Abstract
Background
Live oral rotavirus vaccines have been less immunogenic and efficacious among children in poor developing countries compared with middle income and industrialized countries for reasons that are not yet completely understood. We assessed whether the neutralizing activity of breast milk could lower the titer of vaccine virus and explain this difference in vitro.
Methods
Breast milk samples were collected from mothers who were breast-feeding infants 4 to 29 weeks of age (ie, vaccine eligible age) in India (N = 40), Vietnam (N = 77), South Korea (N = 34), and the United States (N = 51). We examined breast milk for rotavirus-specific IgA and neutralizing activity against 3 rotavirus vaccine strains—RV1, RV5 G1, and 116E using enzyme immunoassays. The inhibitory effect of breast milk on RV1 was further examined by a plaque reduction assay.
Findings
Breast milk from Indian women had the highest IgA and neutralizing titers against all 3 vaccine strains, while lower but comparable median IgA and neutralizing titers were detected in breast milk from Korean and Vietnamese women, and the lowest titers were seen in American women. Neutralizing activity was greatest against the 2 vaccine strains of human origin, RV1 and 116E. This neutralizing activity in one half of the breast milk specimens from Indian women could reduce the effective titer of RV1 by ~2 logs, of 116E by 1.5 logs, and RV5 G1 strain by ~1 log more than that of breast milk from American women.
Interpretation
The lower immunogenicity and efficacy of rotavirus vaccines in poor developing countries could be explained, in part, by higher titers of IgA and neutralizing activity in breast milk consumed by their infants at the time of immunization that could effectively reduce the potency of the vaccine. Strategies to overcome this negative effect, such as delaying breast-feeding at the time of immunization, should be evaluated.
Keywords: rotavirus, RV1, RV5, neutralizing activity, breast milk
Rotavirus is the most common cause of severe diarrhea in children less than 5 years of age. Rotavirus disease is responsible for an estimated 527,000 deaths per year worldwide, with >85% of these deaths occurring in low-income countries.1,2 Two live oral vaccines, a monovalent attenuated human rotavirus strain, RV1 (Rotarix) and a pentavalent human-bovine rotavirus reassortants, RV5 (RotaTeq), have demonstrated high efficacy against severe diarrhea and hospitalization from rotavirus among young children in developed and middle income countries.3,4 They have now been licensed for use in many countries. However, both vaccines have demonstrated significantly lower immunogenicity and efficacy in low income countries of Africa and Asia. RV1 induced an immune response in >90% of infants in Finland,5 ~70% in Latin American,6 and 40% to 60% in South Africa, Malawi, Bangladesh, and India.7–10 Similarly, RV1 induced protection against severe rotavirus disease in >85% of children in Europe and Latin America, ~70% in South Africa and <50% in Malawi.11 Clinical trials of RV5 in Ghana, Kenya, Mali, Bangladesh, and Vietnam have also demonstrated significantly lower efficacy in these resource poor settings (G.E. Armah and K Zaman, personal communication). A postlicensure evaluation of RV5 in Nicaragua demonstrated vaccine effectiveness of ~60% against severe rotavirus disease,12 considerably lower than the efficacy of >90% in the United States and Finland. The lower immunogenicity and efficacy seen for the 2 new rotavirus vaccines is similar to observations made with early versions of rotavirus vaccines and other live oral vaccines—polio and cholera, when they were tested in populations living in low-income countries.13–17
The observed gradient between the immunogenicity and efficacy of rotavirus vaccines and the level of development of a country remains unexplained. One possible explanation of the lower efficacy of these vaccines in low income settings is that mothers more frequently breast-feed their infants in clinic, at the very time that the vaccine is orally administered. In addition, mothers in low income countries appear to have greater natural exposure to rotavirus, as reflected not only in higher titers of neutralizing activity in their breast milk but also higher titers of transplacental IgG in their infants.18 Together these could decrease the effective titer of vaccine virus reaching and replicating in the small intestine of children in low income countries, rendering the vaccine less effective.19,20
In this study, we investigated antibody profiles and neutralizing activities against rotavirus vaccine strains in breast milk specimens collected from lactating women in India, Vietnam, South Korea, and the United States. Our objectives were to assess the potential impact that breast-feeding might have to lower the immunogenicity and efficacy of live oral vaccine in developing countries. If the difference were significant, it would encourage trials to transiently delay breast-feeding at the time of immunization to see whether this strategy could improve the outcome of vaccination in poor developing countries.
METHODS
Subjects and Specimen Collection
From January 2007 to October 2007, we collected 5 to 10 mL of breast milk from mothers in India, Vietnam, Korea, and the United States who were breast-feeding their infants of 4 to 29 weeks, approximately the recommended age for the administration of rotavirus vaccine. Each specimen was assigned a unique identifier to maintain the anonymity of the donor and data on the ages of the mother and infant and the date of collection were recorded. All specimens were kept frozen at −70°C before being shipped and analyzed in the laboratory at CDC. Informed consent was obtained from all participants using the same study protocol. The protocol was reviewed and approved by the institutional review boards of each of the participating institutions. Because the Centers for Disease Control and Prevention (CDC) tested pre-existing, anonymous specimens, this research did not require review by the CDC IRB.
Detection of Rotavirus Specific Antibody
Rotavirus-specific IgA in breast milk samples was determined by ELISA as previously described.21,22 Briefly, microplate wells were coated with rabbit hyperimmune serum to rhesus rotavirus (RRV) and incubated with diluted RRV or blotto (5% skim milk in phosphate-buffered saline [PBS]); after washing, breast milk samples that were serially diluted from 1:2 to 1:2048 in diluent buffer (PBS supplemented with 1% skim milk and 0.5% [vol/vol] of 10% polyoxyethylene ether W1) were added to the wells, followed by horseradish peroxidase (HRP)-conjugated goat antihuman IgA antibodies (Sigma, US). After incubation and washing, the reactions were developed with tetramethylbenzidine (TMB, Sigma) and stopped with 1N HCl. Optical density (OD) was determined at 450 nm with an EIA reader (MRX Revelation, Dynex Technologies, Chantilly, VA). Antibody titers in breast milk were calculated as the reciprocal of the highest dilution that gave a mean OD greater than the cutoff value (3 standard deviations above the mean OD of the blotto wells).
Rotavirus-specific neutralizing activity in breast milk was measured by a microneutralization assay as previously described.21 Briefly, breast milk samples (50 µL) in 2-fold dilutions were mixed with an equal volume of trypsin activated vaccine virus (RV1, RV5 G1, or 116E) to yield a concentration of 4000 FFU/well and incubated at 37°C for 1 hours. Monolayers of MA104 cells grown in 96 well plates were washed with PBS and incubated with diluted the breast milk and virus mixture. Following incubation at 37°C for 1 hours, the plates were washed with PBS and incubated with 100 µL of Iscove’s Modified Dulbecco Medium containing 5 µg/mL trypsin. After 20 hours incubation at 37°C, the plates were fixed with 15 µL of 37% formaldehyde at 4°C for 30 minutes. Rotavirus antigen in the MA104 cells was detected by incubating plates with a rabbit anti-RRV hyperimmune serum, HRP-labeled antirabbit IgG, and then TMB. Neutralizing titer in a breast milk specimen was determined as the reciprocal of the highest dilution that showed a greater than 70% reduction in the absorbance value compared with that in virus-only controls. The cumulative frequency distribution of IgA titers and levels of neutralizing activity against rotavirus in breast milk obtained from women in the 4 countries were plotted.
Plaque Reduction Neutralization Test
Because RV1 demonstrated the highest reduction in virus titer with breast milk from India, we used a plaque reduction neutralization assay to simulate the neutralization of this vaccine virus that might occur in the mouth or gut of a child being breast-fed and simultaneously immunized. Approximately 100 PFU of trypsin-activated vaccine virus in 100 µL was incubated with an equal volume of serially diluted breast milk (4-fold) for 1 hour at 37°C. The mixtures were placed on a monolayer of MA104 cells in 6-well plates and after 1 hours incubation, the wells were overlaid with 0.3% agarose in minimum essential media containing 5 µg/mL of the trypsin. The plates were then stained with 2% neutral red and rotavirus plaques in duplicate wells were counted. The percent reduction in virus titer was calculated by comparing the numbers of plaques in wells of virus-breast milk mixture to those of virus-only controls.
Statistical Analysis
The Kolmogorov-Smirnov goodness-of-fit test was used to statistically compare the distribution of titers in specimens from each country. The Wilcoxon signed-rank test (SPSS 17.0 [SPSS Inc. Chicago, IL]) was used to analyze the difference in antibody titers among milk specimens from mothers in different countries.
RESULTS
A total of 202 breast milk specimens were collected from mothers with infants of 4 to 29 weeks of age in India (n = 40), Vietnam (77), Korea (34), and United States (51) (Table 1). The age of women from Korea, Vietnam, and the United States ranged from 16 to 41 years (median, 28 –35 years), whereas the ages of Indian women were not available. The ages of the infants from all 4 countries ranged from 1.0 to 6.6 months (median, 2.8 –3.5 months).
TABLE 1.
Collection of Breast Milk Specimens From 4 Countries
| Countries | No. Samples | Age of Mothers (yr) |
Age of Infants (mo) |
||
|---|---|---|---|---|---|
| Range | Median | Range | Median | ||
| United States | 51 | 16–41 | 35 | 1.4–6.6 | 2.8 |
| Korea | 34 | 26–34 | 31 | 1–5.4 | 3.15 |
| Vietnam | 77 | 21–41 | 28 | 2–6 | 3.5 |
| India | 40 | NA | NA | 1.2–5.7 | 3.1 |
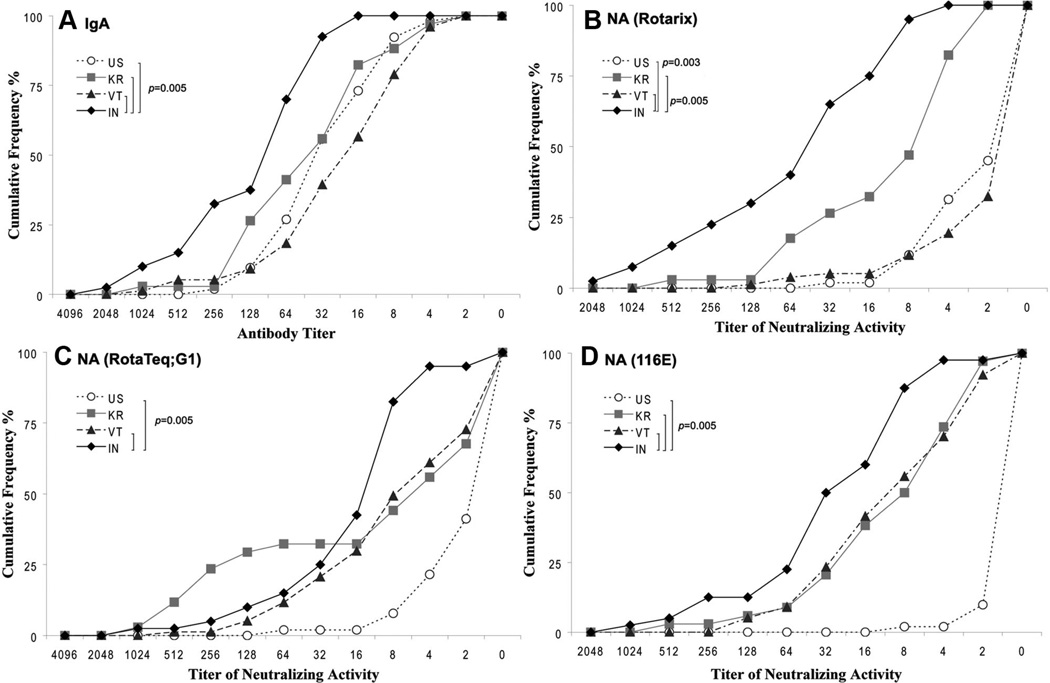
We first examined antirotavirus IgA titers in breast milk of mothers from the 4 countries (Fig. 1A). Almost all women had some IgA in their breast milk. The specimens from Indian women had the highest IgA titers (median, 64, range: 16–2048), whereas lower and comparable titers were detected in specimens from women in Vietnam (median, 16; range: 2–1024), Korea (median, 32; range: 2–1024) and the United States (median, 32, range: 2–512). The titers in Indian women were significantly greater than those of women from the 3 other countries (P = 0.005) and the maximum difference in median titer was about 4-fold.
FIGURE 1.
Cumulative frequency profiles of rotavirus-specific antibodies in breast milk specimens from mothers in India, Vietnam, Korea and the United States. Milk specimens were tested for IgA (A) and neutralizing activity against vaccine strains Rotarix (B), RotaTeq G1 (C), and 116E (D) as described in the text. Nearly all Indian women had an IgA titier >16 versus all other women. The median IgA and neutralizing titers and the distribution of titers for breast milk from Indian women were higher than all others. The values of significance between Indian women and those from Korea, Vietnam, and the United States are indicated. NA indicates neutralizing antibody.
We then measured neutralizing activity in breast milk against 3 rotavirus vaccine strains RV1, RV5 G1 and 116E (Figs. 1B–D). Against RV1 (Fig. 1B), breast milk titers from the Indian women were significantly greater than those of all other women; >50% had high levels (≥64) of neutralizing activity and >30% had a titer ≥128. By contrast, titers in Vietnamese and American mothers (median, 1:2) were significantly lower than the others, and titers of Korean women (median, 1:8) were intermediate. Breast milk of Indian women also had the highest neutralizing titers against RV5 G1 and 116E strains, followed by milk from Korean and Vietnamese women (Figs. 1C, D). Mothers in the United States had low or no neutralizing activity against RV5 G1 and 116E. Of note, the greatest difference in median neutralization titer between Indian and American mothers occurred in the human vaccine strains RV1 (median difference >32-fold) and 116E (16-fold) versus the RV5 G1 strain (~8-fold).
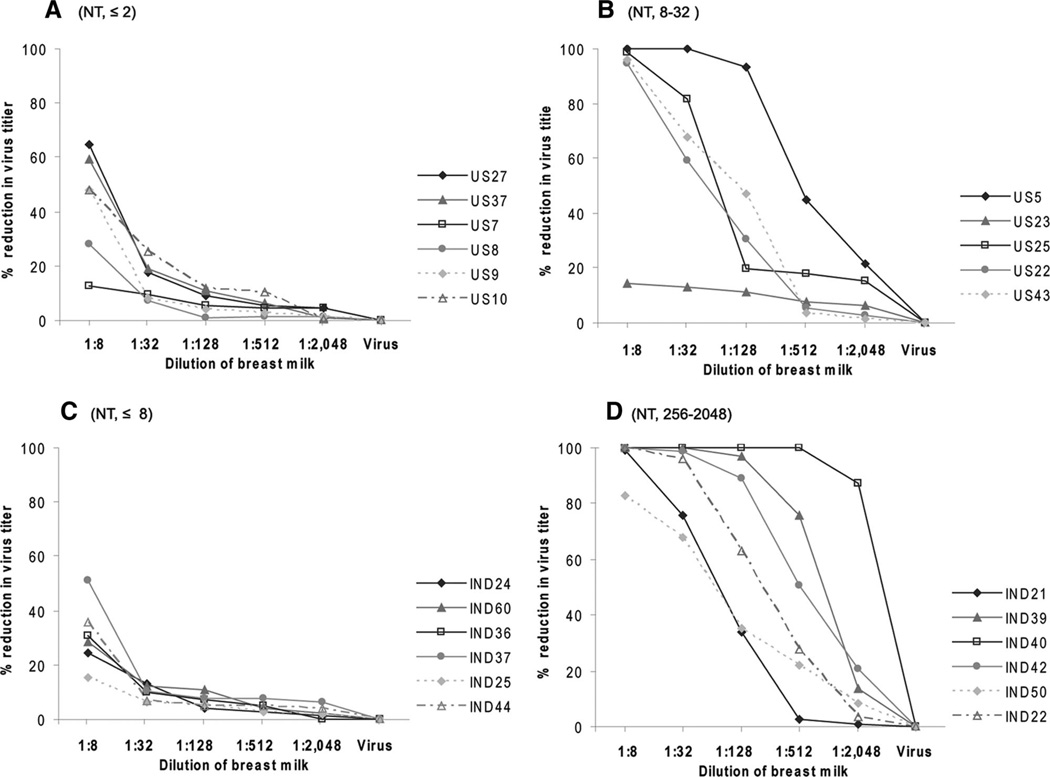
Finally, we selected breast milk specimens with low (≤8) and high (256 –2048) neutralizing titers from India and those with no (≤2) and high (8 –32) titers from the United States to determine how much these samples could reduce the titer of RV1 by using a plaque reduction assay (Fig. 2). Except for some nonspecific reduction observed at the 1:8 dilution, low titer milk specimens from both India and USA did not substantially reduce the titer of the vaccine virus (Figs. 2A, C). However, all but one specimen of high titer milk from India and the United States resulted in a reduction in the titer of RV1. Furthermore, the magnitude of reduction was dependent upon the level of neutralizing activity in the specimens (Figs. 2B, D). For example, at a 1:32 dilution, all 6 Indian specimens resulted in >70% reduction in titer, whereas only 2 of 5 specimens from United States led to a reduction of comparable magnitude. Even at a 1:512 dilution, a >50% reduction was observed with specimens from 3 of the 6 Indian women compared with none from American women.
FIGURE 2.
Reduction in titer of Rotarix by breast milk from India and United States. Selected breast milk specimens with low and high neutralizing titer from the United States (A, B) and India (C, D) were tested for reduction in viral titer as described in the text. While breast milk with low titers demonstrated only modest neutralization activity that was quenched with dilution, breast milk with higher titer, particularly those from India, could be diluted 1:128–1:512 and still neutralize the vaccine virus. NT indicates neutralizing titer.
DISCUSSION
New data on the lower immunogenicity and efficacy of RV1 and RV5 observed among children in low income countries has renewed concern about the performance of live oral rotavirus vaccines in these challenging settings where 85% of the 527,000 rotavirus deaths occur. Many hypotheses have been suggested to explain their lower performance, including differences in gut flora or levels of maternal antibody. A short delay of breast-feeding at the time of immunization might be the least complicated intervention to improve the efficacy of these vaccines.23 Our findings suggest that the neutralizing activity of breast milk could substantially reduce the potency and effectiveness of live oral rotavirus vaccines among infants in resource-poor countries where mothers often breast-feed in clinic at the very time that a vaccine is orally administered. Our results demonstrate that milk specimens from Indian mothers had significantly higher titers of neutralization activity against licensed vaccine strains than those of American, Korean, and Vietnamese women. This effect was more striking with the 2 vaccine strains of human origin, RV1 derived from the most commonly circulating rotavirus strain and 116E, a neonatal strain originally isolated in India, than the bovine-origin RV5 strain, although interference with the latter was also observed.
Results from clinical trials conducted in developed and middle income countries indicated that breast-feeding did not reduce the efficacy of either RV1 or RV5.3,4 However, the investigators only recorded whether or not an infant was breast-fed but not the interval between breast-feeding and the time the vaccine was administered. Our findings that mothers in America and Korea had no or only modest titers of neutralizing activity in breast milk also indicate that breast-feeding may not have a substantial negative impact in these high income settings. However, high titers of neutralizing activities in milk from mothers in low income countries like India could dramatically reduce the effective titers of vaccine in the gut and lead to significantly reduced immunogenicity and efficacy. Our data suggests that the depressed immune response to RV1 in Bangladesh, India, and South Africa and the ~50% efficacy of the vaccine in Malawi might be explained, in part, if infants consumed breast milk with high neutralizing activity at the very time of immunization.8,9,11,24 Lower efficacy was also reported for the early candidate bovine rotavirus vaccine RIT in African and Asian countries15,16 and RV5 in Nicaragua,12 Bangladesh, Ghana, and Mali. Of interest, antibody profiles in breast milk specimens from Vietnamese women were more similar to those in American women than Indian mothers. This finding corroborates other studies in which the epidemiology of rotavirus in Vietnam was strikingly more similar to that in developed countries than in the developing world.25 Of note, the efficacy of RV5 was high in Vietnam compared with the other 4 low income countries (Bangladesh, Ghana, Malawi and Nicaragua) where it was tested. Thus, the potential negative impact of breast-feeding is likely to vary by setting and could be greatest in poor developing country settings with the highest burden of rotavirus and frequent maternal exposure to natural rotavirus infection.
Some caveats should be considered in interpreting our data. First, we only examined antibody and neutralizing activity in breast milk from mothers in 4 countries; more specimens from other parts of the world including Africa and Asia should be examined to fully assess the potential negative impact of breast milk on live oral rotavirus vaccines in different settings. Second, while the observed high titers of antibody and in vitro neutralization activity of breast milk suggest a potential for substantial impact on vaccine performance, it is not possible to directly translate these findings into the real-world impact as this will be affected by many other factors such as the timing and amount of breast milk in the gut at the time of vaccination. Titers of neutralizing activity and antibodies decrease over time, so the effect we measure could also change over time or be neglected by administering multiple doses of the vaccine, perhaps at a time when the infant is not breast-feeding. Finally, we did not assess other factors such as interference of multiple bacterial and viral agents and different enteropathology in the gut of children in poor developing countries that might also potentially inhibit vaccine performance.
In conclusion, our findings indicate that the neutralizing activity of breast milk could be one of the many factors that might explain the lower observed immunogenicity and effectiveness of live oral rotavirus vaccines among children in developing countries. These data should encourage clinical trials to investigate whether delaying breast-feeding for a short period before and after giving the vaccine could reasonably improve the immune response and protective efficacy. Since all live oral rotavirus vaccines are potentially susceptible to interference from breast milk neutralizing activity and other factors such as maternal antibody and other enteric flora, a parenteral vaccine with nonliving rotavirus (eg, inactivated vaccine) should be pursued as an alternative that will provide an insurance policy to the global immunization agenda against rotaviruses.26
ACKNOWLEDGMENTS
The authors thank Drs. H. F. Clark, Guillermo M. Ruiz-Palacios, and Jon R. Gentsch for providing individual RV5, RV1, and 116E strains, respectively.
Supported (in part) by CDC program funds. Also supported (in part) by the NIH National Center for Research Resources, grants K12RR017643 and 1KL2RR025009 (to S.A.L.).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of CDC.
REFERENCES
- 1.Parashar UD, Gibson CJ, Bresse JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285–295. [PubMed] [Google Scholar]
- 3.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Karvonen A, Puustinen L, et al. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23:937–943. doi: 10.1097/01.inf.0000141722.10130.50. [DOI] [PubMed] [Google Scholar]
- 6.Salinas B, Perez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: a randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24:807–816. doi: 10.1097/01.inf.0000178294.13954.a1. [DOI] [PubMed] [Google Scholar]
- 7.Steele AD, De Vos B, Tumbo J, et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. doi: 10.1016/j.vaccine.2008.08.034. In press. [DOI] [PubMed] [Google Scholar]
- 8.Zaman K, Sack DA, Yunus M, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27:1333–1339. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 9.Narang A, Bose A, Pandit AN, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5:414–419. doi: 10.4161/hv.5.6.8176. [DOI] [PubMed] [Google Scholar]
- 10.Meeting of the immunization Strategic Advisory Group of Experts, April 2009—conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:220–236. [PubMed] [Google Scholar]
- 11.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 12.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 13.Gunn RA, Kimball AM, Pollard RA, et al. Bottle feeding as a risk factor for cholera in infants. Lancet. 1979;2:730–732. doi: 10.1016/s0140-6736(79)90653-6. [DOI] [PubMed] [Google Scholar]
- 14.John TJ, Devarajan LV, Luther L, et al. Effect of breast-feeding on seroresponse of infants to oral poliovirus vaccination. Pediatrics. 1976;57:47–53. [PubMed] [Google Scholar]
- 15.Hanlon P, Hanlon L, Marsh V, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1:1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 16.De Mol P, Zissis G, Butzler JP, et al. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986;2:108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- 17.Lanata CF, Black RE, del Aguila R, et al. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989;159:452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- 18.Ray PG, Kelkar SD. Prevalence of neutralizing antibodies against different rotavirus serotypes in children with severe rotavirus-induced diarrhea and their mothers. Clin Diagn Lab Immunol. 2004;11:186–194. doi: 10.1128/CDLI.11.1.186-194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass RI, Stoll BJ, Wyatt RG, et al. Observations questioning a protective role for breast-feeding in severe rotavirus diarrhea. Acta Paediatr Scand. 1986;75:713–718. doi: 10.1111/j.1651-2227.1986.tb10279.x. [DOI] [PubMed] [Google Scholar]
- 20.Glass RI, Ing DJ, Stoll BJ, et al. Immune response to rotavirus vaccines among breast-fed and nonbreast-fed children. Adv Exp Med Biol. 1991;310:249–254. doi: 10.1007/978-1-4615-3838-7_33. [DOI] [PubMed] [Google Scholar]
- 21.Jiang B, Estes MK, Barone C, et al. Heterotypic protection from rotavirus infection in mice vaccinated with virus-like particles. Vaccine. 1999;17:1005–1013. doi: 10.1016/s0264-410x(98)00317-x. [DOI] [PubMed] [Google Scholar]
- 22.Jiang B, Wang Y, Saluzzo JF, et al. Immunogenicity of a thermally inactivated rotavirus vaccine in mice. Hum Vaccin. 2008;4:143–147. doi: 10.4161/hv.4.2.5263. [DOI] [PubMed] [Google Scholar]
- 23.Patel M, Shane AL, Parashar UD, et al. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200(suppl 1):S39–S48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steele AD, De Vos B, Tumbo J, et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. doi: 10.1016/j.vaccine.2008.08.034. In press. [DOI] [PubMed] [Google Scholar]
- 25.Van Man N, Luan le T, Trach DD, et al. Epidemiological profile and burden of rotavirus diarrhea in Vietnam: 5 years of sentinel hospital surveillance, 1998–2003. J Infect Dis. 2005;192(suppl 1):S127–S132. doi: 10.1086/431501. [DOI] [PubMed] [Google Scholar]
- 26.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26:6754–6758. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]