Abstract
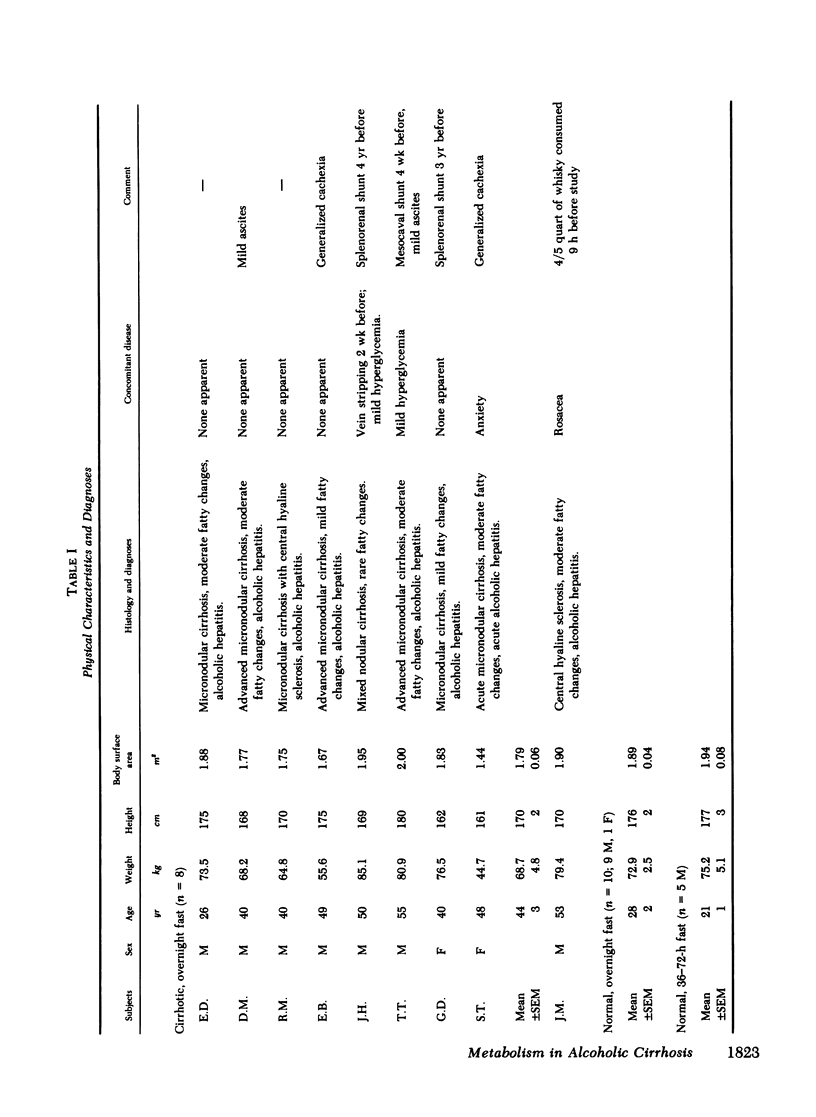
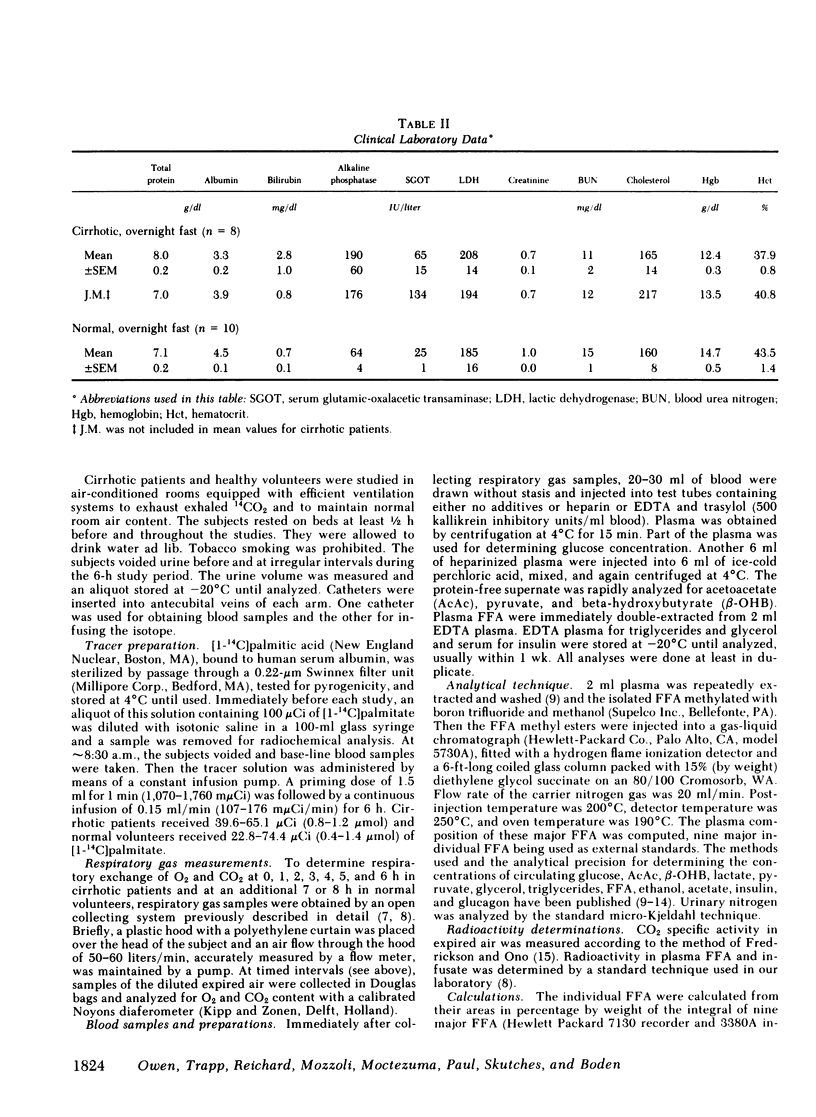
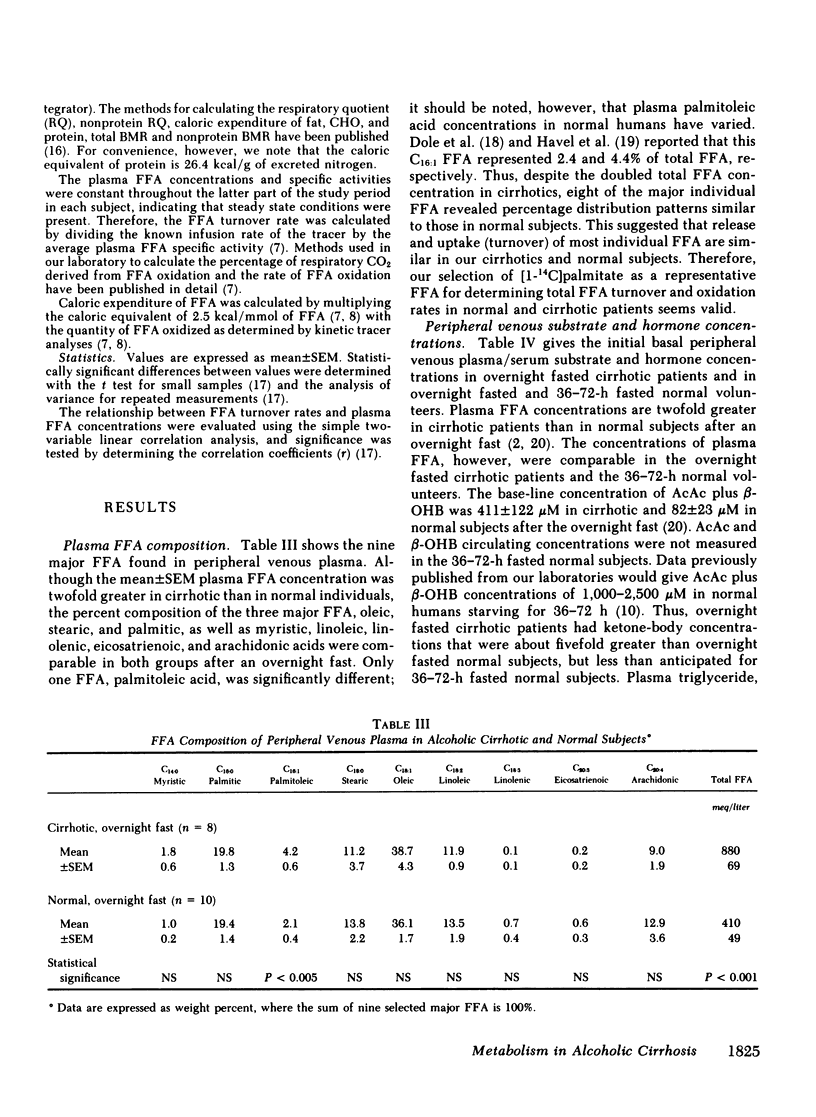
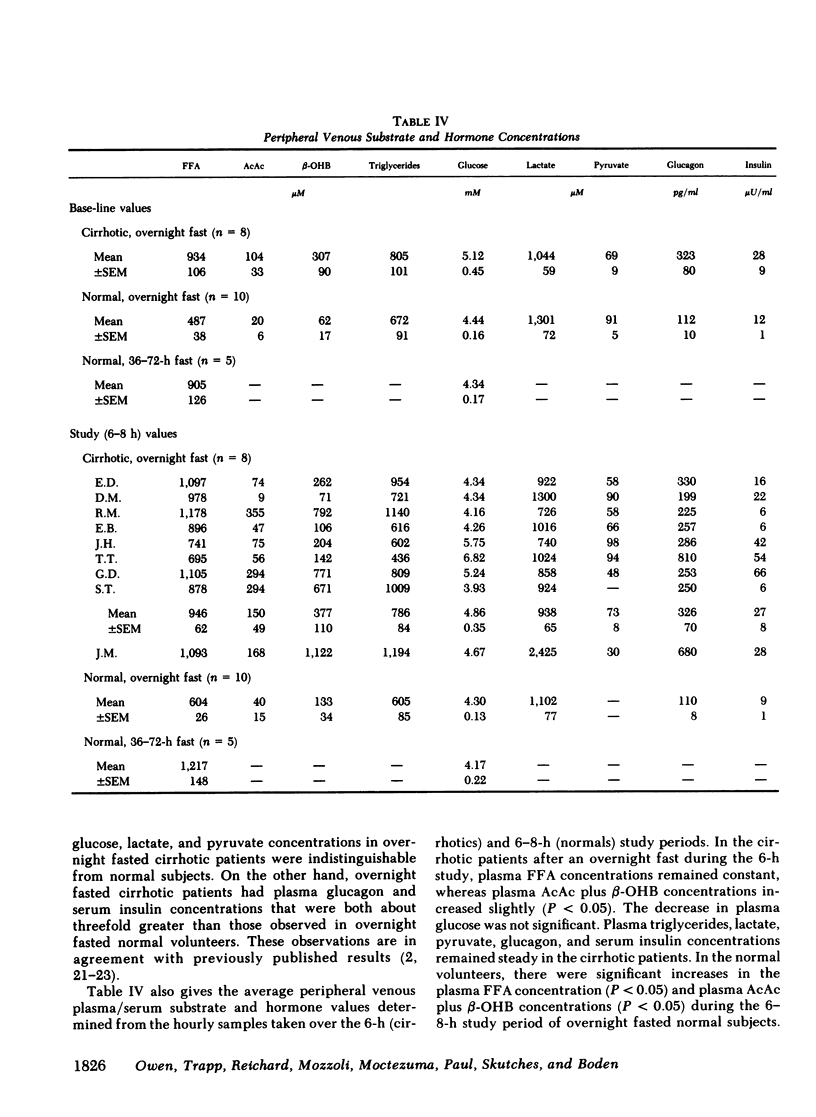
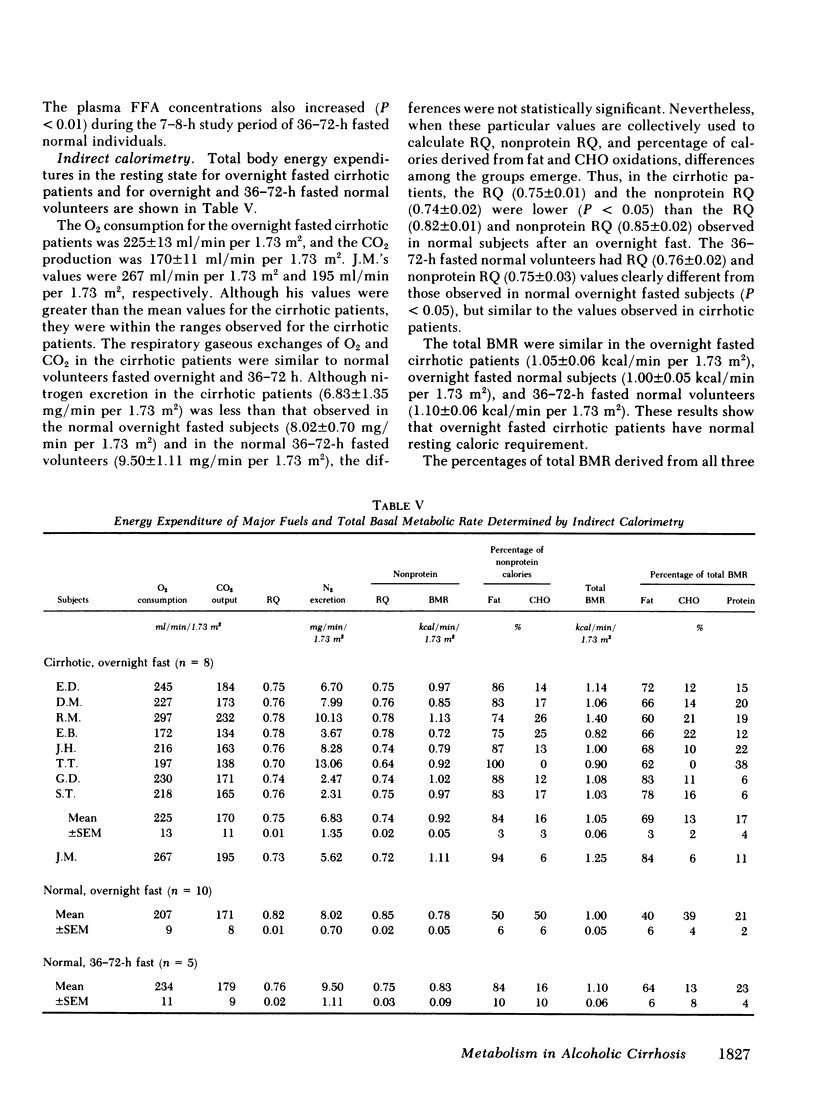
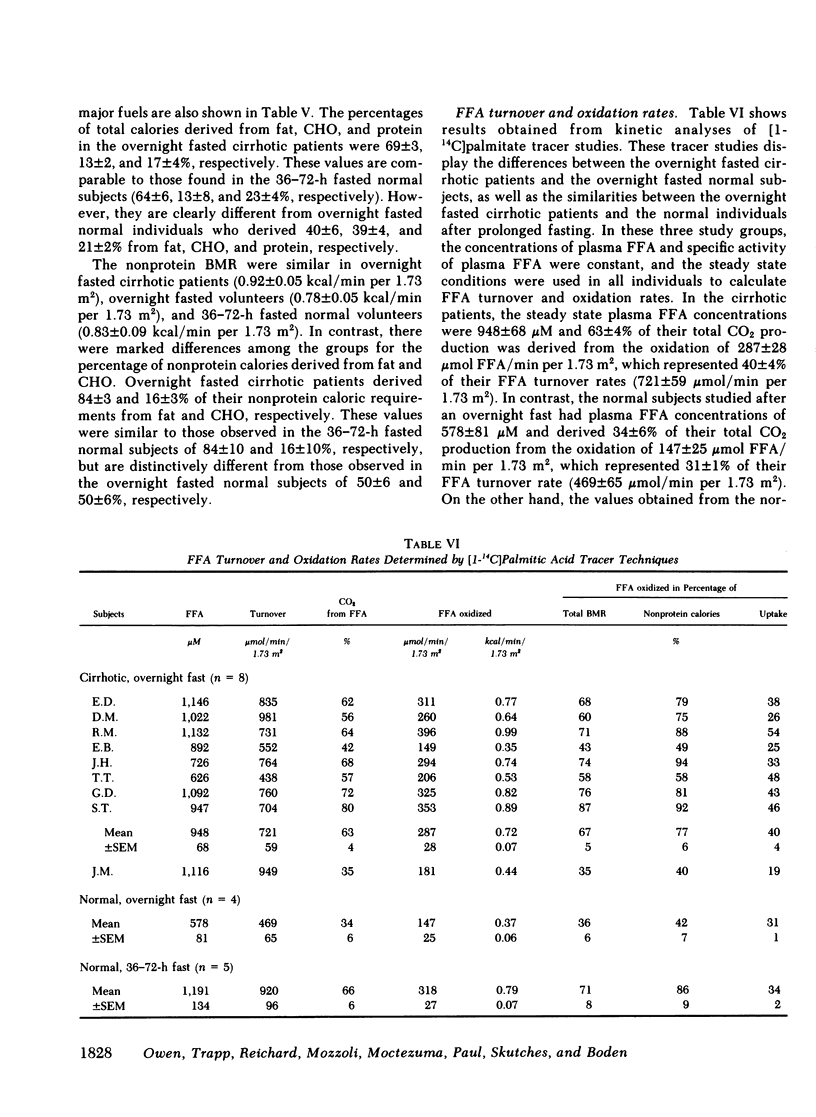
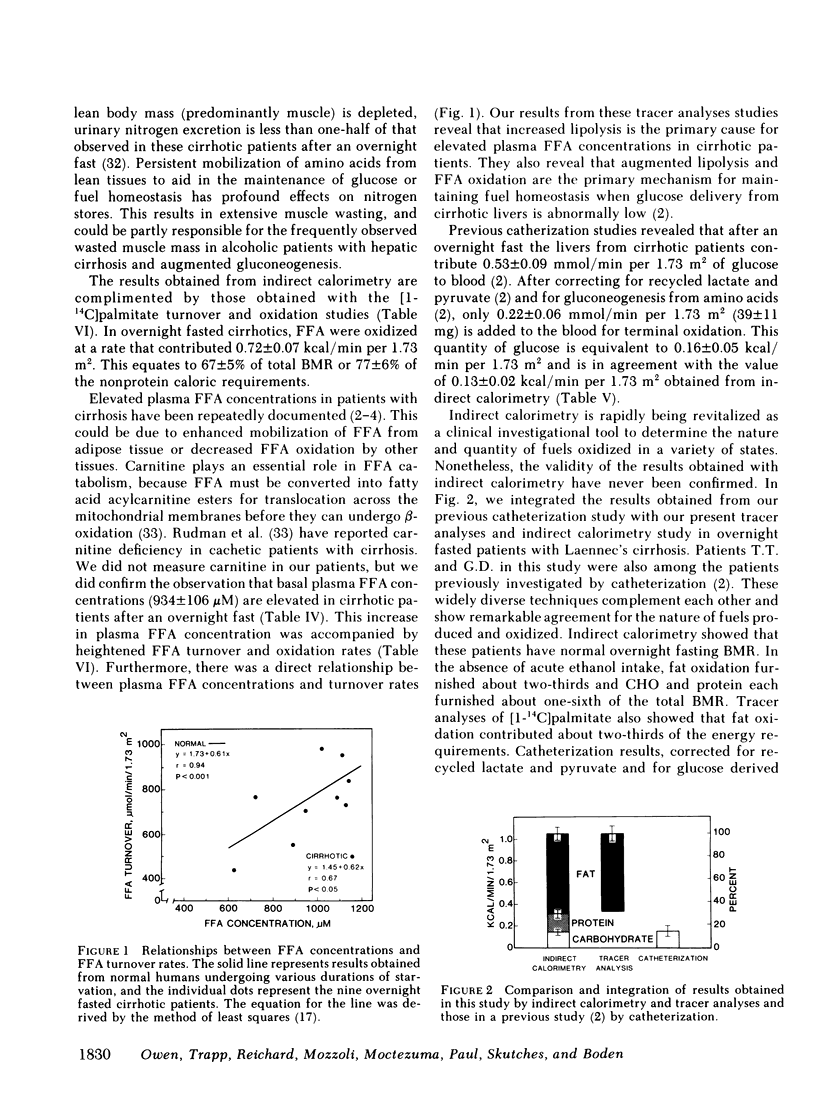
Although alcoholism is a leading cause of morbidity and mortality of middle-aged Americans, there are no data available pertaining to the consequences of Laennec's cirrhosis on total body energy requirements or mechanisms for maintaining fuel homeostasis in this patient population. Therefore, we simultaneously used the techniques of indirect calorimetry and tracer analyses of [14C]palmitate to measure the nature and quantity of fuels oxidized by patients with biopsy-proven alcoholic cirrhosis and compared the results with values obtained from health volunteers. Cirrhotic patients were studied after an overnight fast (10-12 h). Normal volunteers were studied after an overnight fast (12 h) or after a longer period of starvation (36-72 h). Total basal metabolic requirements were similar in overnight fasted cirrhotic patients (1.05 +/- 0.06 kcal/min per 1.73 m2), overnight fasted normal subjects (1.00 +/- 0.05 kcal/min per 1.73 m2), and 36-72-h fasted normal volunteers (1.10 +/- 0.06 kcal/min per 1.73 m2). Indirect calorimetry revealed that in cirrhotic patients the percentages of total calories derived from fat (69 +/- 3%), carbohydrate (13 +/- 2%), and protein (17 +/- 4%) were comparable to those found in 36-72-h fasted subjects, but were clearly different from those of overnight fasted normal individuals who derived 40 +/- 6, 39 +/- 4, and 21 +/- 2% from fat, carbohydrate, and protein, respectively. These data are strikingly similar to data obtained through tracer analyses of [14C]palmitate, which showed that in overnight fasted patients with alcoholic cirrhosis, 63 +/- 4% of their total CO2 production was derived from oxidation of 287 +/- 28 mumol free fatty acids (FFA)/min per 1.73 m2. In contrast, normal overnight fasted humans derived 34 +/- 6% of their total CO2 production from the oxidation of 147 +/- 25 mumol FFA/min per 1.73 m2. On the other hand, values obtained from the normal volunteers fasted 36-72 h were similar to the overnight fasted cirrhotic patients. These results show that after an overnight fast the caloric requirements of patients with alcoholic cirrhosis are normal, but the nature of fuels oxidized are similar to normal humans undergoing 2-3 d of total starvation. Thus, patients with alcoholic cirrhosis develop the catabolic state of starvation more rapidly than do normal humans. This disturbed but compensated pattern for maintaining fuel homeostasis may be partly responsible for the cachexia observed in some patients with alcoholic cirrhosis. This study also showed remarkably good agreement between the results obtained with indirect calorimetry and those obtained with 14C tracer analyses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Record C. O., Williamson D. H., Wright R. Metabolic changes in active chronic hepatitis. Clin Sci. 1972 May;42(5):591–605. doi: 10.1042/cs0420591. [DOI] [PubMed] [Google Scholar]
- BUCHER T., REDETZKI H. Eine spezifische photometrische Bestimmung von Athylalkohol auf fermentativen Wege. Klin Wochenschr. 1951 Sep 15;29(35-36):615–616. doi: 10.1007/BF01485653. [DOI] [PubMed] [Google Scholar]
- Berkowitz D. Glucose tolerance, free fatty acid, and serum insulin responses in patients with cirrhosis. Am J Dig Dis. 1969 Oct;14(10):691–699. doi: 10.1007/BF02233575. [DOI] [PubMed] [Google Scholar]
- Bichet D. G., Van Putten V. J., Schrier R. W. Potential role of increased sympathetic activity in impaired sodium and water excretion in cirrhosis. N Engl J Med. 1982 Dec 16;307(25):1552–1557. doi: 10.1056/NEJM198212163072504. [DOI] [PubMed] [Google Scholar]
- Conn H. O., Daughaday W. H. Cirrhosis and diabetes. V. Serum human growth hormone levels in Laennec's cirrhosis. J Lab Clin Med. 1970 Oct;76(4):678–688. [PubMed] [Google Scholar]
- DOLE V. P., JAMES A. T., WEBB J. P., RIZACK M. A., STURMAN M. F. The fatty acid patterns of plasma lipids during alimentary lipemia. J Clin Invest. 1959 Sep;38:1544–1554. doi: 10.1172/JCI103933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. F., Prudhoe K., Douglas A. P. Plasma cyclic adenosine-3', 5'-monophosphate response to glucagon in patients with liver disease. Br Med J. 1976 Apr 17;1(6015):931–933. doi: 10.1136/bmj.1.6015.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKSON D. S., ONO K. An improved technique for assay of C14O2 in expired air using the liquid scintillation counter. J Lab Clin Med. 1958 Jan;51(1):147–151. [PubMed] [Google Scholar]
- Flatt J. P. On the maximal possible rate of ketogenesis. Diabetes. 1972 Jan;21(1):50–53. doi: 10.2337/diab.21.1.50. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Menzel P. H., Boden G., Owen O. E. Hepatic ketogenesis and gluconeogenesis in humans. J Clin Invest. 1974 Oct;54(4):981–989. doi: 10.1172/JCI107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., CARLSON L. A., EKELUND L. G., HOLMGREN A. TURNOVER RATE AND OXIDATION OF DIFFERENT FREE FATTY ACIDS IN MAN DURING EXERCISE. J Appl Physiol. 1964 Jul;19:613–618. doi: 10.1152/jappl.1964.19.4.613. [DOI] [PubMed] [Google Scholar]
- Harewood M. S., Proietto J., Dudley F., Alford F. P. Insulin action and cirrhosis: insulin binding and lipogenesis in isolated adipocytes. Metabolism. 1982 Dec;31(12):1241–1246. doi: 10.1016/0026-0495(82)90011-7. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Cellular thermogenesis. Annu Rev Physiol. 1976;38:315–351. doi: 10.1146/annurev.ph.38.030176.001531. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Bortz W. M., Miller H. I., Paul P. Turnover rate of plasma FFA in humans and in dogs. Metabolism. 1967 Nov;16(11):1001–1009. doi: 10.1016/0026-0495(67)90093-5. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I., Bortz W. M. Oxidation of plasma FFA in lean and obese humans. Metabolism. 1968 Jan;17(1):62–73. doi: 10.1016/s0026-0495(68)80008-3. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Rubenstein A. H. Circulating glucagon. Plasma profiles and metabolism in health and disease. Diabetes. 1977 Sep;26(9):887–904. doi: 10.2337/diab.26.9.887. [DOI] [PubMed] [Google Scholar]
- Leatherdale B. A., Chase R. A., Rogers J., Alberti K. G., Davies P., Record C. O. Forearm glucose uptake in cirrhosis and its relationship to glucose tolerance. Clin Sci (Lond) 1980 Sep;59(3):191–198. doi: 10.1042/cs0590191. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Liver adaptation and injury in alcoholism. N Engl J Med. 1973 Feb 15;288(7):356–362. doi: 10.1056/NEJM197302152880710. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Pathogenesis and early diagnosis of alcoholic liver injury. N Engl J Med. 1978 Apr 20;298(16):888–893. doi: 10.1056/NEJM197804202981606. [DOI] [PubMed] [Google Scholar]
- MYERS J. D. Net splanchnic glucose production in normal man and in various disease states. J Clin Invest. 1950 Nov;29(11):1421–1429. doi: 10.1172/JCI102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco J., Diego J., Villanueva M. L., Diaz-Fierros M., Valverde I., Segovia J. M. Elevated plasma glucagon levels in cirrhosis of the liver. N Engl J Med. 1973 Nov 22;289(21):1107–1111. doi: 10.1056/NEJM197311222892103. [DOI] [PubMed] [Google Scholar]
- Megyesi C., Samols E., Marks V. Glucose tolerance and diabetes in chronic liver disease. Lancet. 1967 Nov 18;2(7525):1051–1056. doi: 10.1016/s0140-6736(67)90334-0. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Mozzoli M. A., Boden G., Patel M. S., Reichard G. A., Jr, Trapp V., Shuman C. R., Felig P. Substrate, hormone, and temperature responses in males and females to a common breakfast. Metabolism. 1980 Jun;29(6):511–523. doi: 10.1016/0026-0495(80)90076-1. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr Human forearm metabolism during progressive starvation. J Clin Invest. 1971 Jul;50(7):1536–1545. doi: 10.1172/JCI106639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Reichle F. A., Mozzoli M. A., Kreulen T., Patel M. S., Elfenbein I. B., Golsorkhi M., Chang K. H., Rao N. S., Sue H. S. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981 Jul;68(1):240–252. doi: 10.1172/JCI110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Trapp V. E., Reichard G. A., Jr, Mozzoli M. A., Smith R., Boden G. Effects of therapy on the nature and quantity of fuels oxidized during diabetic ketoacidosis. Diabetes. 1980 May;29(5):365–372. doi: 10.2337/diab.29.5.365. [DOI] [PubMed] [Google Scholar]
- Patek A. J., Jr, Toth I. G., Saunders M. G., Castro G. A., Engel J. J. Alcohol and dietary factors in cirrhosis. An epidemiological study of 304 alcoholic patients. Arch Intern Med. 1975 Aug;135(8):1053–1057. [PubMed] [Google Scholar]
- Proietto J., Alford F. P., Dudley F. J. The mechanism of the carbohydrate intolerance of cirrhosis. J Clin Endocrinol Metab. 1980 Nov;51(5):1030–1036. doi: 10.1210/jcem-51-5-1030. [DOI] [PubMed] [Google Scholar]
- Rudman D., Sewell C. W., Ansley J. D. Deficiency of carnitine in cachectic cirrhotic patients. J Clin Invest. 1977 Sep;60(3):716–723. doi: 10.1172/JCI108824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R., Joshi P., Hendler R., Felig P., Conn H. O. Hyperglucagonemia in Laennec's cirrhosis. The role of portal-systemic shunting. N Engl J Med. 1974 Jan 31;290(5):239–242. doi: 10.1056/NEJM197401312900502. [DOI] [PubMed] [Google Scholar]
- Skutches C. L., Holroyde C. P., Myers R. N., Paul P., Reichard G. A. Plasma acetate turnover and oxidation. J Clin Invest. 1979 Sep;64(3):708–713. doi: 10.1172/JCI109513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Ho P. W., Yeung R. T. Down-regulation of insulin receptors in postnecrotic cirrhosis of liver. J Clin Endocrinol Metab. 1982 Sep;55(3):524–530. doi: 10.1210/jcem-55-3-524. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Dobbs R. E., Orci L. Insulin, glucagon, and somatostatin secretion in the regulation of metabolism. Annu Rev Physiol. 1978;40:307–343. doi: 10.1146/annurev.ph.40.030178.001515. [DOI] [PubMed] [Google Scholar]
- Videla L., Bernstein J., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Increased oxidative capacity. Biochem J. 1973 Jun;134(2):507–514. doi: 10.1042/bj1340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C., Peterson W., Jr, Unger R. Blood ammonia levels in advanced cirrhosis during therapeutic elevation of the insulin: glucagon ratio. N Engl J Med. 1974 Jul 25;291(4):168–171. doi: 10.1056/NEJM197407252910403. [DOI] [PubMed] [Google Scholar]



