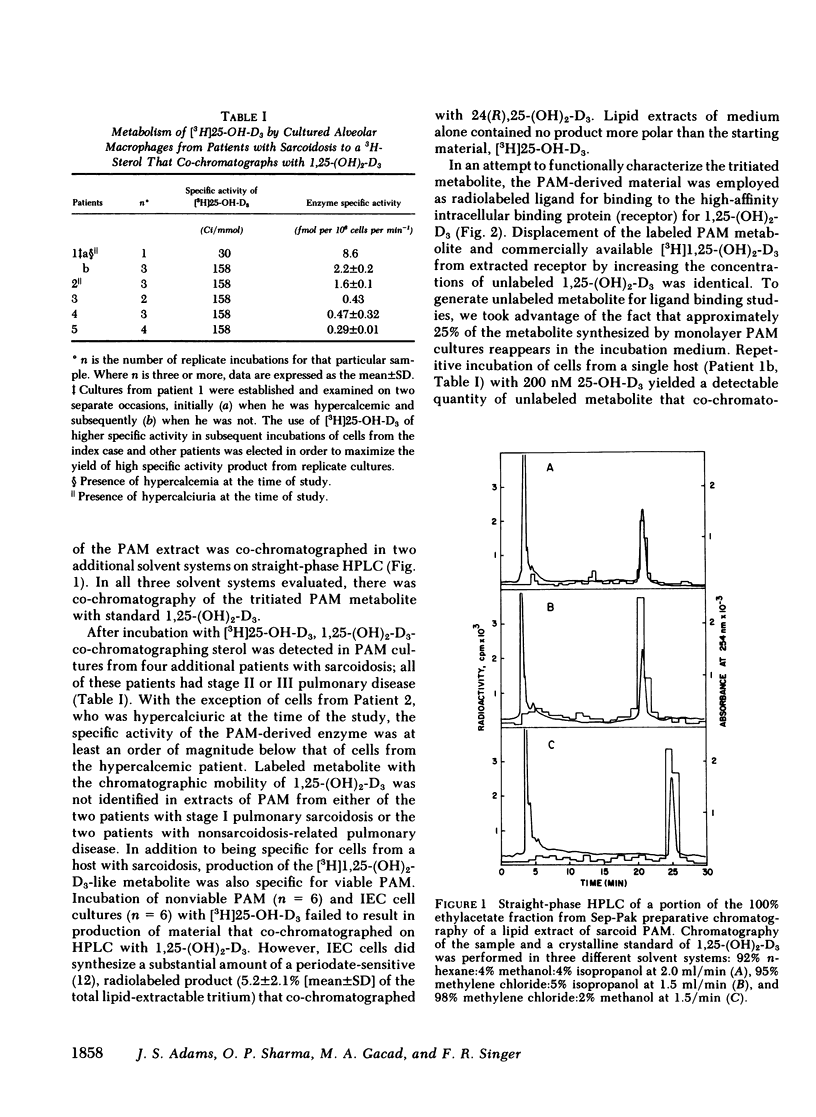
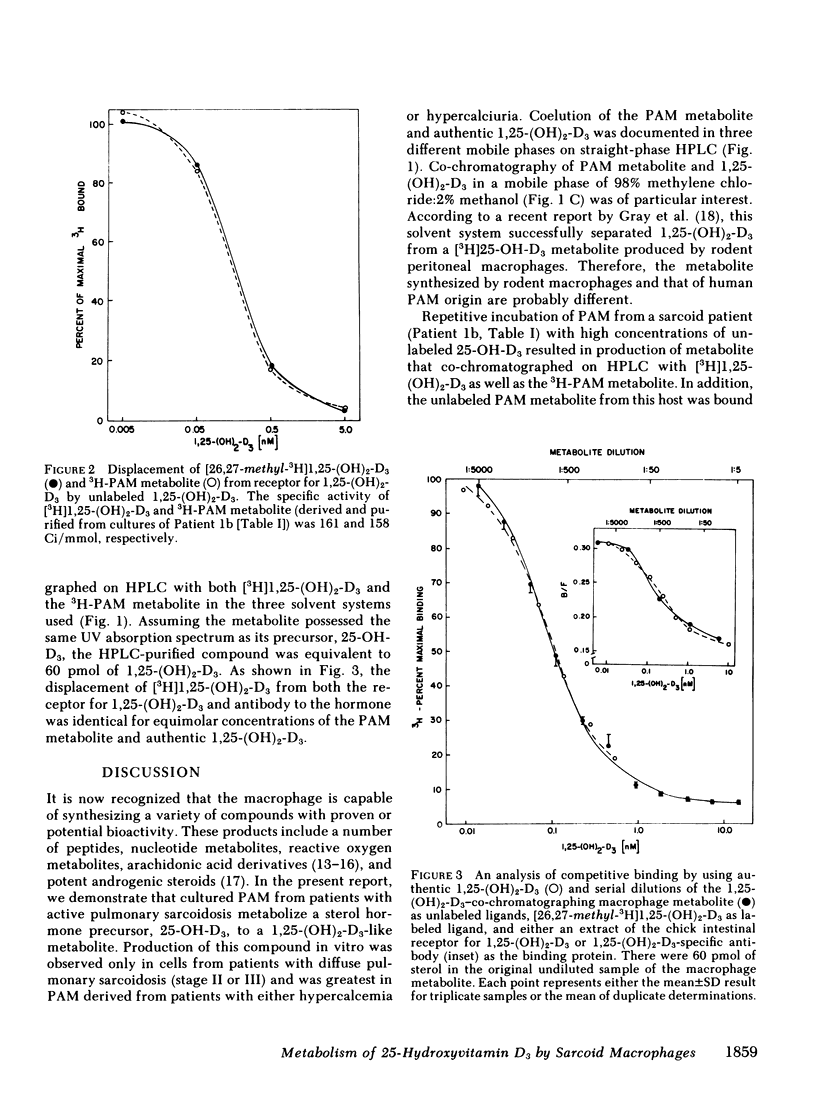
Abstract
Metabolism of [3H]25-hydroxyvitamin D3(25-OH-D3) was studied in primary cultures of pulmonary alveolar macrophages (PAM) from seven patients with sarcoidosis and two patients with idiopathic pulmonary fibrosis. Production of a [3H]1,25-dihydroxyvitamin D3 (1,25-[OH]2-D3)-like metabolite of [3H]25-OH-D3 was detected in lipid extracts of cells from five patients with sarcoidosis. Synthesis of this compound in vitro was limited to viable PAM and was greatest in cells derived from a patient with hypercalcemia and an elevated serum concentration of 1,25-dihydroxyvitamin D. The tritiated PAM metabolite coeluted with authentic 1,25-(OH)2-D3 in three different solvent systems on straight-phase high performance liquid chromatography (HPLC) and demonstrated binding to extracted receptor for 1,25-(OH)2-D3, which was identical to that of commercially available [3H]1,25-(OH)2-D3 of comparable specific activity. Incubation of PAM with high concentrations of 25-OH-D3 resulted in production of an unlabeled metabolite that co-chromatographed with the 3H-PAM metabolite on HPLC and that was bound with high affinity by both the specific receptor for 1,25-(OH)2-D3 and antiserum to 1,25-(OH)2-D3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Clemens T. L., Holick M. F. Silica Sep-Pak preparative chromatography for vitamin D and its metabolites. J Chromatogr. 1981 Nov 13;226(1):198–201. doi: 10.1016/s0378-4347(00)84221-8. [DOI] [PubMed] [Google Scholar]
- Adams J. S., Clemens T. L., Horiuchi N., Quaroni A., Holick M. F. Demonstration of a receptor-like binding protein for 1,25-(OH)2-D3 in cultured intestinal epithelial cells from the adult rat. FEBS Lett. 1982 Jun 7;142(2):247–250. doi: 10.1016/0014-5793(82)80145-2. [DOI] [PubMed] [Google Scholar]
- Barbour G. L., Coburn J. W., Slatopolsky E., Norman A. W., Horst R. L. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981 Aug 20;305(8):440–443. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- Bell N. H., Stern P. H., Pantzer E., Sinha T. K., DeLuca H. F. Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979 Jul;64(1):218–225. doi: 10.1172/JCI109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens T. L., Hendy G. N., Graham R. F., Baggiolini E. G., Uskokovic M. R., O'Riordan J. L. A radioimmunoassay for 1,25-dihydroxycholecalciferol. Clin Sci Mol Med. 1978 Mar;54(3):329–332. doi: 10.1042/cs0540329. [DOI] [PubMed] [Google Scholar]
- Gray T. K., Maddux F. W., Lester G. E., Williams M. E. Rodent macrophages metabolize 25-hydroxyvitamin D3 in vitro. Biochem Biophys Res Commun. 1982 Dec 15;109(3):723–729. doi: 10.1016/0006-291x(82)92000-9. [DOI] [PubMed] [Google Scholar]
- Gupta R. G., Oparil S., Szidon J., Daise M. Clinical significance of serum angiotensin-converting enzyme levels in sarcoidosis. J Lab Clin Med. 1979 Jun;93(6):940–949. [PubMed] [Google Scholar]
- Hinman L. M., Stevens C., Matthay R. A., Bernard J., Gee L. Angiotensin convertase activities in human alveolar macrophages: effects of cigarette smoking and sarcoidosis. Science. 1979 Jul 13;205(4402):202–203. doi: 10.1126/science.221980. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Gray R. W., Boyle I. T., Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972 Nov 7;11(23):4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Szapiel S. V., Strumpf I. J., Kawanami O., Ferrans V. J., Keogh B. A., Crystal R. G. The human alveolar macrophage. Methods Cell Biol. 1980;21A:95–105. doi: 10.1016/s0091-679x(08)60760-8. [DOI] [PubMed] [Google Scholar]
- James D. G., Williams W. J. Immunology of sarcoidosis. Am J Med. 1982 Jan;72(1):5–8. doi: 10.1016/0002-9343(82)90564-2. [DOI] [PubMed] [Google Scholar]
- MAYOCK R. L., BERTRAND P., MORRISON C. E., SCOTT J. H. MANIFESTATIONS OF SARCOIDOSIS. ANALYSIS OF 145 PATIENTS, WITH A REVIEW OF NINE SERIES SELECTED FROM THE LITERATURE. Am J Med. 1963 Jul;35:67–89. doi: 10.1016/0002-9343(63)90165-7. [DOI] [PubMed] [Google Scholar]
- Milewich L., Kaimal V., Toews G. B. Androstenedione metabolism in human alveolar macrophages. J Clin Endocrinol Metab. 1983 May;56(5):920–924. doi: 10.1210/jcem-56-5-920. [DOI] [PubMed] [Google Scholar]
- Quaroni A., May R. J. Establishment and characterizaton of intestinal epithelial cell cultures. Methods Cell Biol. 1980;21B:403–427. [PubMed] [Google Scholar]
- Werb Z. How the macrophage regulates its extracellular environment. Am J Anat. 1983 Mar;166(3):237–256. doi: 10.1002/aja.1001660302. [DOI] [PubMed] [Google Scholar]
- Winnacker J. L., Becker K. L., Katz S. Endocrine aspects of sarcoidosis. N Engl J Med. 1968 Feb 22;278(8):427–434. doi: 10.1056/NEJM196802222780805. [DOI] [PubMed] [Google Scholar]



