Abstract
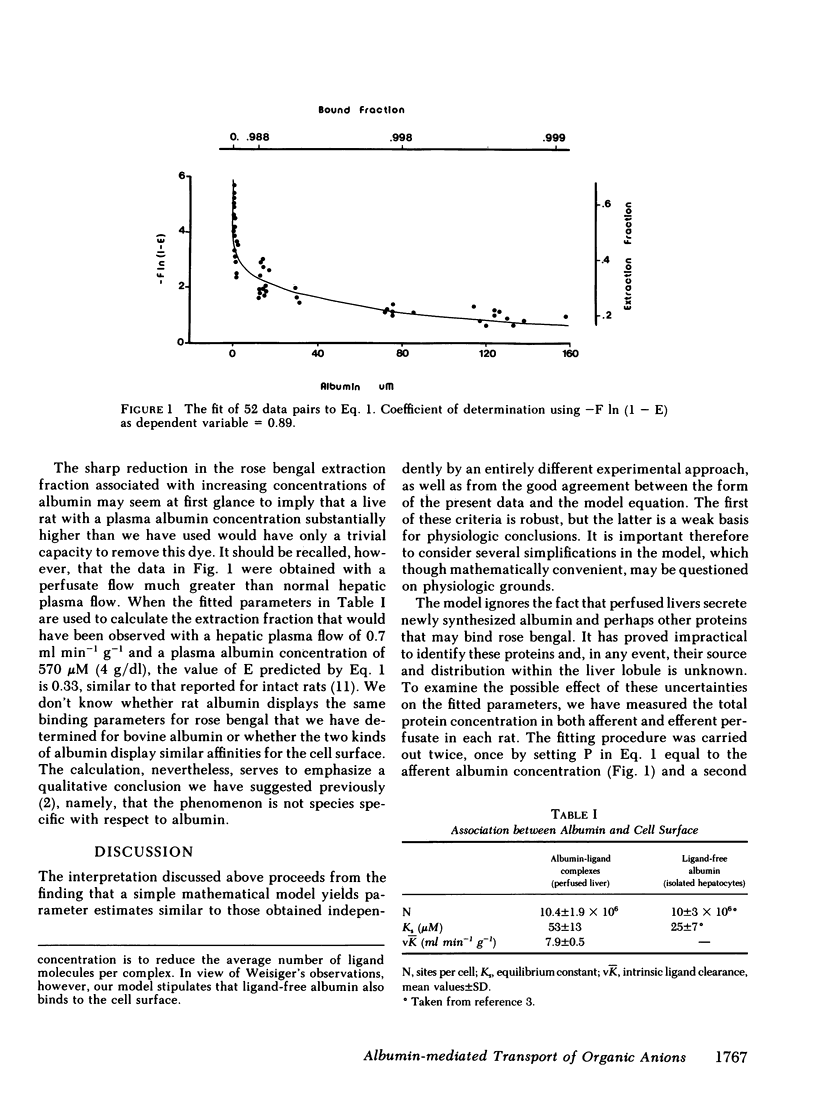
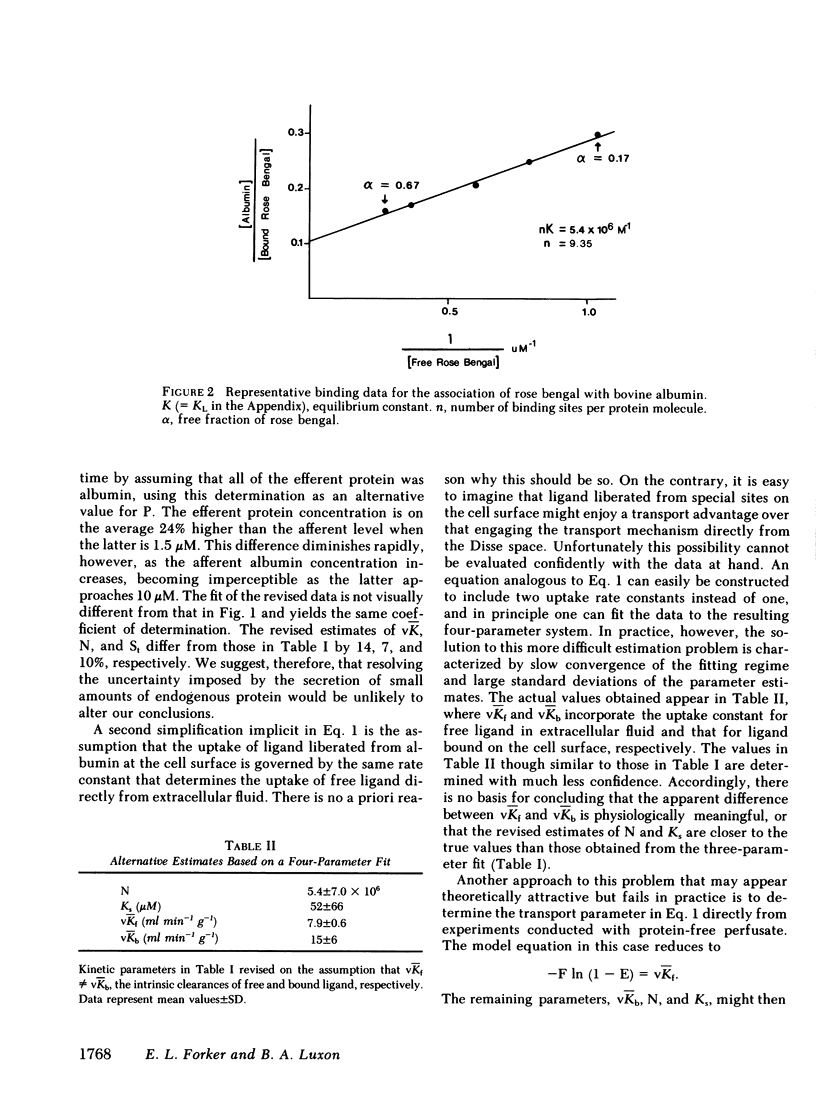
Rapid dissociation of organic anions from plasma albumin maximizes the presentation of free ligand to the cell surface and thus favors its efficient hepatic extraction. Even assuming these optimal conditions, however, taurocholate and rose bengal have hepatic extraction fractions that are higher than can be accounted for by spontaneous dissociation of their albumin-ligand complexes. In this study we developed a transport model that attributes this behavior to sites on the hepatocyte plasma membrane that bind the albumin-ligand complexes, promoting the transport of ligand into the hepatocyte. Fitting this model to rose bengal removal rates measured over a wide range of albumin concentrations yields estimates of the number of cell surface sites and their affinity for albumin. These estimates are in good agreement with those reported by Weisiger, Gollan, and Ockner for the binding of ligand-free albumin to isolated hepatocytes. We conclude that both experiments measure the same phenomenon and, accordingly, that the binding of albumin to the cell surface is the functional equivalent of albumin-mediated transport.
Full text
PDF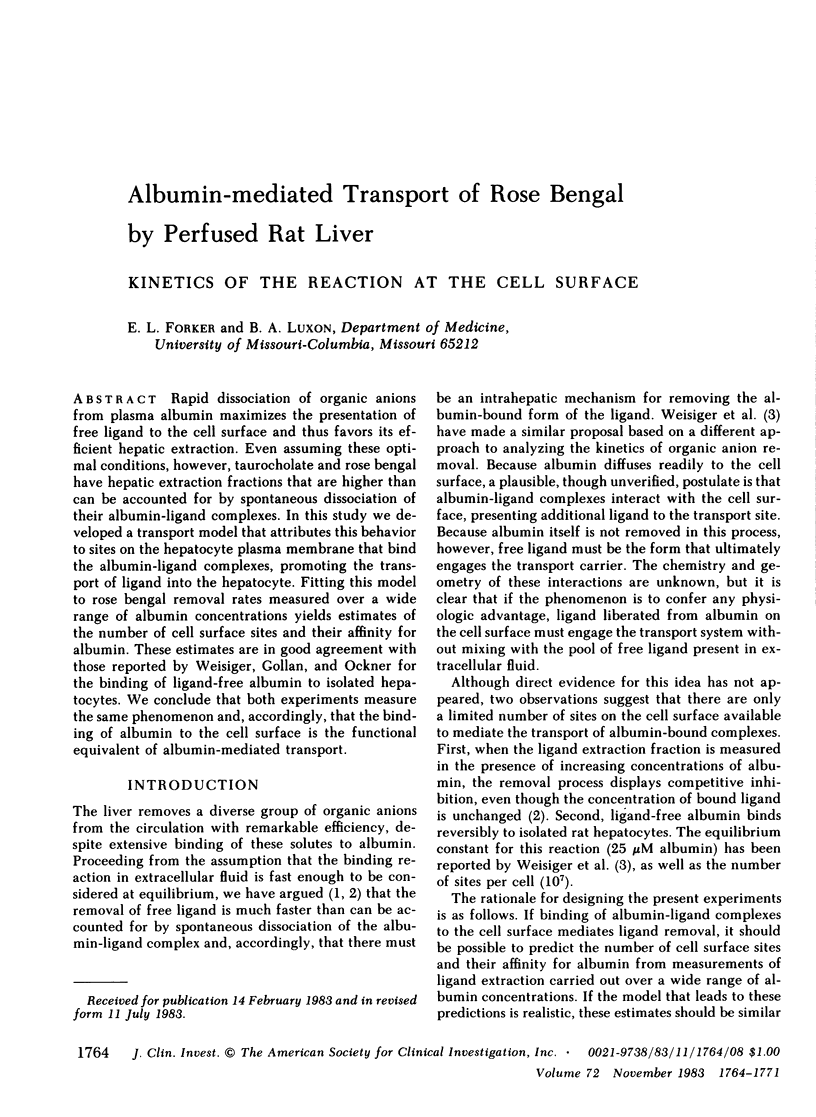





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin helps mediate removal of taurocholate by rat liver. J Clin Invest. 1981 May;67(5):1517–1522. doi: 10.1172/JCI110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A., Snell M., Shurmantine W. O. Effect of albumin binding on the hepatic transport of rose bengal: surface-mediated dissociation of limited capacity. J Pharmacol Exp Ther. 1982 Nov;223(2):342–347. [PubMed] [Google Scholar]
- Goresky C. A., Bach G. G., Nadeau B. E. On the uptake of materials by the intact liver. The transport and net removal of galactose. J Clin Invest. 1973 May;52(5):991–1009. doi: 10.1172/JCI107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]



