Abstract
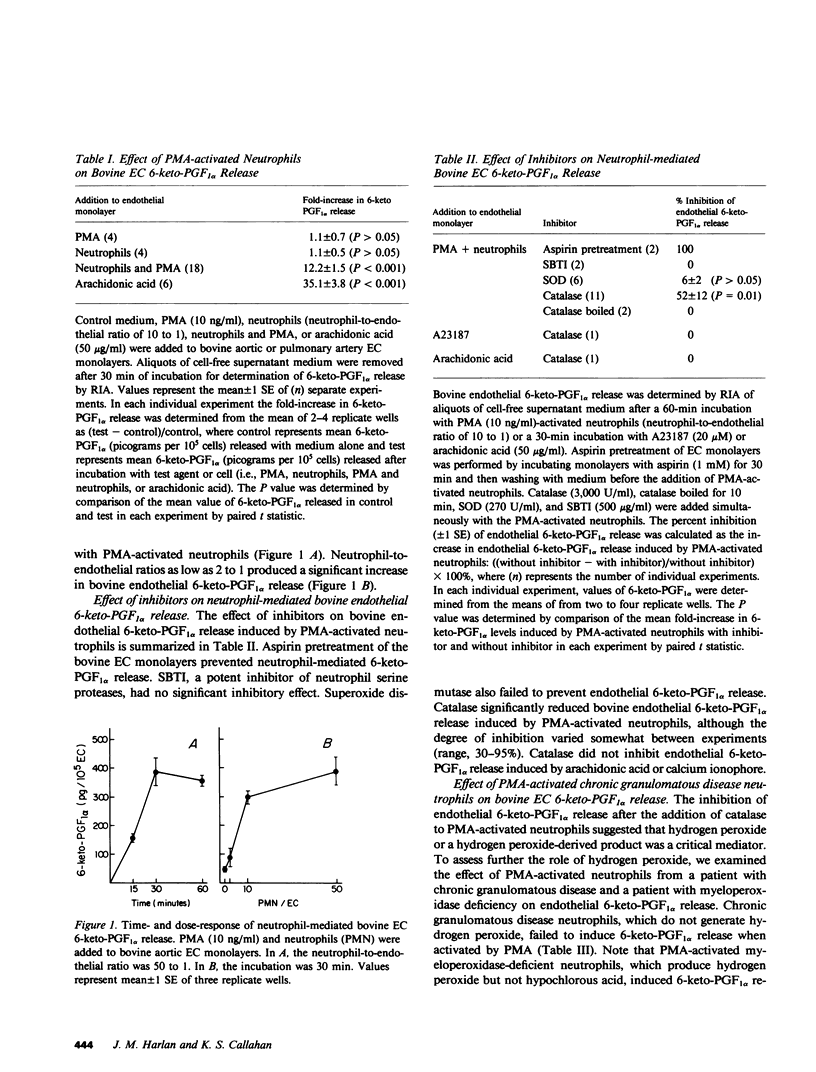
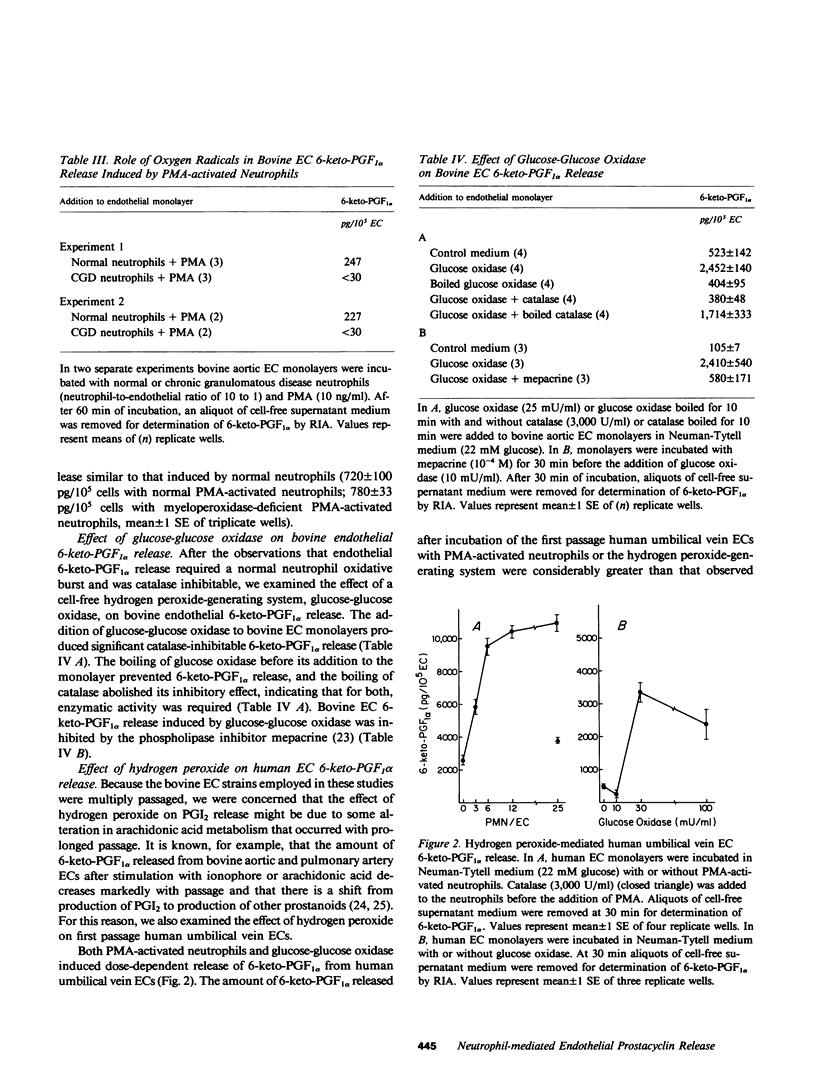
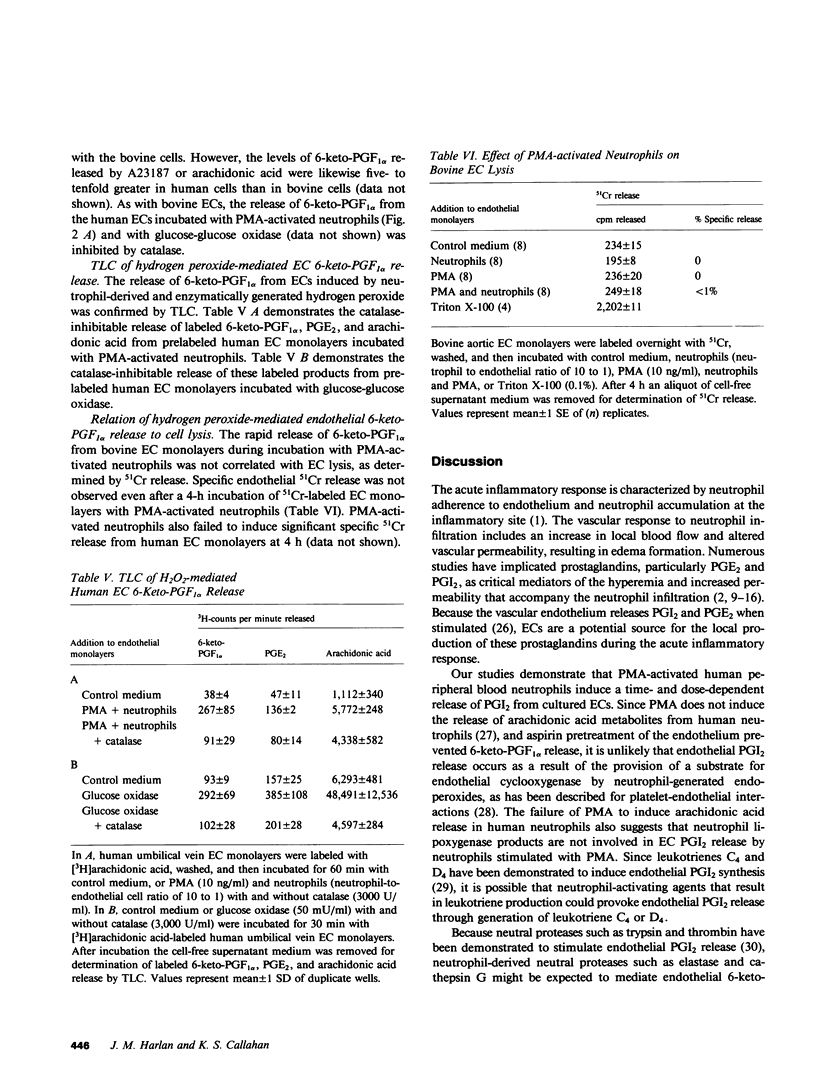
We have examined the effect of activated neutrophils on the release of prostacyclin (PGI2) from cultured endothelial cells by radioimmunoassay and thin layer chromatography of its stable metabolite, 6-keto-prostaglandin F1 alpha (6-keto-PGF1 alpha). Phorbol myristate acetate-activated neutrophils induced a time- and dose-dependent release of 6-keto-PGF1 alpha from human and bovine endothelial cell monolayers, whereas phorbol myristate acetate alone and neutrophils alone did not. Pretreatment of the endothelial cells with aspirin prevented neutrophil-mediated 6-keto-PGF1 alpha release, indicating that it did not depend upon neutrophil-generated endoperoxides. Phorbol myristate acetate-activated neutrophils from a patient with chronic granulomatous disease failed to induce endothelial 6-keto-PGF1 alpha release. Addition of catalase but not of superoxide dismutase significantly reduced human and bovine endothelial 6-keto-PGF1 alpha release by phorbol myristate acetate-activated neutrophils. Catalase-inhibitable endothelial 6-keto-PGF1 alpha release was also observed after the addition of the hydrogen peroxide-generating system, glucose-glucose oxidase, to bovine and human endothelial cell monolayers. Bovine endothelial 6-keto-PGF1 alpha release induced by exogenously generated hydrogen peroxide was attenuated by the phospholipase inhibitor mepacrine, suggesting that hydrogen peroxide may act by triggering endothelial membrane phospholipase activation. The release of 6-keto-PGF1 alpha by enzymatically or neutrophil-generated hydrogen peroxide was not associated with endothelial cell lysis as assessed by 51Cr release. We conclude that exogenously generated hydrogen peroxide or a hydrogen peroxide-derived product mediates rapid nonlytic release of PGI2 from cultured endothelial cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhenc-Gelas F., Tsai S. J., Callahan K. S., Campbell W. B., Johnson A. R. Stimulation of prostaglandin formation by vasoactive mediators in cultured human endothelial cells. Prostaglandins. 1982 Nov;24(5):723–742. doi: 10.1016/0090-6980(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Benjamin C. W., Hopkins N. K., Oglesby T. D., Gorman R. R. Agonist specific desensitization of leukotriene C4-stimulated PGI2 biosynthesis in human endothelial cells. Biochem Biophys Res Commun. 1983 Dec 28;117(3):780–787. doi: 10.1016/0006-291x(83)91665-0. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Schmidt M., Yoder M., Baehner R. L. Inhibition of polymorphonuclear leukocyte adherence by prostacyclin. J Lab Clin Med. 1980 May;95(5):672–678. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Egan R. W., Paxton J., Kuehl F. A., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [PubMed] [Google Scholar]
- Flick M. R., Perel A., Staub N. C. Leukocytes are required for increased lung microvascular permeability after microembolization in sheep. Circ Res. 1981 Mar;48(3):344–351. doi: 10.1161/01.res.48.3.344. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Walker J. R., Davidson E. M., Smith M. J. PGI2: a potential mediator of inflammation. Prostaglandins. 1978 Aug;16(2):253–258. doi: 10.1016/0090-6980(78)90027-8. [DOI] [PubMed] [Google Scholar]
- Goldsmith J. C., Jafvert C. T., Lollar P., Owen W. G., Hoak J. C. Prostacyclin release from cultured and ex vivo bovine vascular endothelium. Studies with thrombin, arachidonic acid, and ionophore A23187. Lab Invest. 1981 Aug;45(2):191–197. [PubMed] [Google Scholar]
- Goldsmith J. C., Needleman S. W. A comparative study of thromboxane and prostacyclin release from ex vivo and cultured bovine vascular endothelium. Prostaglandins. 1982 Aug;24(2):173–178. doi: 10.1016/0090-6980(82)90143-5. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Striker G. E., Weaver L. J. Effects of lipopolysaccharide on human endothelial cells in culture. Thromb Res. 1983 Jan 1;29(1):15–26. doi: 10.1016/0049-3848(83)90121-4. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Cook H. W., Lands W. E. Prostaglandin biosynthesis can be triggered by lipid peroxides. Arch Biochem Biophys. 1979 Apr 1;193(2):340–345. doi: 10.1016/0003-9861(79)90038-9. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Lands W. E. Evidence for a peroxide-initiated free radical mechanism of prostaglandin biosynthesis. J Biol Chem. 1980 Jul 10;255(13):6253–6261. [PubMed] [Google Scholar]
- Higgs E. A., Moncada S., Vane J. R. Inflammatory effects of prostacyclin (PGI2) and 6-oxo-PGF1alpha in the rat paw. Prostaglandins. 1978 Aug;16(2):153–162. doi: 10.1016/0090-6980(78)90018-7. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh P. D., Hillis L. D., Campbell W. B., Firth B. G., Willerson J. T. Release of prostaglandins and thromboxane into the coronary circulation in patients with ischemic heart disease. N Engl J Med. 1981 Mar 19;304(12):685–691. doi: 10.1056/NEJM198103193041201. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C. Vascular responses during acute neutrophilic inflammation. Their relationship to in vivo neutrophil emigration. Lab Invest. 1981 Nov;45(5):435–441. [PubMed] [Google Scholar]
- Johnson A., Malik A. B. Effect of granulocytopenia on extravascular lung water content after microembolization. Am Rev Respir Dis. 1980 Oct;122(4):561–566. doi: 10.1164/arrd.1980.122.4.561. [DOI] [PubMed] [Google Scholar]
- Kent R. S., Diedrich S. L., Whorton A. R. Regulation of vascular prostaglandin synthesis by metabolites of arachidonic acid in perfused rabbit aorta. J Clin Invest. 1983 Aug;72(2):455–465. doi: 10.1172/JCI110993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoriya K., Ohmori H., Azuma A., Kurozumi S., Hashimoto Y., Nicolaou K. C., Barnette W. E., Magolda R. L. Prostaglandin I2 as a potentiator of acute inflammation in rats. Prostaglandins. 1978 Apr;15(4):557–564. doi: 10.1016/0090-6980(78)90052-7. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A., Broekman M. J. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980 Nov;66(5):979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S. Prostacyclin and arterial wall biology. Arteriosclerosis. 1982 May-Jun;2(3):193–207. doi: 10.1161/01.atv.2.3.193. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Moncada S. Prostacyclin release and the modulation of some vasoactive hormones. Prostaglandins. 1980 Jul;20(1):25–49. doi: 10.1016/0090-6980(80)90004-0. [DOI] [PubMed] [Google Scholar]
- Polgar P., Taylor L. Stimulation of prostaglandin synthesis by ascorbic acid via hydrogen peroxide formation. Prostaglandins. 1980 May;19(5):693–700. doi: 10.1016/0090-6980(80)90168-9. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Taylor L., Menconi M. J., Polgar P. The participation of hydroperoxides and oxygen radicals in the control of prostaglandin synthesis. J Biol Chem. 1983 Jun 10;258(11):6855–6857. [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Quadracci L. J., Striker G. E. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol. 1978 Aug;96(2):203–213. doi: 10.1002/jcp.1040960209. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Waite B. M., Thomas M. J., DeChatelet L. R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981 Jul 25;256(14):7228–7234. [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Turk J., Needleman P. A mechanism for the hydroperoxide-mediated inactivation of prostacyclin synthetase. Blood. 1979 Jun;53(6):1191–1196. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Bralove D. A., Gallin J. I. The differential mobilization of human neutrophil granules. Effects of phorbol myristate acetate and ionophore A23187. Am J Pathol. 1977 May;87(2):273–284. [PMC free article] [PubMed] [Google Scholar]



