Abstract
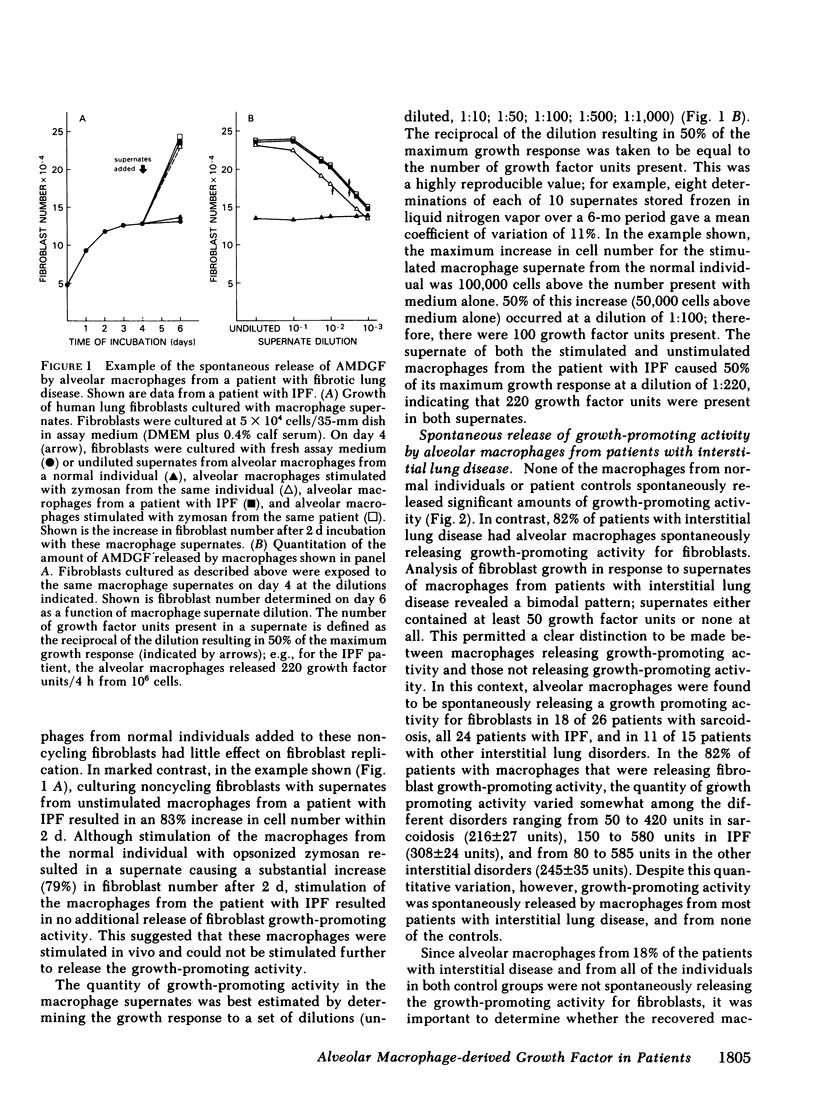
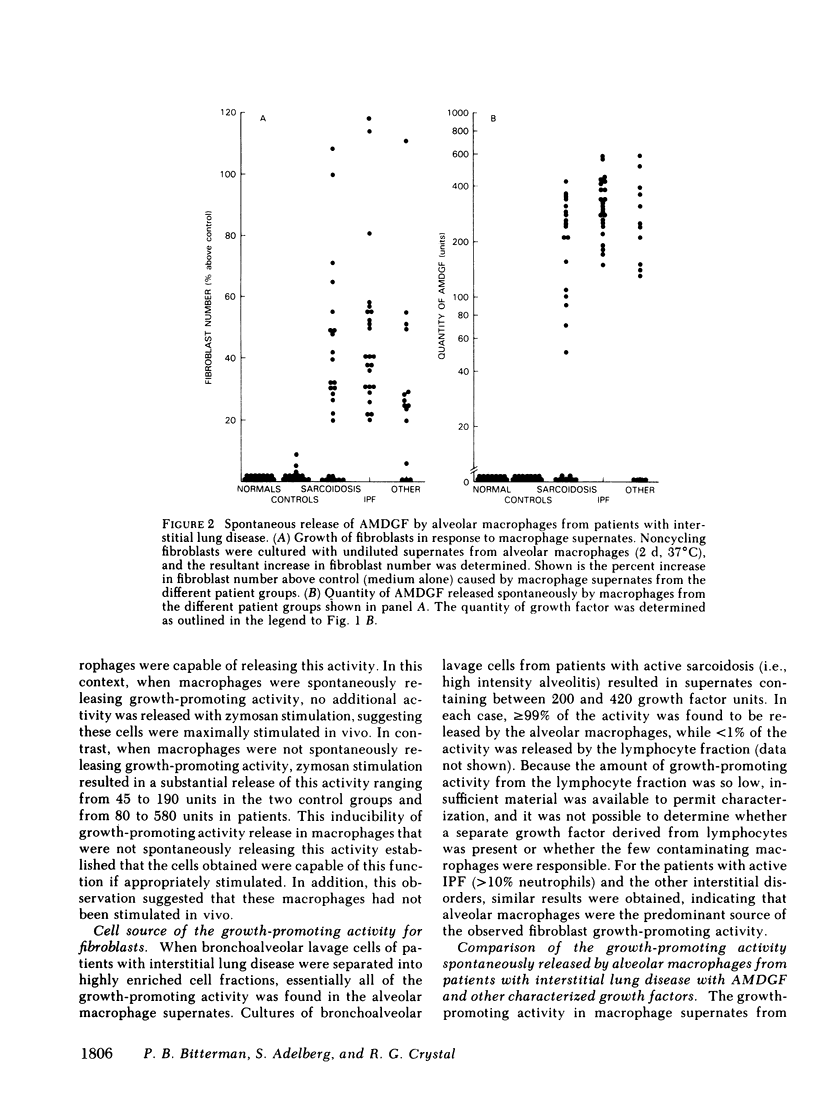
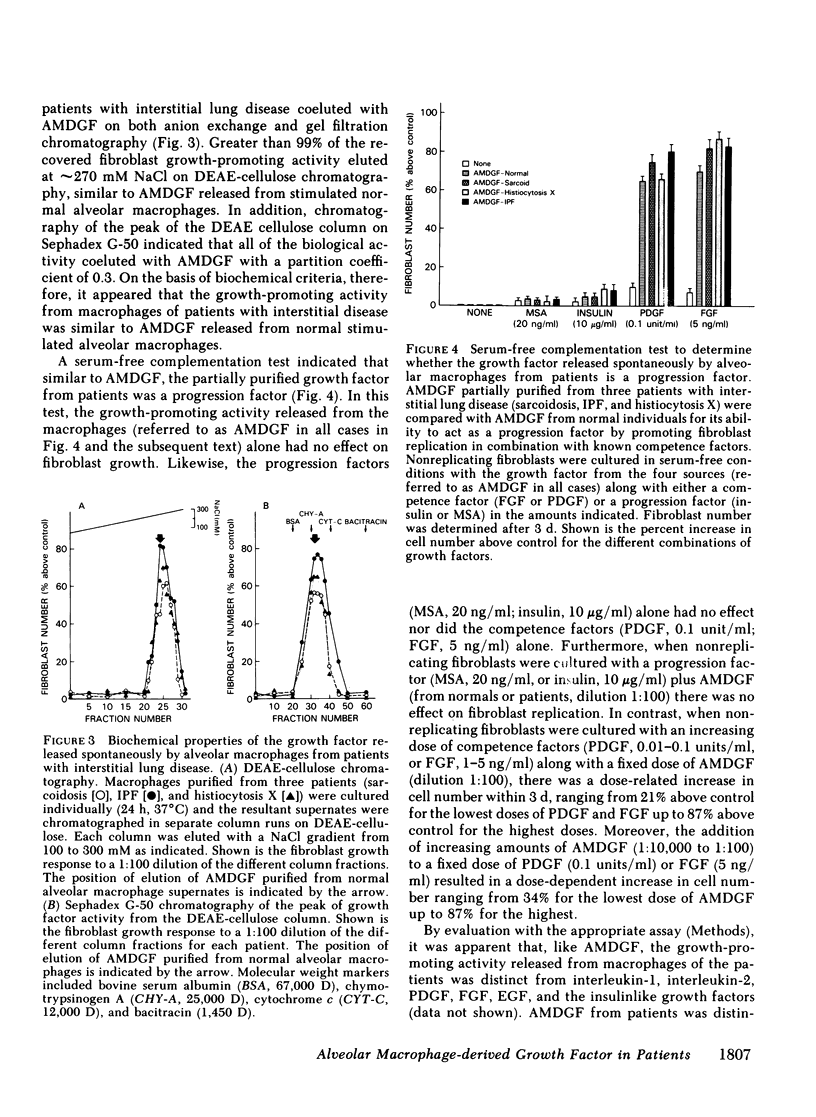
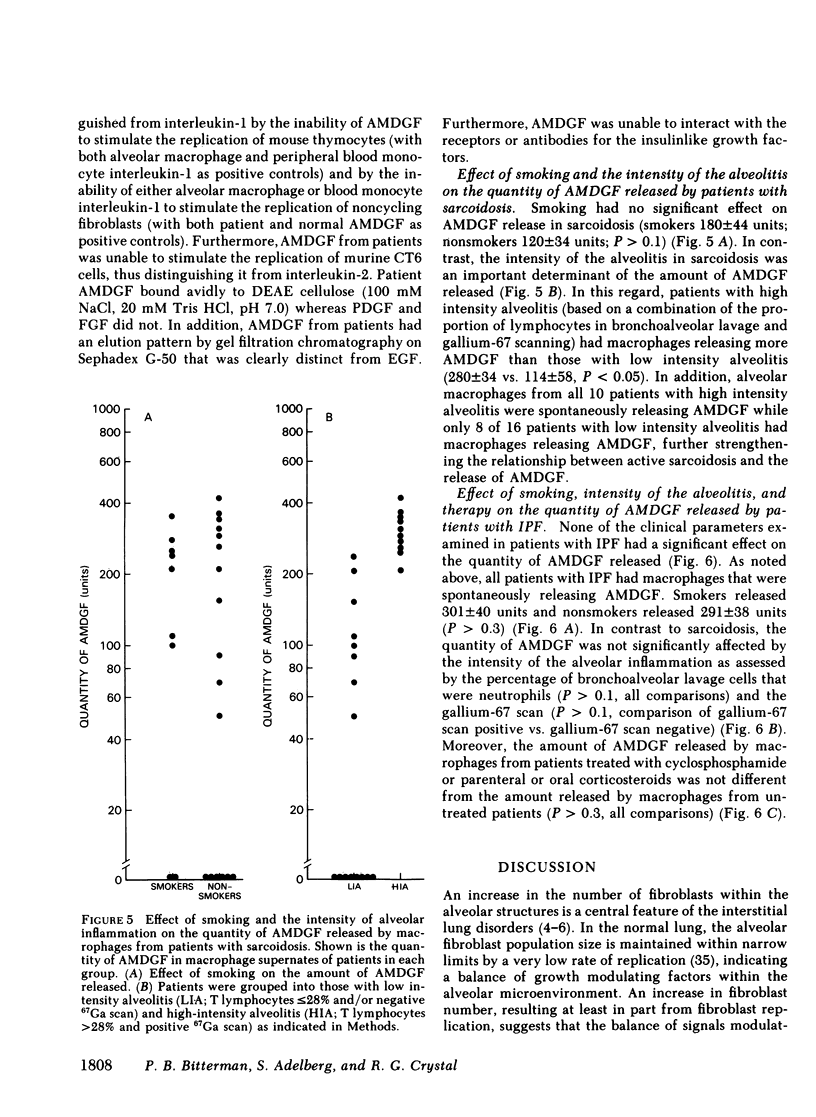
Interstitial lung disorders are characterized both by a chronic inflammation of the lower respiratory tract that includes increased numbers of activated alveolar macrophages and by increased numbers of fibroblasts within the alveolar wall. Since alveolar macrophages from normal individuals can be activated to release a growth factor for lung fibroblasts (alveolar macrophage-derived growth factor [AMDGF]), we hypothesized that the activated alveolar macrophages within the lower respiratory tract of patients with fibrotic lung disorders might be spontaneously releasing AMDGF. To evaluate this hypothesis, alveolar macrophages (suspension culture, 4 h, 37 degrees) from 65 patients with interstitial lung disorders and 30 control subjects were examined for the spontaneous release of fibroblast growth-promoting activity, with human lung fibroblasts as the target. Whereas none of the controls had macrophages spontaneously releasing a growth-promoting activity for fibroblasts, 82% of the patients with interstitial lung disease had alveolar macrophages that were spontaneously releasing a growth-promoting activity for fibroblasts. In common with AMDGF, the fibroblast growth-promoting activity released by these macrophages eluted from DEAE cellulose at 270 mM NaCl, had a partition coefficient of 0.3 by gel filtration on Sephadex G-50, was distinct from other characterized growth factors, and acted as a progression factor for fibroblast replication in a serum-free complementation test. These data suggest that the expansion of fibroblast numbers within the alveolar structures in interstitial lung disorders may result, in part, from the release of AMDGF by alveolar macrophages stimulated in vivo.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basset F., Corrin B., Spencer H., Lacronique J., Roth C., Soler P., Battesti J. P., Georges R., Chrétien J. Pulmonary histiocytosis X. Am Rev Respir Dis. 1978 Nov;118(5):811–820. doi: 10.1164/arrd.1978.118.5.811. [DOI] [PubMed] [Google Scholar]
- Beck G. J., Doyle C. A., Schachter E. N. Smoking and lung function. Am Rev Respir Dis. 1981 Feb;123(2):149–155. doi: 10.1164/arrd.1981.123.2.149. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Craighead J. E. Interstitial associations of cells lining air spaces in human pulmonary fibrosis. Virchows Arch A Pathol Anat Histol. 1976 Nov 22;372(1):39–49. doi: 10.1007/BF00429715. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Van Wyk J. J. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. J Clin Invest. 1981 Jan;67(1):10–19. doi: 10.1172/JCI110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Rossman M. D. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980 Mar;92(3):406–416. doi: 10.7326/0003-4819-92-3-406. [DOI] [PubMed] [Google Scholar]
- DeLustro F., LeRoy E. C. Characterization of the release of human monocyte regulators of fibroblast proliferation. J Reticuloendothel Soc. 1982 Apr;31(4):295–305. [PubMed] [Google Scholar]
- DeLustro F., Sherer G. K., LeRoy E. C. Human monocyte stimulation of fibroblast growth by a soluble mediator(s). J Reticuloendothel Soc. 1980 Dec;28(6):519–532. [PubMed] [Google Scholar]
- Dearden L. C., Fairshter R. D., McRae D. M., Smith W. R., Glauser F. L., Wilson A. F. Pulmonary ultrastructure of the late aspects of human paraquat poisoning. Am J Pathol. 1978 Dec;93(3):667–680. [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Bils R. F. Identification of cells labeled with tritiated thymidine in the pulmonary alveolar walls of the mouse. Am Rev Respir Dis. 1969 Sep;100(3):372–378. doi: 10.1164/arrd.1969.100.3.372. [DOI] [PubMed] [Google Scholar]
- Foley T. P., Jr, Nissley S. P., Stevens R. L., King G. L., Hascall V. C., Humbel R. E., Short P. A., Rechler M. M. Demonstration of receptors for insulin and insulin-like growth factors on Swarm rat chondrosarcoma chondrocytes. Evidence that insulin stimulates proteoglycan synthesis through the insulin receptor. J Biol Chem. 1982 Jan 25;257(2):663–669. [PubMed] [Google Scholar]
- Freiman D. G., Hardy H. L. Beryllium disease. The relation of pulmonary pathology to clinical course and prognosis based on a study of 130 cases from the U.S. beryllium case registry. Hum Pathol. 1970 Mar;1(1):25–44. doi: 10.1016/s0046-8177(70)80003-x. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D., Bienkowski R. S., Cowan M. J., Breul S. D., Bradley K. M., Ferrans V. J., Roberts W. C., Crystal R. G. Collagen concentration and rates of synthesis in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1980 Aug;122(2):289–301. doi: 10.1164/arrd.1980.122.2.289. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D., Roberts W. C., von Gal E. R., Grystal R. G. Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest. 1977 Sep;60(3):595–610. doi: 10.1172/JCI108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAENSLER E. A., MOISTER V. B., HAMM J. OPEN-LUNG BIOPSY IN DUFFUSE PULMONARY DISEASE. N Engl J Med. 1964 Jun 18;270:1319–1331. doi: 10.1056/NEJM196406182702501. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Harington J. S., Ritchie M., King P. C., Miller K. The in-vitro effects of silica-treated hamster macrophages on collagen production by hamster fibroblasts. J Pathol. 1973 Jan;109(1):21–37. doi: 10.1002/path.1711090104. [DOI] [PubMed] [Google Scholar]
- Haschek W. M., Reiser K. M., Klein-Szanto A. J., Kehrer J. P., Smith L. H., Last J. A., Witschi H. P. Potentiation of butylated hydroxytoluene-induced acute lung damage by oxygen. Cell kinetics and collagen metabolism. Am Rev Respir Dis. 1983 Jan;127(1):28–34. doi: 10.1164/arrd.1983.127.1.28. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Styles J. A. Activity of a macrophage factor in collagen formation by silica. Nature. 1967 Apr 29;214(5087):521–522. doi: 10.1038/214521a0. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis. 1979 Mar;119(3):471–503. doi: 10.1164/arrd.1979.119.3.471. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Lawley T. J., Crystal R. G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981 Jul;68(1):259–269. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Jones A. W., Reeve N. L. Ultrastructural study of bleomycin-induced pulmonary changes in mice. J Pathol. 1978 Apr;124(4):227–233. doi: 10.1002/path.1711240407. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Van Obberghen E., Nissley S. P., Rechler M. M. Demonstration of two subtypes of insulin-like growth factor receptors by affinity cross-linking. J Biol Chem. 1981 Jun 10;256(11):5305–5308. [PubMed] [Google Scholar]
- Keogh B. A., Bernardo J., Hunninghake G. W., Line B. R., Price D. L., Crystal R. G. Effect of intermittent high dose parenteral corticosteroids on the alveolitis of idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1983 Jan;127(1):18–22. doi: 10.1164/arrd.1983.127.1.18. [DOI] [PubMed] [Google Scholar]
- Keogh B. A., Hunninghake G. W., Line B. R., Crystal R. G. The alveolitis of pulmonary sarcoidosis. Evaluation of natural history and alveolitis-dependent changes in lung function. Am Rev Respir Dis. 1983 Aug;128(2):256–265. doi: 10.1164/arrd.1983.128.2.256. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Line B. R., Fulmer J. D., Reynolds H. Y., Roberts W. C., Jones A. E., Harris E. K., Crystal R. G. Gallium-67 citrate scanning in the staging of idiopathic pulmonary fibrosis: Correlation and physiologic and morphologic features and bronchoalveolar lavage. Am Rev Respir Dis. 1978 Aug;118(2):355–365. doi: 10.1164/arrd.1978.118.2.355. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosentreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by a macrophage cell line, P388D1. II. Biochemical characterization of LAF induced by activated T cells and LPS. J Immunol. 1978 May;120(5):1504–1508. [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M. Immunological cross-reactivity of multiplication-stimulating activity polypeptides. Eur J Biochem. 1980 Jan;103(2):401–408. doi: 10.1111/j.1432-1033.1980.tb04326.x. [DOI] [PubMed] [Google Scholar]
- Nourse L. D., Nourse P. N., Botes H., Schwartz H. M. The effects of macrophages isolated from the lungs of guinea pigs dusted with silica on collagen biosynthesis by guinea pig fibroblasts in cell culture. Environ Res. 1975 Apr;9(2):115–127. doi: 10.1016/0013-9351(75)90056-0. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M., Nissley S. P. Characterization of the binding of multiplication-stimulating activity to a receptor for growth polypeptides in chick embryo fibroblasts. J Biol Chem. 1977 Jun 10;252(11):3898–3910. [PubMed] [Google Scholar]
- Reiser K. M., Last J. A. Silicosis and fibrogenesis: fact and artifact. Toxicology. 1979 May;13(1):51–72. [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. C., Moore V. L. Immunopathogenesis of hypersensitivity pneumonitis. Am Rev Respir Dis. 1977 Dec;116(6):1075–1090. doi: 10.1164/arrd.1977.116.6.1075. [DOI] [PubMed] [Google Scholar]
- Rutherford B., Steffin K., Sexton J. Activated human mononuclear phagocytes release a substance(s) that induces replication of quiescent human fibroblasts. J Reticuloendothel Soc. 1982 Apr;31(4):281–293. [PubMed] [Google Scholar]
- Scadding J. G. Diffuse pulmonary alveolar fibrosis. Thorax. 1974 May;29(3):271–281. doi: 10.1136/thx.29.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadding J. G., Hinson K. F. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967 Jul;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Schoenberger C. I., Hunninghake G. W., Kawanami O., Ferrans V. J., Crystal R. G. Role of alveolar macrophages in asbestosis: modulation of neutrophil migration to the lung after acute asbestos exposure. Thorax. 1982 Nov;37(11):803–809. doi: 10.1136/thx.37.11.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. Interstitial pneumonia. Annu Rev Med. 1967;18:423–442. doi: 10.1146/annurev.me.18.020167.002231. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]



