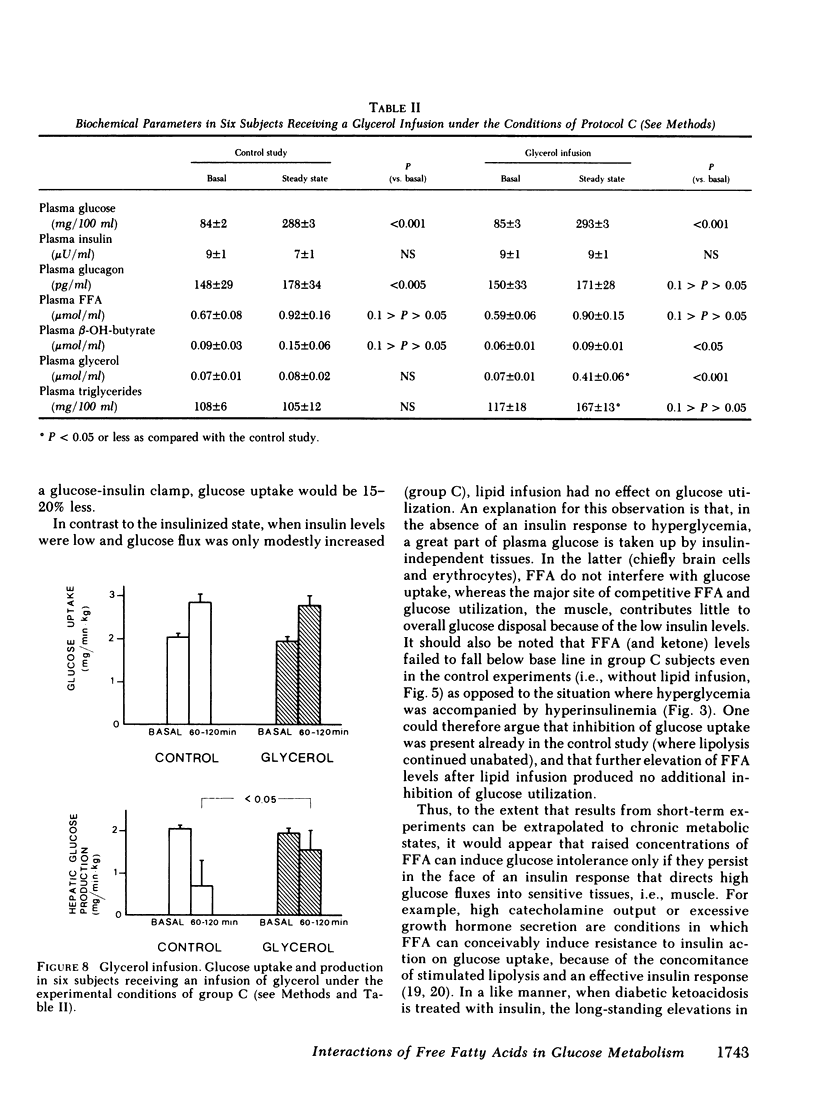
Since the initial proposal of the glucose fatty acid cycle, considerable controversy has arisen concerning its physiologic significance in vivo. In the present study, we examined the effect of acute, physiologic elevations of FFA concentrations on glucose production and uptake in normal subjects under three controlled experimental conditions. In group A, plasma insulin levels were raised and maintained at approximately 100 microU/ml above base line by an insulin infusion, while holding plasma glucose at the fasting level by a variable glucose infusion. In group B, plasma glucose concentration was raised by 125 mg/100 ml and plasma insulin was clamped at approximately 50 microU/ml by a combined infusion of somatostatin and insulin. In group C, plasma glucose was raised by 200 mg/100 ml above the fasting level, while insulin secretion was inhibited with somatostatin and peripheral glucagon levels were replaced with a glucagon infusion (1 ng/min X kg). Each protocol was repeated in the same subject in combination with a lipid-heparin infusion designed to raise plasma FFA levels by 1.5-2.0 mumol/ml. With euglycemic hyperinsulinemia (study A), lipid infusion caused a significant inhibition of total glucose uptake (6.3 +/- 1.3 vs. 7.4 +/- 0.6 mg/min X kg, P less than 0.02). Endogenous glucose production (estimated by the [3-3H]glucose technique) was completely suppressed both with and without lipid infusion. With hyperglycemic hyperinsulinemia (study B), lipid infusion also induced a marked impairment in glucose utilization (6.2 +/- 1.1 vs. 9.8 +/- 1.9 mg/min X kg, P less than 0.05); endogenous glucose production was again completely inhibited despite the increase in FFA concentrations. Under both conditions (A and B), the percentage inhibition of glucose uptake by FFA was positively correlated with the total rate of glucose uptake (r = 0.69, P less than 0.01). In contrast, when hyperglycemia was associated with relative insulinopenia and hyperglucagonemia (study C), thus simulating a diabetic state, lipid infusion had no effect on glucose uptake (2.9 +/- 0.2 vs. 2.6 +/- 0.2 mg/min X kg) but markedly stimulated endogenous glucose production (1.4 +/- 0.5 vs. 0.5 +/- 0.4 mg/min X kg, P less than 0.005). Under the same conditions as study C, a glycerol infusion producing plasma glycerol levels similar to those achieved with lipid-heparin, enhanced endogenous glucose production (1.5 +/- 0.5 vs. 0.7 +/- 0.6 mg/min X kg, P less than 0.05). We conclude that, in the well-insulinized state raised FFA levels effectively compete with glucose for uptake by peripheral tissues, regardless of the presence of hyperglycemia. When insulin is deficient, on the other hand, elevated rates of lipolysis may contribute to hyperglycemia not by competition for fuel utilization, but through an enhancement of endogenous glucose output.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS H. Metabolic production of sucrose from fat. Nature. 1961 Jul 29;191:433–436. doi: 10.1038/191433a0. [DOI] [PubMed] [Google Scholar]
- Balasse E. O. Effect of free fatty acids and ketone bodies on glucose uptake and oxidation in the dog. Horm Metab Res. 1971 Nov;3(6):403–409. doi: 10.1055/s-0028-1094129. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Havel R. J. Evidence for an effect of inulin on the peripheral utilization of ketone bodies in dogs. J Clin Invest. 1971 Apr;50(4):801–813. doi: 10.1172/JCI106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasse E. O., Neef M. A. Influence of nicotinic acid on the rates of turnover and oxidation of plasma glucose in man. Metabolism. 1973 Sep;22(9):1193–1204. doi: 10.1016/0026-0495(73)90207-2. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Neef M. A. Operation of the "glucose-fatty acid cycle" during experimental elevations of plasma free fatty acid levels in man. Eur J Clin Invest. 1974 Aug;4(4):247–252. doi: 10.1111/j.1365-2362.1974.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Beatty C. H., Bocek R. M. Interrelation of carbohydrate and palmitate metabolism in skeletal muscle. Am J Physiol. 1971 Jun;220(6):1928–1934. doi: 10.1152/ajplegacy.1971.220.6.1928. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman O., Felig P., Wahren J. The contrasting responses of splanchnic and renal glucose output to gluconeogenic substrates and to hypoglucagonemia in 60-h-fasted humans. Diabetes. 1980 Aug;29(8):610–616. doi: 10.2337/diab.29.8.610. [DOI] [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- Cassens R. G., Bocek R. M., Beatty C. H. Effect of octanoate on carbohydrate metabolism in red and white muscle of the rhesus monkey. Am J Physiol. 1969 Sep;217(3):715–719. doi: 10.1152/ajplegacy.1969.217.3.715. [DOI] [PubMed] [Google Scholar]
- Costill D. L., Coyle E., Dalsky G., Evans W., Fink W., Hoopes D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1977 Oct;43(4):695–699. doi: 10.1152/jappl.1977.43.4.695. [DOI] [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Effect of sodium linoleate infusion on plasma free fatty acids, glucose, insulin, and ketones in unanesthetized dogs. Diabetes. 1972 Dec;21(12):1179–1184. doi: 10.2337/diab.21.12.1179. [DOI] [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969 Oct;48(10):1934–1943. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Park C. R. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969 Aug 10;244(15):4095–4102. [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- FELBER J. P., VANNOTTI A. EFFECTS OF FAT INFUSION ON GLUCOSE TOLERANCE AND INSULIN PLASMA LEVELS. Med Exp Int J Exp Med. 1964;10:153–156. doi: 10.1159/000135410. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of maturity-onset diabetes on splanchnic glucose balance after oral glucose ingestion. Diabetes. 1978 Feb;27(2):121–126. doi: 10.2337/diab.27.2.121. [DOI] [PubMed] [Google Scholar]
- Ferré P., Pegorier J. P., Marliss E. B., Girard J. R. Influence of exogenous fat and gluconeogenic substrates on glucose homeostasis in the newborn rat. Am J Physiol. 1978 Feb;234(2):E129–E136. doi: 10.1152/ajpendo.1978.234.2.E129. [DOI] [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Effects of insulin and fatty acids on gluconeogenesis in the rat. J Biol Chem. 1967 Aug 25;242(16):3620–3627. [PubMed] [Google Scholar]
- GELLHORN A., MARKS P. A. The composition and biosynthesis of lipids in human adipose tissues. J Clin Invest. 1961 Jun;40:925–932. doi: 10.1172/JCI104331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 9. Effects of fatty acids and ketone bodies, and of alloxan-diabetes and starvation, on pyruvate metabolism and on lactate-pyruvate and L-glycerol 3-phosphate-dihydroxyacetone phosphate concentration ratios in rat heart and rat diaphragm muscles. Biochem J. 1964 Dec;93(3):665–678. doi: 10.1042/bj0930665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C., Vranic M., Hetenyi G., Jr Nonhypoglycemic glucoregulation: role of glycerol and glucoregulatory hormones. Am J Physiol. 1983 Apr;244(4):E373–E379. doi: 10.1152/ajpendo.1983.244.4.E373. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Effects of low-carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose-tolerance tests. Lancet. 1963 Apr 13;1(7285):790–794. doi: 10.1016/s0140-6736(63)91501-0. [DOI] [PubMed] [Google Scholar]
- Hall S. E., Hall A. J., Layberry R. A., Berman M., Hetenyi G., Jr Effects of age and fasting on gluconeogenesis from glycerol in dogs. Am J Physiol. 1976 Feb;230(2):362–367. doi: 10.1152/ajplegacy.1976.230.2.362. [DOI] [PubMed] [Google Scholar]
- Herrera M. G., Kamm D., Ruderman N., Cahill Non-hormonal factors in the control of gluconeogenesis. Adv Enzyme Regul. 1966;4:225–235. doi: 10.1016/0065-2571(66)90017-3. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Koehler J. O., Morgan H. E. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci U S A. 1972 Apr;69(4):816–820. doi: 10.1073/pnas.69.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller U., Chiasson J. L., Liljenquist J. E., Cherrington A. D., Jennings A. S., Crofford O. S. The roles of insulin, glucagon, and free fatty acids in the regulation of ketogenesis in dogs. Diabetes. 1977 Nov;26(11):1040–1051. doi: 10.2337/diab.26.11.1040. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B., Mancini M., Mattock M., Chait A., Fraser T. R. Plasma triglyceride and fatty acid metabolism in diabetes mellitus. Eur J Clin Invest. 1972 Nov;2(6):445–453. doi: 10.1111/j.1365-2362.1972.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- NESTEL P. J., CARROLL K. F., SILVERSTEIN M. S. INFLUENCE OF FREE-FATTY-ACID METABOLISM ON GLUCOSE TOLERANCE. Lancet. 1964 Jul 18;2(7351):115–117. doi: 10.1016/s0140-6736(64)90125-4. [DOI] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Paul P., Issekutz B., Jr, Miller H. I. Interrelationship of free fatty acids and glucose metabolism in the dog. Am J Physiol. 1966 Dec;211(6):1313–1320. doi: 10.1152/ajplegacy.1966.211.6.1313. [DOI] [PubMed] [Google Scholar]
- Paul P., Issekutz B., Jr, Miller H. I. Interrelationship of free fatty acids and glucose metabolism in the dog. Am J Physiol. 1966 Dec;211(6):1313–1320. doi: 10.1152/ajplegacy.1966.211.6.1313. [DOI] [PubMed] [Google Scholar]
- Pelkonen R., Miettinen T. A., Taskinen M. R., Nikkilä E. A. Effect of acute elevation of plasma glycerol, triglyceride and FFA levels on glucose utilization and plasma insulin. Diabetes. 1968 Feb;17(2):76–82. doi: 10.2337/diab.17.2.76. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Newsholme E. A., Hales C. N. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann N Y Acad Sci. 1965 Oct 8;131(1):324–333. doi: 10.1111/j.1749-6632.1965.tb34800.x. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M. J., Holloszy J. O. Inhibition of glucose uptake and glycogenolysis by availability of oleate in well-oxygenated perfused skeletal muscle. Biochem J. 1977 Nov 15;168(2):161–170. doi: 10.1042/bj1680161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M. J., Winder W. W., Holloszy J. O. A sparing effect of increased plasma fatty acids on muscle and liver glycogen content in the exercising rat. Biochem J. 1976 Jun 15;156(3):647–655. doi: 10.1042/bj1560647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Goodman M. N., Conover C. A., Berger M. Substrate utilization in perfused skeletal muscle. Diabetes. 1979 Jan;28 (Suppl 1):13–17. doi: 10.2337/diab.28.1.s13. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Schalch D. S., Kipnis D. M. Abnormalities in carbohydrate tolerance associated with elevated plasma nonesterified fatty acids. J Clin Invest. 1965 Dec;44(12):2010–2020. doi: 10.1172/JCI105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld G., Kipnis D. M. Effects of fatty acids on carbohydrate and fatty acid metabolism of rat diaphragm. Am J Physiol. 1968 Aug;215(2):513–522. doi: 10.1152/ajplegacy.1968.215.2.513. [DOI] [PubMed] [Google Scholar]
- Seyffert W. A., Jr, Madison L. L. Physiologic effects of metabolic fuels on carbohydrate metabolism. I. Acute effect of elevation of plasma free fatty acids on hepatic glucose output, peripheral glucose utilization, serum insulin, and plasma glucagon levels. Diabetes. 1967 Nov;16(11):765–776. doi: 10.2337/diab.16.11.765. [DOI] [PubMed] [Google Scholar]
- Shaw W. A., Issekutz T. B., Issekutz B., Jr Gluconeogenesis from glycerol at rest and during exercise in normal, diabetic, and methylprednisolone-treated dogs. Metabolism. 1976 Mar;25(3):329–339. doi: 10.1016/0026-0495(76)90091-3. [DOI] [PubMed] [Google Scholar]
- Shaw W. A., Issekutz T. B., Issekutz B., Jr Interrelationship of FFA and glycerol turnovers in resting and exercising dogs. J Appl Physiol. 1975 Jul;39(1):30–36. doi: 10.1152/jappl.1975.39.1.30. [DOI] [PubMed] [Google Scholar]
- Struck E., Ashmore J., Wieland O. Effects of glucagon and long chain fatty acids on glucose production by isolated perfused rat liver. Adv Enzyme Regul. 1966;4:219–224. doi: 10.1016/0065-2571(66)90016-1. [DOI] [PubMed] [Google Scholar]
- Struck E., Ashmore J., Wieland O. Stimulierung der Gluconeogenese durch langkettige Fettsäuren und Glucagon. Biochem Z. 1965 Nov 5;343(1):107–110. [PubMed] [Google Scholar]
- Thiebaud D., Jacot E., DeFronzo R. A., Maeder E., Jequier E., Felber J. P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982 Nov;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]



