β-Catenin provides stability to epithelia exposed to mechanical stress in part by strengthening adherens junctions in response to tension.
Abstract
Many tissues in our body experience mechanical stresses caused by both internal and external forces. The skin, for example, must tolerate diverse mechanical insults. In this paper, we report a role for β-catenin in providing stability to epithelia under stress. Loss of β-catenin during epidermal development caused perinatal lethality. Mutant embryos up-regulated stress responses at sites of active morphogenesis, which became more widespread after the stresses associated with birth. In addition, selective loss of tight junctions occurred in focal regions. This was recapitulated in cultured β-catenin–null cells exposed to externally applied forces. In addition, mutant cells were defective in tension-induced engagement of adherens junctions. We found that β-catenin was required to recruit vinculin to the cell cortex and to strengthen the junction’s association with the underlying cytoskeleton in response to tension. These data demonstrate that a complete understanding of the functions of cell adhesion proteins must take into account their roles in response to mechanical stresses.
Introduction
Throughout development and homeostasis, tissues are exposed to multiple physical stresses from forces developed both within the organism and from external sources. Tissues that experience the greatest physical assaults are mechanically robust, caused in part by robust cell adhesions that connect to the underlying cytoskeleton (Perez-Moreno et al., 2003; Simpson et al., 2011). Some of these adhesive structures, such as adherens junctions, are mechanosensitive and responsive structures that strengthen their connection to the actin cytoskeleton when force is applied to them (le Duc et al., 2010; Liu et al., 2010; Yonemura et al., 2010). This is thought to be mediated, in part, by the association of the actin-binding protein vinculin with adherens junctions. However, neither the molecular requirements for this strengthening nor the role it plays in tissue physiology has been fully addressed.
Genetic evidence suggests that β-catenin is dispensable for interfollicular epidermal function (Huelsken et al., 2001; Valenta et al., 2012). Loss of epidermal β-catenin resulted in loss of hair follicle specification, but interfollicular function was apparently normal (Huelsken et al., 2001). This was explained by (a) the lack of Wnt signaling in epidermal differentiation and (b) the ability of plakoglobin, a paralogue of β-catenin, to rescue adhesive functions of β-catenin. This is supported by work in cultured cells and other tissues (Posthaus et al., 2002; Zhou et al., 2007). However, β-catenin ablation was mosaic in these embryos and was not complete until early postnatal stages, precluding conclusions on its role during embryonic development.
Here, we demonstrate an unexpected role for β-catenin in protecting the epidermis from mechanical stresses. Using two in vitro assays, we demonstrate that loss of β-catenin results in loss of response to mechanical stimuli. These functions may underlie the essential role that we find for β-catenin in function of the epidermis during embryogenesis and neonatal stages.
Results and discussion
Epidermal ablation of β-catenin leads to barrier defects and neonatal death
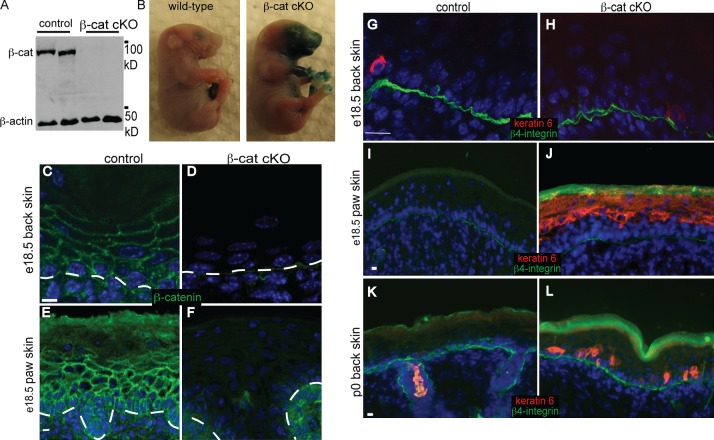
To better understand the role of β-catenin in embryonic epidermal development, we used a keratin 14-Cre mouse line that allows for early (embryonic day 14.5 [e14.5]) and ubiquitous recombination throughout the basal layer of the epidermis (Vasioukhin et al., 1999). Using these mice, β-catenin was quantitatively lost from the epidermis by e16.5 (Fig. S1, A and B) and remained absent in e18.5 embryos by both Western blot and immunofluorescence analysis (Fig. 1, A and C–F). No adult β-catenin conditional null animals (conditional knockouts [cKOs]) were obtained as a result of fully penetrant neonatal lethality within hours of birth, demonstrating an essential role for β-catenin in epidermal function.
Figure 1.
Loss of β-catenin in the embryonic epidermis resulted in spatially restricted loss of barrier function. (A) Western blot analysis of e18.5 back skin lysates confirmed quantitative loss of β-catenin (β-cat). (B) An X-gal penetration assay to measure barrier function was performed on e18.5 WT and β-catenin cKO embryos. This analysis showed skin barrier defects restricted to the epidermis covering the mouth and forepaw regions of β-catenin cKO animals. (C–F) Immunofluorescence localization of β-catenin in tissue sections, as indicated. Note the loss of β-catenin from the epidermis in both back skin and paw skin. The dotted lines indicate the basement membrane. (G–L) Immunofluorescence localization of keratin 6. β4-Integrin staining marks the basement membrane. Bars, 5 µm.
Macroscopic examination of the knockout animals did not reveal any blistering of the skin, and littermates were comparable in body size. A fully formed epidermal barrier is required at birth to prevent dehydration of neonates. To determine whether the barrier function of the epidermis was affected by loss of β-catenin, we performed a dye-penetration assay. Embryos (e18.5) were immersed in a solution containing X-gal, which can be converted into a blue precipitate in the dermis if there is no barrier. Much of the epidermis, including the back skin of the β-catenin cKO animals, had a functional barrier, similar to that seen in the wild-type (WT) littermates. However, there was a clear loss of barrier activity over the paws and facial areas that likely contributed to neonatal lethality (Fig. 1 B).
The restricted pattern of the barrier defects could be caused by a delay in barrier formation in the extremities or could reflect specific requirements for β-catenin in these regions. To begin to assess this, we analyzed different skin regions for the expression of keratin 6 in e18.5 embryos. This protein is not normally expressed in interfollicular epidermis during development but is often induced under conditions of stress. We found a clear up-regulation of keratin 6 only in the mutant paw skin, but not in back skin, consistent with the barrier defects (Fig. 1, G–J). As keratin 6 is not normally expressed at these levels in the WT paw, this demonstrates that the barrier defect is not simply a developmental delay.
Loss of β-catenin does not result in epidermal differentiation defects
Epidermal barrier loss could be attributed to multiple factors. Defects in epidermal differentiation or lipid metabolism can lead to incomplete formation of cornified envelopes, which are essential for barrier activity (Segre, 2003; Fehrenschild et al., 2012). However, no significant differentiation defects in the paw skin were observed as determined by immunostaining for the suprabasal and granular layer markers keratin 1 and loricrin, respectively (Fig. S2, A–D). Additionally, the Wnt–β-catenin–Lef1 signaling pathway has been implicated in skin barrier formation via regulation of epidermal lipid metabolism (Fehrenschild et al., 2012). To test whether the observed defects could be caused by a perturbation of the Wnt signaling pathway, Wnt reporter expression was observed using TCF/Lef-H2B-GFP reporter mice (Ferrer-Vaquer et al., 2010). Consistent with a previous study, strong Wnt reporter expression was observed in the hair follicles of the back skin (DasGupta and Fuchs, 1999). However, no Wnt reporter expression was observed in the paw epidermis of WT newborn animals (Fig. S2, E and F). Although we cannot rule out β-catenin transcriptional activity that is not reported by the TCF/Lef-H2B-GFP mice, these results suggest a Wnt-independent role of β-catenin in the paw epidermal barrier function.
Cell–cell contacts are maintained in β-catenin knockout epidermis
The regional specificity of the epidermal barrier loss observed in the β-catenin knockout embryos was similar to that previously observed in E/P-cadherin–deficient epidermis that showed notable defects in intercellular junction formation (Tinkle et al., 2008). These previous observations suggested that intercellular junction integrity could be perturbed in β-catenin cKO animals. However, in contrast to E/P-cadherin–deficient epidermis, β-catenin cKO epidermis maintained cortical localization of other adherens junction components at intercellular borders in paw and back skin epidermis with no intercellular gaps in either area (Fig. 2, A–D). It is likely that the β-catenin paralogue plakoglobin partially functionally complements for β-catenin in the cKO epidermis, allowing for proper adherens junction protein localization (Huelsken et al., 2000). Consistent with this hypothesis, cortical localization of plakoglobin was observed in both the back and paw skin of the β-catenin cKO animals (Fig. S1, C and D; and not depicted). Previous work has demonstrated plakoglobin association with adherens junctions both biochemically and ultrastructurally (Ozawa et al., 1989; Huelsken et al., 2000). These results demonstrate that despite the decreased barrier integrity of the paw epidermis at e18.5, there were no notable alterations in the localization of adherens junction components in the β-catenin cKO animals. The barrier defects are unlikely to be caused by small decreases in adherens junction complexes as loss of p120-catenin (which caused a large decrease in total and cortical E-cadherin) did not cause barrier defects or neonatal lethality (Perez-Moreno et al., 2006).
Figure 2.

Defects in tight junction protein localization in the paw skin of β-catenin cKOs. (A–D) E-cadherin localization in e18.5 back and paw epidermis in both WT and β-catenin (β-cat) cKO embryos, as indicated. (E–H) ZO-1 localization in e18.5 back and paw epidermis in both WT and β-catenin cKO embryos, as indicated. Dotted lines mark the basement membrane. Bars, 10 µm.
Tight junction protein localization is diminished in β-catenin cKO paw epidermis
Animals with epidermal knockout of tight junction components die soon after birth from severe water loss as a result of a compromised barrier (Furuse et al., 2002). Therefore, impaired tight junction integrity could provide a possible explanation for the neonatal lethality in the β-catenin knockout mice. We found normal levels and localization of tight junction components in the back skin of β-catenin cKO embryos (Fig. 2, E and F). In contrast, the paw epidermis had severely compromised cortical localization of the tight junction proteins ZO-1 and occludin (Figs. 2, G and H; and S1, G and H).
Because of the perinatal lethality of the β-catenin cKOs, we wondered whether epidermal defects were exacerbated in newborn pups. We reasoned that the stresses associated with birth and exposure to a terrestrial environment might lead to additional defects. Indeed, we found that keratin 6, which is absent in the back skin of late stage embryos, is up-regulated in a salt and pepper manner in newborn mutant back skin (compare Fig. 1, H and L). This was less severe than effects seen in the paw skin and was not associated with a dramatic loss of tight junction proteins (unpublished data).
The aforementioned defects were associated with sites of active growth, morphogenesis, and movement (in the case of paws) or were compounded by additional stresses, such as birth (in the back skin). There are several potential causes of these defects, both shared and unique. Active morphogenesis, movement, and birth are all expected to generate mechanical forces on the epidermis. Paws become motile in midgestation and undergo rapid movements in utero (Kodama and Sekiguchi, 1984). Furthermore, the paws are areas of particularly active morphogenesis during development and are more sensitive to loss of cell adhesion/cytoskeletal proteins than other areas of the skin (Raghavan et al., 2000; Tinkle et al., 2008; Sumigray et al., 2012). The observed barrier defects in mice with loss of cell adhesion proteins in the epidermis suggest an impaired ability to resist mechanical forces. We thus hypothesized that one function of β-catenin is to help maintain barrier integrity by supporting tight junction stability under mechanical stress.
β-Catenin is essential to maintain tight junctions under externally applied mechanical stretch
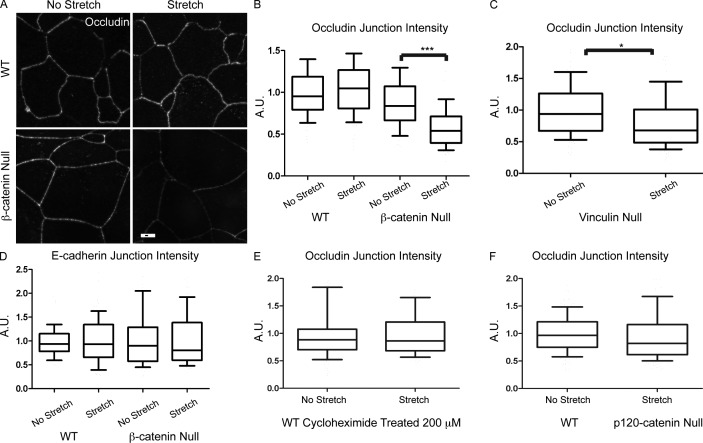
Our data are consistent with β-catenin providing protection to the actively growing limbs during development and to the epidermis more widely at birth. Strong support for this would be provided by a role for β-catenin in mechanical stress responses in isolated keratinocytes. A previous study demonstrated that adherens junction complexes assembled normally and contained increased amounts of plakoglobin in β-catenin–null cells (unpublished data; Posthaus et al., 2002). However, the effects of mechanical stress on cell adhesion structures in these cells were not addressed. We therefore established WT and matched β-catenin–null cells from the back skins of e18.5 pups. To test the role of β-catenin in resisting external mechanical stresses, an in vitro culture system was used involving uniaxial cyclic stretch of these keratinocytes. As in intact back skin, WT and β-catenin–null cells showed no significant difference in the localization or intensity of the tight junction protein occludin after a switch to high calcium media to promote cell adhesion (Fig. 3, A and B). However, upon the application of a cyclic mechanical stretch, β-catenin–null cells showed a significant decrease in junctional occludin intensity compared with WT stretched and KO unstretched cells (Fig. 3, A and B). The maintenance of tight junctions under stretch did not require new protein synthesis, as determined by treatment of WT cells with cycloheximide before stretch (Fig. 3 E). Therefore, transcriptional activities of β-catenin are unlikely to play a role in the stabilization of tight junction components. To determine whether this defect was tight junction specific, the effect of stretch on the adherens junction protein E-cadherin was also observed. No significant change was detected between the stretched and unstretched conditions in either WT or β-catenin–null cells (Fig. 3 D). Therefore, unlike WT cells, cultured β-catenin–null cells showed stretch-induced tight junction loss. This effect could be caused by changes in general adherens junction function or could reflect a specific function for β-catenin. To test this, we performed the same assay on p120-catenin–null keratinocytes, which have decreased cortical E-cadherin (Perez-Moreno et al., 2006). This function appears to be specific for β-catenin because loss of p120-catenin did not make tight junction protein localization sensitive to stretch (Fig. 3 F).
Figure 3.
β-Catenin–null keratinocytes are unable to maintain tight junction protein localization upon exposure to uniaxial stretch. (A) Confluent monolayers of WT and β-catenin–null cells were stained for the tight junction protein occludin, in both the control and stretch conditions. Stretch was 20% at 0.5 Hz for 30 min on polydimethylsiloxane substrates coated with fibronectin. Bar, 10 µm. (B) Mean junctional intensity of occludin staining was quantified, demonstrating a significant decrease in the β-catenin–null stretched cells (***, P < 0.001). (C) Mean junctional intensity of occludin staining was quantified in vinculin-null cells with or without uniaxial stretch (*, P < 0.05). (D) Junctional levels of the adherens junction protein E-cadherin were not affected by stretch in either WT or β-catenin–null cells. (E) Junctional occludin levels were quantified in control and stretched WT cells treated with 200 µM of the translational inhibitor cycloheximide. (F) Junctional levels of occludin in p120-catenin–null cells were quantitated in control and stretched samples. Horizontal lines are the median. Boxes are 25–75%, and whiskers are 10–90%. A.U., arbitrary units.
β-Catenin is required for mechanotransduction at adherens junctions
Although the aforementioned work demonstrates a requirement for β-catenin in resisting externally applied forces, adherens junctions must also respond to internal forces generated by the actomyosin cytoskeleton. Recent work has demonstrated that adherens junctions are mechanosensitive and responsive structures (le Duc et al., 2010; Liu et al., 2010; Yonemura et al., 2010). In response to forces, adherens junctions can become more strongly engaged with the underlying cytoskeleton. The engagement of adherens junctions can be induced in cultured keratinocytes by treating cells with taxol (Sumigray et al., 2012). Microtubule stabilization by taxol induces their reorganization to the cell cortex and results in the recruitment of myosin II to cell–cell junctions. Myosin II–dependent forces cause engagement of adherens junctions and strengthening of epithelial sheets (Sumigray et al., 2012). Whether β-catenin is required for this mechanotransduction has not been addressed previously.
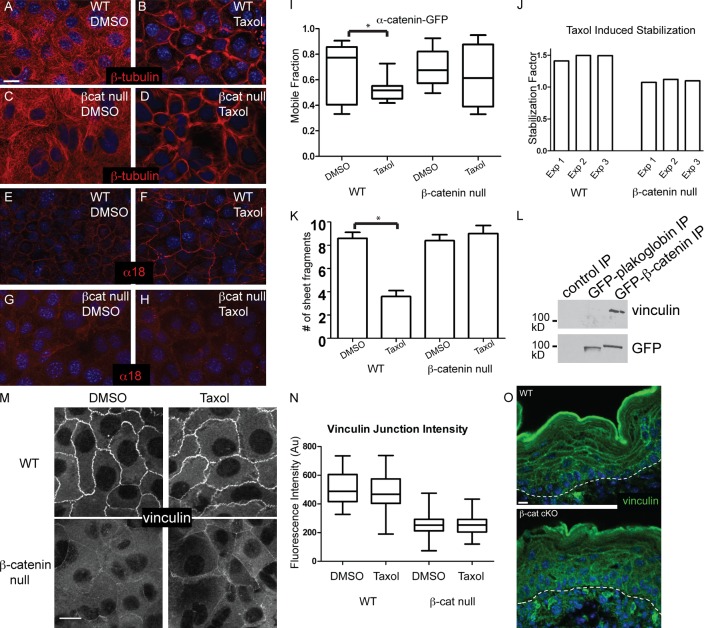
β-Catenin was not required for the cortical reorganization of microtubules after taxol treatment (Fig. 4, A–D). This is consistent with our previous findings that loss of other adherens junction components did not have an effect on the establishment of a cortical microtubule array (Lechler and Fuchs, 2007; Sumigray et al., 2012). We next tested whether the downstream effects of cortical microtubules on adherens junction engagement occurred normally in β-catenin–null cells. In WT cells, taxol treatment caused the exposure of the tension-sensitive α18 epitope of α-catenin (Fig. 4, E and F; Yonemura et al., 2010; Sumigray et al., 2012). In β-catenin–null cells, there was no significant increase in α18 staining upon taxol treatment (Fig. 4, G and H).
Figure 4.
β-Catenin–null cells do not undergo microtubule-dependent adherens junction engagement and epithelial sheet strengthening. (A–D) WT or β-catenin (βcat)–null keratinocytes were treated with DMSO or 10 µM taxol for 1 h before fixation and staining with anti–β-tubulin antibodies. (E–H) WT or β-catenin–null cells (control and taxol treated) were stained with α18 antibodies, which recognize a tension-sensitive epitope of α-catenin. (I) FRAP was performed on WT and β-catenin–null cells expressing α-catenin–GFP. Mobile fraction was determined, and a representative experiment is shown. A significant decrease in mobile fraction was detected only in WT cells treated with taxol, as compared with control (*, P < 0.05). Horizontal lines are the median. Boxes are 25–75%, and whiskers are 10–90%. (J) We determined the stabilization factor (median mobile fraction of control cells/median mobile fraction of taxol-treated cells) for three independent experiments for both WT and β-catenin–null cells. Exp, experiment. (K) Confluent monolayers of WT or β-catenin–null cells were treated with DMSO or 10 µM taxol for 1 h before dispase treatment to release the monolayer from the underlying substrate. After mechanical disruption by pipetting, the number of cell sheet fragments was counted. Larger numbers indicate more fragile sheets, whereas fewer is indicative of increased mechanical integrity. Error bars are standard deviations. (L) Immunoprecipitates (IP) of GFP-tagged plakoglobin or β-catenin were probed with antibodies again vinculin and GFP. (M) WT and β-catenin–null cells were either DMSO or taxol treated for 1 h before fixation and staining with anti-vinculin antibodies. (N) Quantitation of vinculin cortical intensity under the indicated conditions. Horizontal lines are the median. Boxes are 25–75%, and whiskers are 10–90%. Au, arbitrary units. (O) Vinculin localization in WT and β-catenin–null epidermis. The dotted lines indicate the basement membrane. Bars, 10 µm.
Previous studies have demonstrated that engagement of adherens junctions with the underlying cytoskeleton results in decreased turnover of α-catenin at cell junctions, as measured by FRAP (Yonemura et al., 2010; Sumigray et al., 2012). Consistent with a previous study (Sumigray et al., 2012), the mobile fraction of α-catenin decreased when WT cells were treated with taxol (Fig. 4, I and J). Under control conditions, the turnover of α-catenin in β-catenin–null cells was similar to WT cells, again suggesting that basal adherens junction function is normal in these cells (presumably via plakoglobin complementation). However, the robust decrease in the mobile fraction of α-catenin did not occur in β-catenin–null cells (Fig. 4, I and J). Rather, we reproducibly saw a slight, but not statistically significant, decrease in α-catenin mobile fraction upon taxol treatment.
Stabilization of adherens junctions has been reported to increase the mechanical integrity of epithelial sheets (Sumigray et al., 2012). Using a semiquantitative assay for epithelial sheet strength, we found that β-catenin–null cell sheets were not more fragile than WT cells under untreated conditions, consistent with a previous study (Posthaus et al., 2002). However, although WT sheets show enhanced integrity after taxol treatment, this increase in mechanical strength was absent in β-catenin–null cells (Fig. 4 K). These data demonstrate that both adherens junction stabilization and the corresponding increase in keratinocyte sheet strength required β-catenin.
The tension-induced stabilization of adherens junctions is dependent on vinculin, which can bind directly to both α- and β-catenin (le Duc et al., 2010; Peng et al., 2010; Yonemura et al., 2010). The vinculin–β-catenin interaction requires amino-terminal residues in β-catenin that are not conserved in plakoglobin (Peng et al., 2010). In fact, we found little association of plakoglobin-GFP with vinculin, whereas β-catenin–vinculin interactions were noted (Fig. 4 L). In addition, we found a significant decrease in the cortical localization of vinculin in β-catenin–null cells, as compared with WT controls (Fig. 4, M and N). This was true in the presence or absence of taxol. A decrease in cortical localization of vinculin was also noted in β-catenin cKO epidermal tissue sections (Fig. 4 O).
We therefore tested whether the mechanical robustness of tight junctions in cultured keratinocytes required vinculin. Vinculin-null keratinocytes were generated and assayed for their response to cyclic stretch, as previously described for β-catenin–null cells. We found a significant decrease in cortical occludin levels after stretch in vinculin-null cells (Fig. 3 C). This decrease was not as dramatic as for β-catenin–null cells, suggesting that additional proteins may work redundantly with vinculin downstream of β-catenin. In total, these data demonstrate a unique function for β-catenin in adherens junction engagement, mediated in part through the recruitment of vinculin.
This study has identified a specific function for β-catenin at adherens junctions that cannot be complemented by plakoglobin. Although our data demonstrate distinct interactions of these two proteins, we cannot rule out that some of the in vivo effects are caused by dose-dependent loss of total β-catenin/plakoglobin levels. Our data suggest that tension-induced engagement of adherens junctions requires β-catenin. This is consistent with an earlier study that demonstrated a preferential localization of β-catenin over plakoglobin at zonula adherens in cultured cells (Näthke et al., 1994). Zonula adherens are thought to be more highly engaged and under more tension than lateral adherens junctions (Taguchi et al., 2011). Our data demonstrate a requirement for β-catenin in vinculin localization. A similar result has been reported previously in mammary epithelial cells (Peng et al., 2010). Furthermore, our finding that plakoglobin cannot interact with vinculin may explain why it can only partially rescue adherens junction activity. Whether β-catenin simply recruits vinculin to the membrane to increase its local concentration for handoff to α-catenin or whether this complex can be force bearing will require further investigation. Additionally, it remains unclear how adherens junctions and tight junctions are coupled molecularly by β-catenin and whether the observed tight junction defects are secondary to the decrease in adherens junction engagement. Finally, this work demonstrates the value of perturbing junctions with mechanical stresses to model forces experienced in vivo. In tissues, such as the epidermis, that experience a myriad of externally and internally applied forces, this is likely to uncover physiologically important roles for cell adhesion molecules.
Materials and methods
Barrier assay
e18.5 embryos were immersed in X-gal–containing solution (1.3 mM MgCl2, 100 mM NaPO4, 3 mM K3Fe[CN]6, 0.01% sodium deoxycholate, 0.2% NP-40, and 1 mg/ml X-gal) and incubated overnight at 30°C as described previously (Sumigray et al., 2012).
Mice
Keratin 14-Cre mice were provided by E. Fuchs (The Rockefeller University, New York, NY), and β-catenin floxed mice were obtained from Jackson ImmunoResearch Laboratories, Inc.
Stretch experiments
Stretch assays were conducted using a custom-built stretching device, modeled on those previously described (Suzuki et al., 1997; Naruse et al., 1998). Keratinocytes were plated onto 10 µg/ml fibronectin-coated polydimethylsiloxane chambers (Sylgard 184 cure and base mixed at 1:20). Cells grew in the chambers in E (epidermal) low Ca2+ media until confluent. Confluent cells were then switched to media with 1.2 mM calcium to induce junction formation. After 16–20 h in this media, cells were stretched. During stretch, one clamp remained stationary, and the other was moved by a computer-controlled actuator allowing for control of both amplitude and frequency using custom-written MATLAB software (MathWorks). Tracking the position of fluorescent beads and cells confirmed that cellular strain matched substrate strain. Cells were stretched at an amplitude of 20% strain and a frequency of 0.5 Hz for 30 min. Unstretched controls remained in the incubator for the same duration as the stretched samples. After stretch, cells were immediately fixed in 4% paraformaldehyde for 8 min or in methanol for 2 min at −20°C. After fixing, cells were processed for immunofluorescence staining. p120-null keratinocytes were provided by M. Perez-Moreno (Centro Nacional de Investigaciones Oncológicas, Madrid, Spain).
Immunofluorescence staining
Antibodies used were rabbit antioccludin (Abcam), rabbit anti–α-catenin, mouse anti–β-catenin, mouse anti–β-actin, mouse anti-vinculin, and mouse anti–β-tubulin (all obtained from Sigma-Aldrich), rabbit anti–ZO-1 (Invitrogen), rabbit anti-K6 (Covance), rabbit antiplakoglobin (Santa Cruz Biotechnology, Inc.), rat anti–β4-integrin (BD), rat anti-α18 (gift from A. Nagafuchi, Kumamoto University, Chuo Ward, Kumamoto, Japan), rabbit anti-K1, mouse antiloricrin, and rat anti–E-cadherin (gifts from C. Jamora, inStem, Bangalore, India). Imaging was performed on a microscope (AxioImager Z1; Carl Zeiss) with a structured-illumination attachment (ApoTome; Carl Zeiss) and camera (AxioCam MRm; Carl Zeiss) using AxioVision software (Carl Zeiss). A 63×, 1.4 NA Plan Apochromat objective and Immersol 518F immersion oil were used (Carl Zeiss). Imaging was performed at room temperature through either 12-mm round coverslips (No. 1) for cells or No. 1.5 coverslips (VWR Laboratory Products) for tissue sections. For quantification, junctional intensity was measured using custom-written MATLAB code. The code found the maximum profile along each junction, and then, the mean intensity of that maximum profile was determined. The means of >100 junctions for at least two separate trials in each condition were measured. For a given trial, all intensity values were normalized so that the mean no-stretch intensity was unity. Statistical analyses were performed using a Mann–Whitney test with Prism software (GraphPad Software).
FRAP
WT and β-catenin–null keratinocytes were grown on 35-mm glass-bottom culture dishes (MatTek Corporation) in E low Ca2+ media. Cells were transfected with an α-catenin–GFP plasmid using transfection reagent (TransIT-LT1; Mirus) and switched to high calcium (1.2 mM) media to promote cell adhesion for 16–20 h. A confocal scanning light microscope (LSM 710; Carl Zeiss) with a 63×, 1.4 NA objective and Zen software (Carl Zeiss) was used to determine the mobile fraction of α-catenin at cell cortical regions. Imaging was performed at 37°C. Percent recovery was calculated by first adjusting the fluorescent intensity for each region for the background intensity and then normalizing the intensity at each time point to the initial intensity. Mobile fraction was calculated as MF = (Imax − I0)/(1 − I0), in which Imax is the maximal fluorescence recovery, and I0 is the initial fluorescence intensity immediately after photobleaching (Shen et al., 2008).
Online supplemental material
Fig. S1 shows localization of tight and adherens junction proteins in WT and β-catenin–null epidermis. Fig. S2 includes analysis of Wnt signaling and differentiation in β-catenin–null epidermis. Fig. S3 diagrams the cell stretch apparatus used in Fig. 3. A ZIP file is provided that contains two MATLAB programs that identify the cell–cell junction and determine its mean intensity. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201212140/DC1.
Supplementary Material
Acknowledgments
We thank Elaine Fuchs, Colin Jamora, Mirna Perez-Moreno, and Akira Nagafuchi for reagents, Julie Underwood for excellent care of the mice, and members of the Lechler Laboratory, Brent Hoffman, and Scott Soderling for comments on the manuscript. We are grateful to Eric Dufresne (Yale University, New Haven, CT) for advice on design of the cell stretch apparatus and to Richard Nappi for help with construction.
This work was supported by a grant from the National Institutes of Health (R01AR055926) to T. Lechler.
Footnotes
Abbreviations used in this paper:
- cKO
- conditional knockout
- WT
- wild type
References
- DasGupta R., Fuchs E. 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 126:4557–4568 [DOI] [PubMed] [Google Scholar]
- Fehrenschild D., Galli U., Breiden B., Bloch W., Schettina P., Brodesser S., Michels C., Günschmann C., Sandhoff K., Niessen C.M., Niemann C. 2012. TCF/Lef1-mediated control of lipid metabolism regulates skin barrier function. J. Invest. Dermatol. 132:337–345 10.1038/jid.2011.301 [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A., Piliszek A., Tian G., Aho R.J., Dufort D., Hadjantonakis A.K. 2010. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse. BMC Dev. Biol. 10:121 10.1186/1471-213X-10-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S. 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1–deficient mice. J. Cell Biol. 156:1099–1111 10.1083/jcb.200110122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J., Vogel R., Brinkmann V., Erdmann B., Birchmeier C., Birchmeier W. 2000. Requirement for β-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 148:567–578 10.1083/jcb.148.3.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J., Vogel R., Erdmann B., Cotsarelis G., Birchmeier W. 2001. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 105:533–545 10.1016/S0092-8674(01)00336-1 [DOI] [PubMed] [Google Scholar]
- Kodama N., Sekiguchi S. 1984. The development of spontaneous body movement in prenatal and perinatal mice. Dev. Psychobiol. 17:139–150 10.1002/dev.420170205 [DOI] [PubMed] [Google Scholar]
- Lechler T., Fuchs E. 2007. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J. Cell Biol. 176:147–154 10.1083/jcb.200609109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Duc Q., Shi Q., Blonk I., Sonnenberg A., Wang N., Leckband D., de Rooij J. 2010. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II–dependent manner. J. Cell Biol. 189:1107–1115 10.1083/jcb.201001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Tan J.L., Cohen D.M., Yang M.T., Sniadecki N.J., Ruiz S.A., Nelson C.M., Chen C.S. 2010. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA. 107:9944–9949 10.1073/pnas.0914547107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K., Yamada T., Sokabe M. 1998. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am. J. Physiol. 274:H1532–H1538 [DOI] [PubMed] [Google Scholar]
- Näthke I.S., Hinck L., Swedlow J.R., Papkoff J., Nelson W.J. 1994. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J. Cell Biol. 125:1341–1352 10.1083/jcb.125.6.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. 1989. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 8:1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Cuff L.E., Lawton C.D., DeMali K.A. 2010. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J. Cell Sci. 123:567–577 10.1242/jcs.056432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M., Jamora C., Fuchs E. 2003. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 112:535–548 10.1016/S0092-8674(03)00108-9 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M., Davis M.A., Wong E., Pasolli H.A., Reynolds A.B., Fuchs E. 2006. p120-catenin mediates inflammatory responses in the skin. Cell. 124:631–644 10.1016/j.cell.2005.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthaus H., Williamson L., Baumann D., Kemler R., Caldelari R., Suter M.M., Schwarz H., Müller E. 2002. beta-Catenin is not required for proliferation and differentiation of epidermal mouse keratinocytes. J. Cell Sci. 115:4587–4595 10.1242/jcs.00141 [DOI] [PubMed] [Google Scholar]
- Raghavan S., Bauer C., Mundschau G., Li Q., Fuchs E. 2000. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 150:1149–1160 10.1083/jcb.150.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre J. 2003. Complex redundancy to build a simple epidermal permeability barrier. Curr. Opin. Cell Biol. 15:776–782 10.1016/j.ceb.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Shen L., Weber C.R., Turner J.R. 2008. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 181:683–695 10.1083/jcb.200711165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C.L., Patel D.M., Green K.J. 2011. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 12:565–580 10.1038/nrm3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumigray K.D., Foote H.P., Lechler T. 2012. Noncentrosomal microtubules and type II myosins potentiate epidermal cell adhesion and barrier formation. J. Cell Biol. 199:513–525 10.1083/jcb.201206143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Naruse K., Asano Y., Okamoto T., Nishikimi N., Sakurai T., Nimura Y., Sokabe M. 1997. Up-regulation of integrin beta 3 expression by cyclic stretch in human umbilical endothelial cells. Biochem. Biophys. Res. Commun. 239:372–376 10.1006/bbrc.1997.7364 [DOI] [PubMed] [Google Scholar]
- Taguchi K., Ishiuchi T., Takeichi M. 2011. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J. Cell Biol. 194:643–656 10.1083/jcb.201104124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle C.L., Pasolli H.A., Stokes N., Fuchs E. 2008. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc. Natl. Acad. Sci. USA. 105:15405–15410 10.1073/pnas.0807374105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T., Hausmann G., Basler K. 2012. The many faces and functions of β-catenin. EMBO J. 31:2714–2736 10.1038/emboj.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V., Degenstein L., Wise B., Fuchs E. 1999. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA. 96:8551–8556 10.1073/pnas.96.15.8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Wada Y., Watanabe T., Nagafuchi A., Shibata M. 2010. alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12:533–542 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- Zhou J., Qu J., Yi X.P., Graber K., Huber L., Wang X., Gerdes A.M., Li F. 2007. Upregulation of gamma-catenin compensates for the loss of beta-catenin in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 292:H270–H276 10.1152/ajpheart.00576.2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.