Abstract
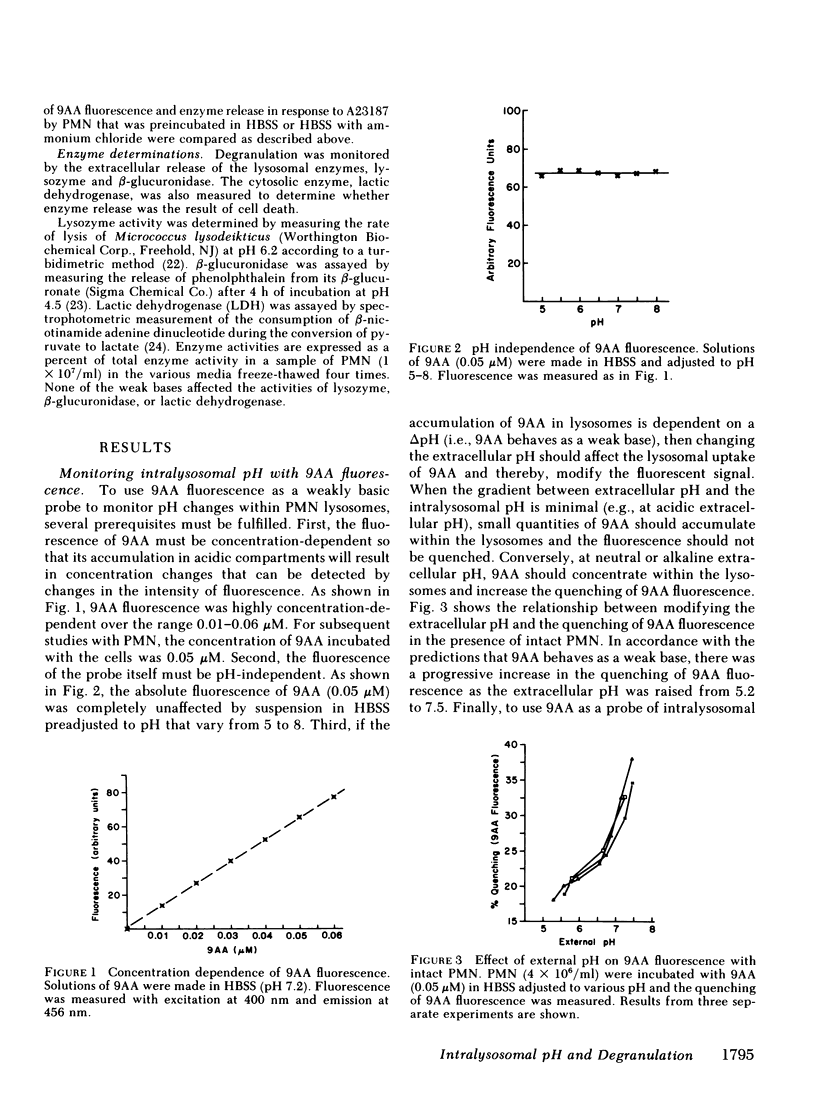
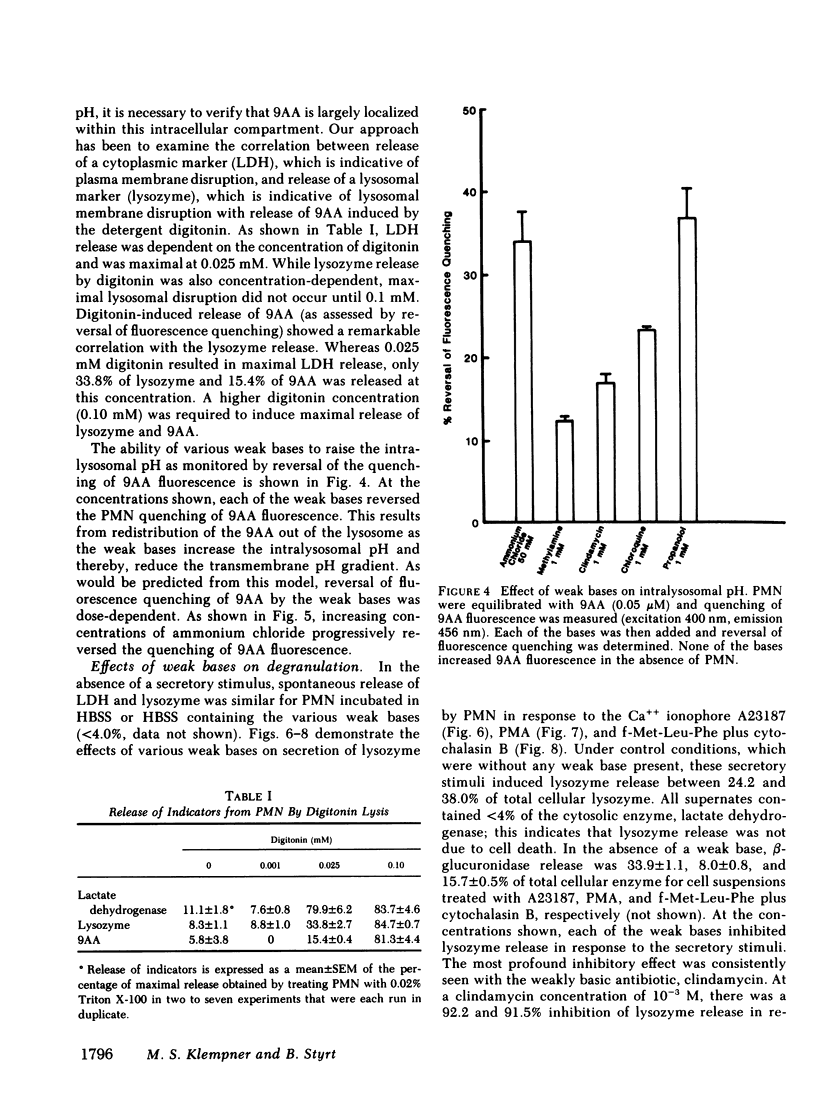
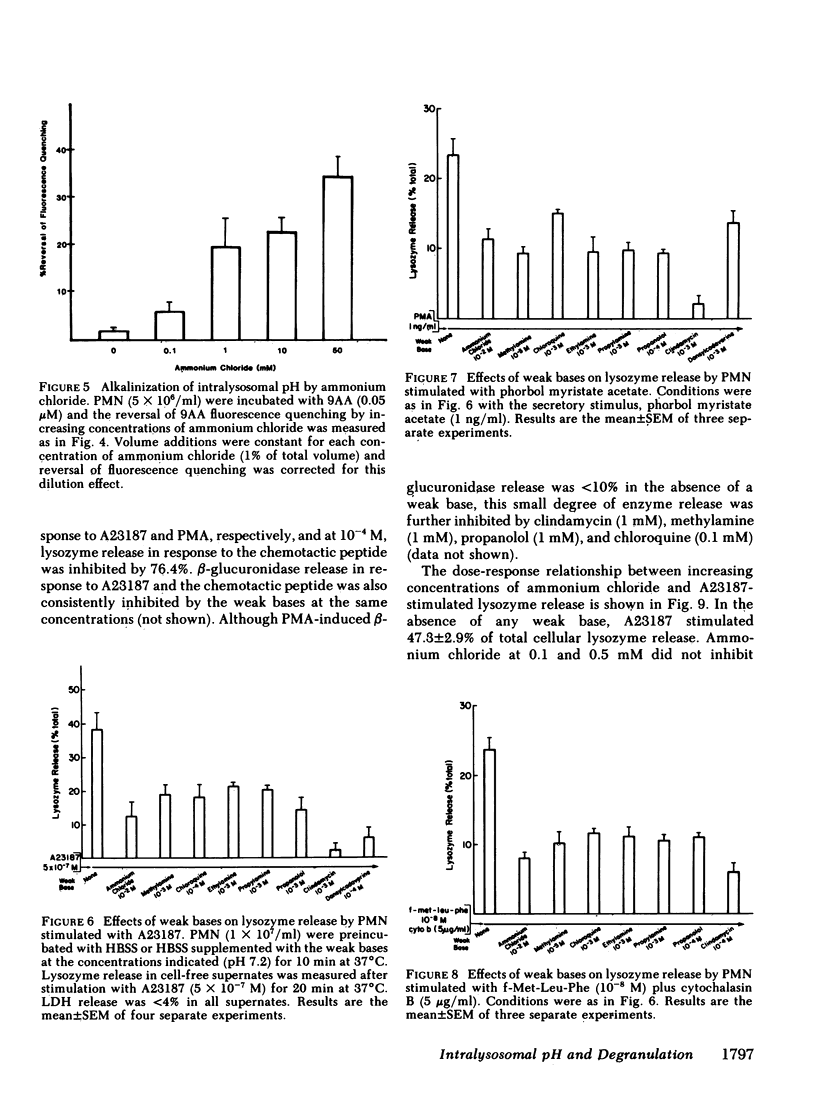
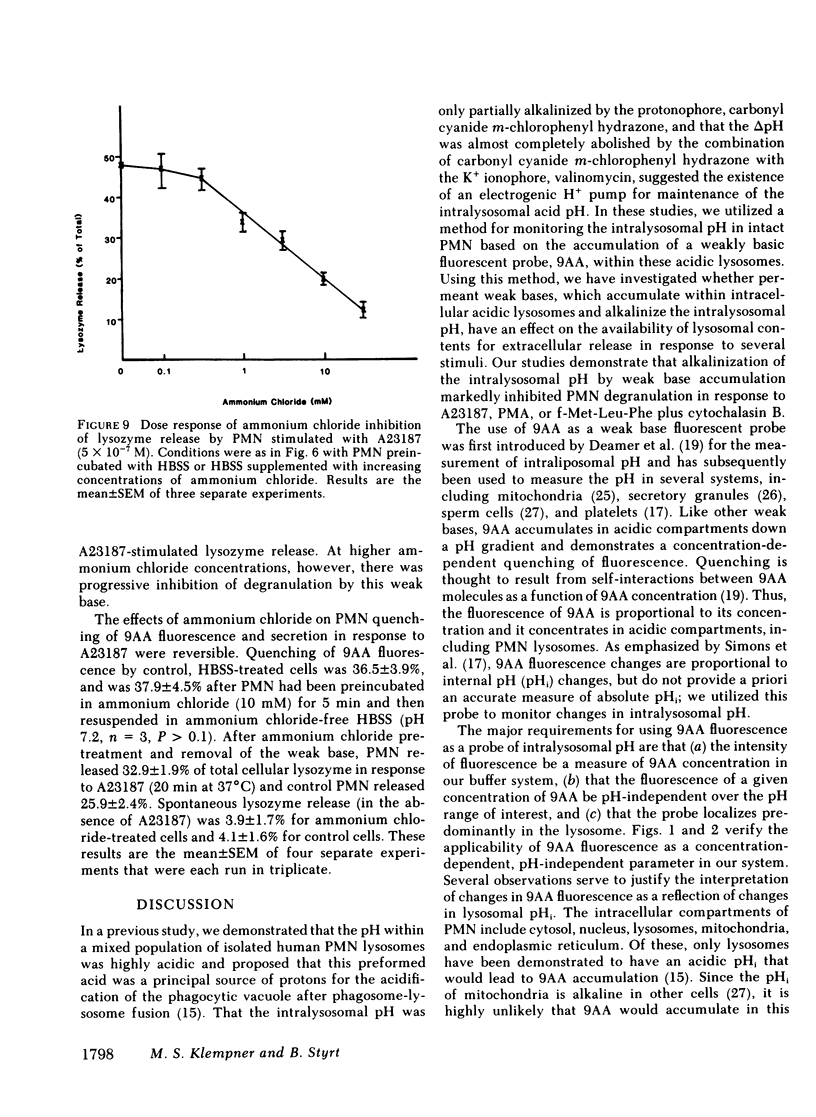
Degranulation of lysosomes is one of the consequences of neutrophil activation. Regulatory mechanisms of lysosomal secretion are thought to be localized largely in the plasma membrane and cytosol, with the lysosome playing a passive role in secretion. Recent evidence indicates that the intralysosomal pH is highly acidic (pH congruent to 5.5) and is maintained by active transport of H+. We investigated whether changes in the intralysosomal pH altered the availability of lysosomes for exocytosis. Intralysosomal pH in intact neutrophils was monitored with the weakly basic fluorescent probe, 9-aminoacridine (9AA). The weak bases, methylamine, chloroquine, clindamycin, propanolol, and ammonium chloride (0.1-50 mM), caused an alkalinization of the intralysosomal pH as determined by reversal of quenching of 9AA fluorescence. Similarly, each of the weak bases, including ammonium chloride, methylamine, chloroquine, ethylamine, propylamine, propanolol, clindamycin, and dansylcadaverine, inhibited neutrophil degranulation in response to the calcium ionophore A23187, phorbol myristate acetate, or the chemotactic peptide, formyl-methionine-leucine-phenylalanine plus cytochalasin B. These studies indicate that an acid intralysosomal pH is important to the neutrophil secretory response and suggest that the lysosome may play an active part in control of degranulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkerman J. W., Ebberink R. H., Lips J. P., Christiaens G. C. Rapid separation of cytosol and particle fraction of human platelets by digitonin-induced cell damage. Br J Haematol. 1980 Feb;44(2):291–300. doi: 10.1111/j.1365-2141.1980.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Deamer D. W., Prince R. C., Crofts A. R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim Biophys Acta. 1972 Aug 9;274(2):323–335. doi: 10.1016/0005-2736(72)90180-0. [DOI] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Nichols W. K., Hill H. R. Cyclic nucleotide changes in human neutrophils induced by chemoattractants and chemotactic modulators. J Immunol. 1977 Aug;119(2):450–456. [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S. T. Ultrastructural demonstration of calcium loss from local regions of the plasma membrane of surface-stimulated human granulocytes. J Immunol. 1979 Sep;123(3):1395–1402. [PubMed] [Google Scholar]
- Hoffstein S., Soberman R., Goldstein I., Weissmann G. Concanavalin A induces microtubule assembly and specific granule discharge in human polymorphonuclear leukocytes. J Cell Biol. 1976 Mar;68(3):781–787. doi: 10.1083/jcb.68.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., George W. J. Hormonal control of lysosomal enzyme release from human neutrophils: elevation of cyclic nucleotide levels by autonomic neurohormones. Proc Natl Acad Sci U S A. 1974 May;71(5):2027–2031. doi: 10.1073/pnas.71.5.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Eisenstat B. A., Smolen J. E., Rutherford L. E., Dunham P. B., Weissmann G. Stimulus-response coupling in the human neutrophil. The role of anion fluxes in degranulation. J Biol Chem. 1982 Jun 25;257(12):6916–6922. [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITWACK G. Photometric determination of lysozyme activity. Proc Soc Exp Biol Med. 1955 Jul;89(3):401–403. doi: 10.3181/00379727-89-21824. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molski T. F., Naccache P. H., Volpi M., Wolpert L. M., Sha'afi R. I. Specific modulation of the intracellular pH of rabbit neutrophils by chemotactic factors. Biochem Biophys Res Commun. 1980 May 30;94(2):508–514. doi: 10.1016/0006-291x(80)91260-7. [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama G., Johnson R. G., Scarpa A. Spectrophotometric measurements of transmembrane potential and pH gradients in chromaffin granules. J Gen Physiol. 1980 Feb;75(2):109–140. doi: 10.1085/jgp.75.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature. 1978 Nov 30;276(5687):515–517. doi: 10.1038/276515a0. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. The effects of extracellular K+, Na+ and Ca++ on lysosomal enzyme secretion from polymorphonuclear leukocytes. J Immunol. 1977 Sep;119(3):804–811. [PubMed] [Google Scholar]
- Smetana K., Lejnar J., Kasík P. Cytochemical studies on histones in the condensed chromatin of mature human leucocytes. Histochemie. 1973 Oct 26;37(2):141–148. doi: 10.1007/BF00305585. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. Increased levels of cyclic adenosine-3',5'-monophosphate in human polymorphonuclear leukocytes after surface stimulation. J Clin Invest. 1980 May;65(5):1077–1085. doi: 10.1172/JCI109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The kinetics of lysosomal degranulation of human neutrophils as measured by 9-aminoacridine quenching. Biochim Biophys Acta. 1983 Apr 5;762(2):145–153. doi: 10.1016/0167-4889(83)90066-6. [DOI] [PubMed] [Google Scholar]
- Styrt B., Klempner M. S. Internal pH of human neutrophil lysosomes. FEBS Lett. 1982 Nov 22;149(1):113–116. doi: 10.1016/0014-5793(82)81083-1. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Hartwig J. H., Maruyama K., Stossel T. P. Ca2+ control of actin filament length. Effects of macrophage gelsolin on actin polymerization. J Biol Chem. 1981 Sep 25;256(18):9693–9697. [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]



