Abstract
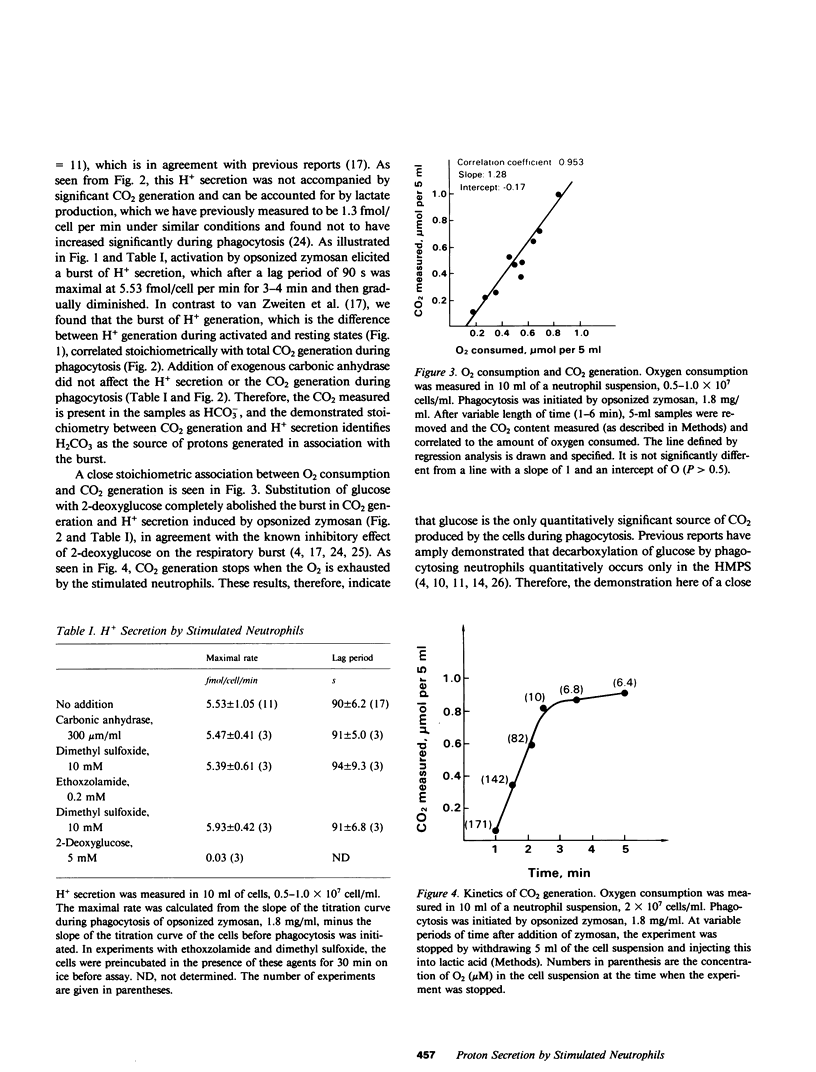
Phagocytosis by neutrophils is accompanied by a burst in O2 consumption and activation of the hexose monophosphate shunt (HMPS). Proton secretion equal to the amount of O2 consumed is an additional feature of the respiratory burst, but its source has not been identified, nor has the source of all electrons donated to O2 in the respiratory burst. We chemically quantitated total CO2 generation in human neutrophils and found that proton secretion elicited by phagocytosis was accompanied by a stoichiometric increase in CO2 generation. Addition of carbonic anhydrase and its inhibitors had no effect on either the quantities of CO2 measured or the quantities of protons secreted. Therefore, the CO2 generated in the respiratory burst of stimulated neutrophils is hydrated to form H2CO3, which then dissociates, accounting for the observed proton secretion. Furthermore, the CO2 generated corresponds to the O2 consumed with a respiratory quotient of nearly 1. We conclude on the basis of this and previous studies that the HMPS activity is the source of both the electrons for the NADPH oxidase and of protons secreted in association with the respiratory burst.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr, Nathan D. G. Comparative study of the metabolic and bactericidal characteristics of severely glucose-6-phosphate dehydrogenase-deficient polymorphonuclear leukocytes and leukocytes from children with chronic granulomatous disease. J Reticuloendothel Soc. 1972 Aug;12(2):150–169. [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982 Sep;70(3):550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Johansen K. S., Esmann V. Quantitation of superoxide production in human polymorphonuclear leukocytes from normals and 3 types of chronic granulomatous disease. Biochem Biophys Res Commun. 1979 Sep 12;90(1):214–219. doi: 10.1016/0006-291x(79)91612-7. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Kragballe K. Role of oxygen in antibody-dependent cytotoxicity mediated by monocytes and neutrophils. J Clin Invest. 1980 Oct;66(4):676–683. doi: 10.1172/JCI109904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Cooper M. R., DeChatelet L. R., McCall C. E., LaVia M. F., Spurr C. L., Baehner R. L. Complete deficiency of leukocyte glucose-6-phosphate dehydrogenase with defective bactericidal activity. J Clin Invest. 1972 Apr;51(4):769–778. doi: 10.1172/JCI106871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dri P., Bellavite P., Berton G., Rossi F. Interrelationship between oxygen consumption, superoxide anion and hydrogen peroxide formation in phagocytosing guinea pig polymorphonuclear leucocytes. Mol Cell Biochem. 1979 Jan 26;23(2):109–122. [PubMed] [Google Scholar]
- Herlin T., Borregaard N. Early changes in cyclic AMP and calcium efflux during phagocytosis by neutrophils from normals and patients with chronic granulomatous disease. Immunology. 1983 Jan;48(1):17–26. [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNOHAN J. C. THE PH-ACTIVITY CURVE OF BOVINE CARBONIC ANHYDRASE AND ITS RELATIONSHIP TO THE INHIBITION OF THE ENZYME BY ANIONS. Biochim Biophys Acta. 1965 Feb 22;96:304–317. [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- Lönnerholm G. Carbonic anhydrase in rat liver and rabbit skeletal muscle: further evidence for the specificity of the histochemical cobalt-phosphate method of Hansson. J Histochem Cytochem. 1980 May;28(5):427–433. doi: 10.1177/28.5.6769996. [DOI] [PubMed] [Google Scholar]
- Maffly R. H. A conductometric method for measuring micromolar quantities of carbon dioxide. Anal Biochem. 1968 May;23(2):252–262. doi: 10.1016/0003-2697(68)90357-6. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Dri P., Kakinuma K., Tedesco F., Rossi F. Studies on the mechanism of metabolic stimulation in polymorphonuclear leucocytes during phagocytosis. I. Evidence for superoxide anion involvement in the oxidation of NADPH2. Biochim Biophys Acta. 1975 Apr 7;385(2):380–386. doi: 10.1016/0304-4165(75)90367-0. [DOI] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. The influence of phorbol myristate acetate on oxygen consumption by polymorphonuclear leukocytes. J Lab Clin Med. 1974 Jun;83(6):911–920. [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Stjernholm R. L., Manak R. C. Carbohydrate metabolism in leukocytes. XIV. Regulation of pentose cycle activity and glycogen metabolism during phagocytosis. J Reticuloendothel Soc. 1970 Dec;8(6):550–560. [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- van Zwieten R., Wever R., Hamers M. N., Weening R. S., Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981 Jul;68(1):310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]


