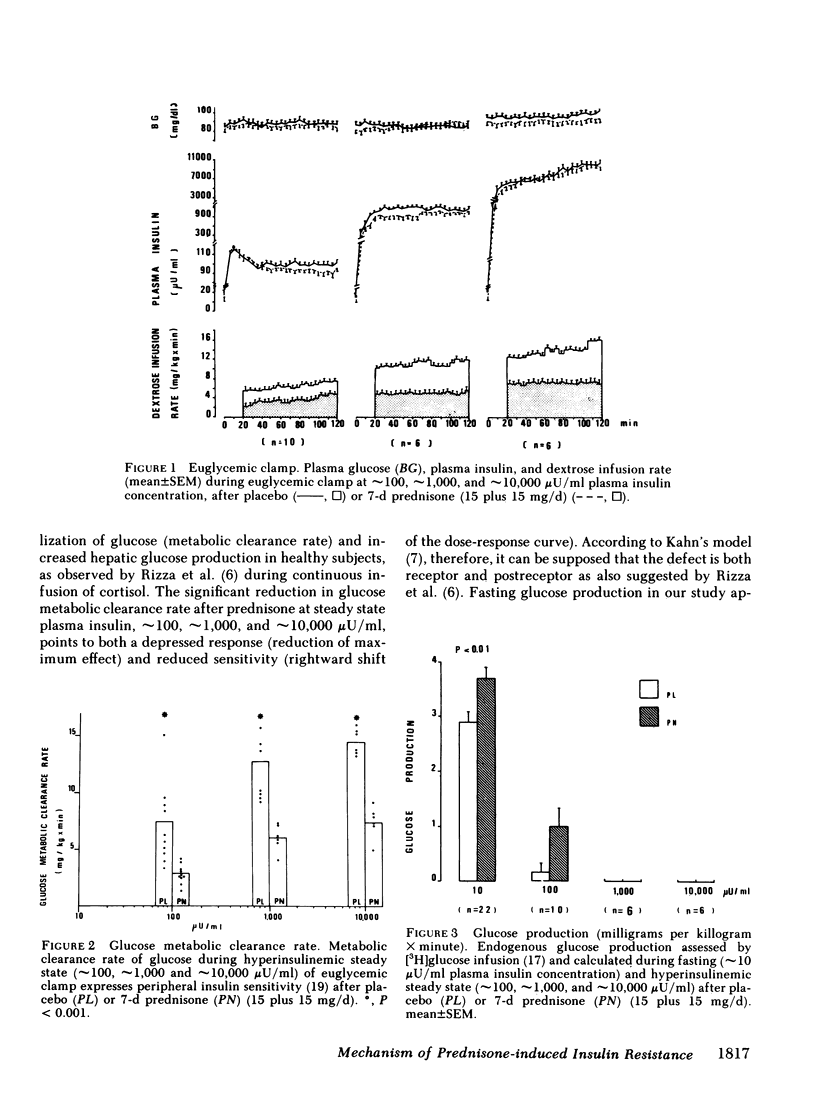
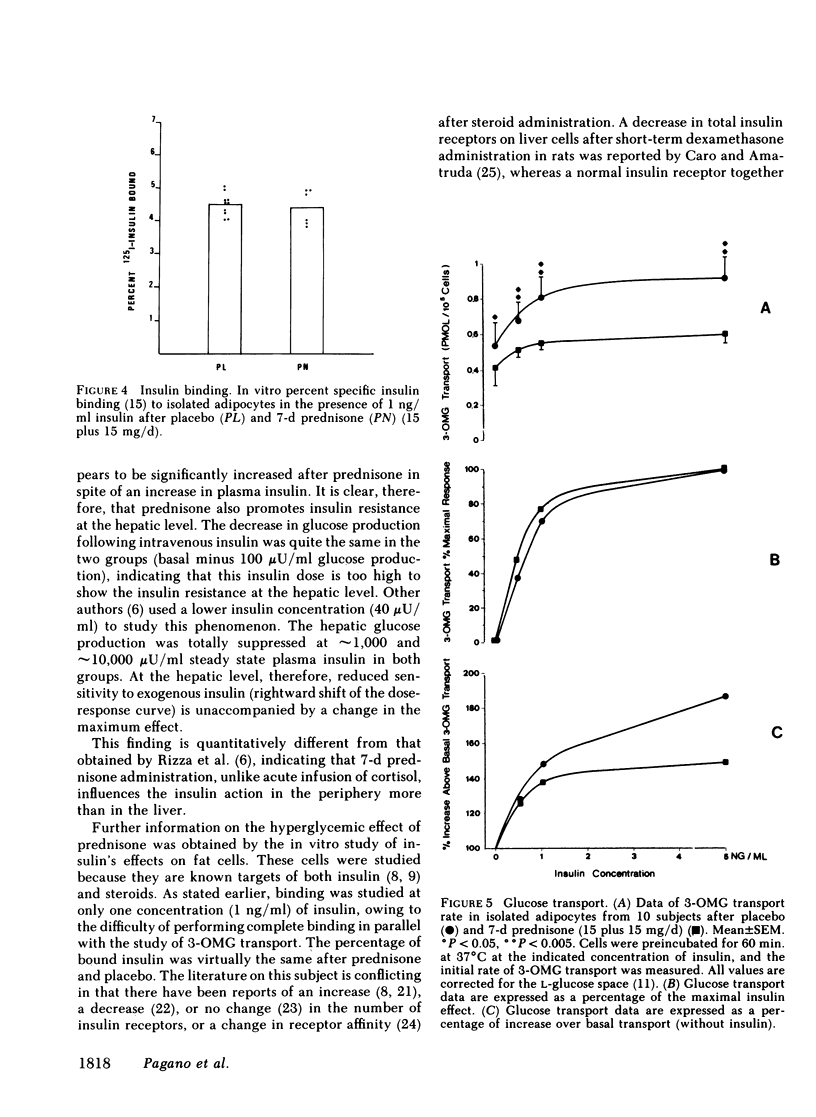
Abstract
Prednisone-induced insulin resistance may depend on either reduced sensitivity (receptor defect) or reduced response to insulin (postreceptor defect). To clarify the mechanism of prednisone-induced insulin resistance, a [3H]glucose infusion (1 microCi/min) was performed for 120 min before and during a euglycemic clamp repeated at approximately 100, approximately 1,000, and approximately 10,000 microU/ml steady state plasma insulin concentration in 10 healthy, normal weight subjects, aged 35 +/- 7 yr. Each test was repeated after 7-d administration of placebo or prednisone (15 plus 15 mg/d per subject), in a randomized sequence with an interval of 1 mo between the two tests. Mean fasting blood glucose (89.5 +/- 2.1 vs. 83.7 +/- 1.9 mg/dl) and mean fasting plasma insulin values (17.8 +/- 1.2 vs. 14.3 +/- 0.8 microU/ml) were significantly higher (P less than 0.01) after prednisone. The insulin sensitivity index (glucose metabolic clearance rate in ml/kg per min) was significantly lower (P less than 0.001) after prednisone at all three steady state plasma insulin levels: 2.8 +/- 0.3 vs. 7.4 +/- 1.1 at approximately 100 microU/ml; 6.0 +/- 0.5 vs. 12.2 +/- 1.1 at approximately 1,000 microU/ml; 7.4 +/- 0.6 vs. 14.4 +/- 0.5 at approximately 10,000 microU/ml. Fasting glucose production (in mg/kg per min) was significantly higher after prednisone: 3.7 +/- 0.2 vs. 2.9 +/- 0.2, P less than 0.001. Suppression of glucose production at steady state plasma insulin level of approximately 100 microU/ml was less after prednisone (1.01 +/- 0.35 vs. 0.14 +/- 0.13, NS), and total at approximately 1,000 and approximately 10,000 microU/ml after both prednisone and placebo. The metabolic kinetic parameters of insulin after prednisone were not significantly different from those after placebo. In addition, insulin binding and 3-ortho-methyl-glucose transport were studied in vitro on fat cells from 16 normal-weight surgical candidates aged 40 +/- 8 yr (10 treated with placebo and 6 with prednisone as above). No significant difference was observed with regard to specific insulin binding (tested with 1 ng/ml hormone only), whereas significant transport differences were noted at the basal level (0.40 +/- 0.10 vs. 0.54 +/- 0.12 pmol/10(5) cells, P less than 0.05), and at increasing concentrations up to the maximum stimulation values (5 ng/ml): 0.59 +/- 0.04 vs. 0.92 +/- 0.12 pmol/10(5) cells, P less than 0.005. These results suggest that (a) administration of an anti-inflammatory dose of prednisone for 7 d induces insulin resistance in man; (b) this is more dependent on depressed peripheral glucose utilization than on increased endogenous production; (c) total insulin binding on isolated adipocytes is not significantly affected; (d) insulin resistance is primarily the outcome of postreceptor defect (impaired glucose transport).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Funder J. W. Hormone receptors. N Engl J Med. 1979 Nov 22;301(21):1149–1161. doi: 10.1056/NEJM197911223012104. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H., De Pirro R., Pedersen O. Prednisone increases the number of insulin receptors on monocytes from normal subjects. J Clin Endocrinol Metab. 1980 Jan;50(1):1–4. doi: 10.1210/jcem-50-1-1. [DOI] [PubMed] [Google Scholar]
- CONN J. W., FAJANS S. S. Influence of adrenal cortical steroids on carbohydrate metabolism in man. Metabolism. 1956 Mar;5(2):114–127. [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Glucocorticoid-induced insulin resistance: the importance of postbinding events in the regulation of insulin binding, action, and degradation in freshly isolated and primary cultures of rat hepatocytes. J Clin Invest. 1982 Apr;69(4):866–875. doi: 10.1172/JCI110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Siegel J. A., Olefsky J. M. Insulin-stimulated glucose transport in human adipocytes. Am J Physiol. 1979 Jun;236(6):E621–E625. doi: 10.1152/ajpendo.1979.236.6.E621. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Czech M. P. Insulin action and the regulation of hexose transport. Diabetes. 1980 May;29(5):399–409. doi: 10.2337/diab.29.5.399. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Fantus I. G., Ryan J., Hizuka N., Gorden P. The effect of glucocorticoids on the insulin receptor: an in vivo and in vitro study. J Clin Endocrinol Metab. 1981 May;52(5):953–960. doi: 10.1210/jcem-52-5-953. [DOI] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Greenfield M. S., Doberne L., Rosenthal M., Schulz B., Widstrom A., Reaven G. M. Effect of sulfonylurea treatment on in vivo insulin secretion and action in patients with non-insulin-dependent diabetes mellitus. Diabetes. 1982 Apr;31(4 Pt 1):307–312. doi: 10.2337/diab.31.4.307. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Allen M., Borkow I. Estimation of glucose turnover in the dog with glucose-2-T and glucose-U- 14 C. Am J Physiol. 1972 Mar;222(3):710–712. doi: 10.1152/ajplegacy.1972.222.3.710. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Allen M. Effect of catecholamines and methylprednisolone on carbohydrate metabolism of dogs. Metabolism. 1972 Jan;21(1):48–59. doi: 10.1016/0026-0495(72)90019-4. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Goldfine I. D., Neville D. M., Jr, De Meyts P. Alterations in insulin binding induced by changes in vivo in the levels of glucocorticoids and growth hormone. Endocrinology. 1978 Oct;103(4):1054–1066. doi: 10.1210/endo-103-4-1054. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Megyesi K., Bar R. S., Eastman R. C., Flier J. S. Receptors for peptide hormones. New insights into the pathophysiology of disease states in man. Ann Intern Med. 1977 Feb;86(2):205–219. doi: 10.7326/0003-4819-86-2-205. [DOI] [PubMed] [Google Scholar]
- Klotz I. M. Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science. 1982 Sep 24;217(4566):1247–1249. doi: 10.1126/science.6287580. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco J., Calle C., Román D., Díaz-Fierros M., Villanueva M. L., Valverde I. Hyperglucagonism induced by glucocorticoid treatment in man. N Engl J Med. 1973 Jan 18;288(3):128–131. doi: 10.1056/NEJM197301182880305. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Tobin J. D., Berman M., Andres R. Kinetics of native insulin in diabetic, obese, and aged men. Diabetes. 1979 Feb;28(2):110–120. doi: 10.2337/diab.28.2.110. [DOI] [PubMed] [Google Scholar]
- Munck A. Glucocorticoid inhibition of glucose uptake by peripheral tissues: old and new evidence, molecular mechanisms, and physiological significance. Perspect Biol Med. 1971 Winter;14(2):265–269. doi: 10.1353/pbm.1971.0002. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Cahill G. F., Jr Metabolic effects of exogenous glucocorticoids in fasted man. J Clin Invest. 1973 Oct;52(10):2596–2605. doi: 10.1172/JCI107452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G., Cassader M., Lenti G. Insulin receptors in adipocytes of non-diabetic and diabetic subjects. Preliminary report. Acta Diabetol Lat. 1977 May-Aug;14(3-4):164–169. doi: 10.1007/BF02581404. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982 Jan;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Soman V., Sherwin R. S. The influence of acute physiological increments of cortisol on fuel metabolism and insulin binding to monocytes in normal humans. J Clin Endocrinol Metab. 1980 Mar;50(3):495–501. doi: 10.1210/jcem-50-3-495. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk C. A., Rizza R. A., Gerich J. E. Effects of plasma glucose concentration on glucose utilization and glucose clearance in normal man. Diabetes. 1981 Jun;30(6):535–537. doi: 10.2337/diab.30.6.535. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Kitabchi A. E. Decreased insulin binding of human erythrocytes after dexamethasone or prednisone ingestion. Diabetes. 1980 Oct;29(10):811–814. doi: 10.2337/diacare.20.10.811. [DOI] [PubMed] [Google Scholar]
- de Pirro R., Bertoli A., Fusco A., Testa I., Greco A. V., Lauro R. Effect of dexamethasone and cortisone on insulin receptors in normal human male. J Clin Endocrinol Metab. 1980 Sep;51(3):503–507. doi: 10.1210/jcem-51-3-503. [DOI] [PubMed] [Google Scholar]



