Abstract
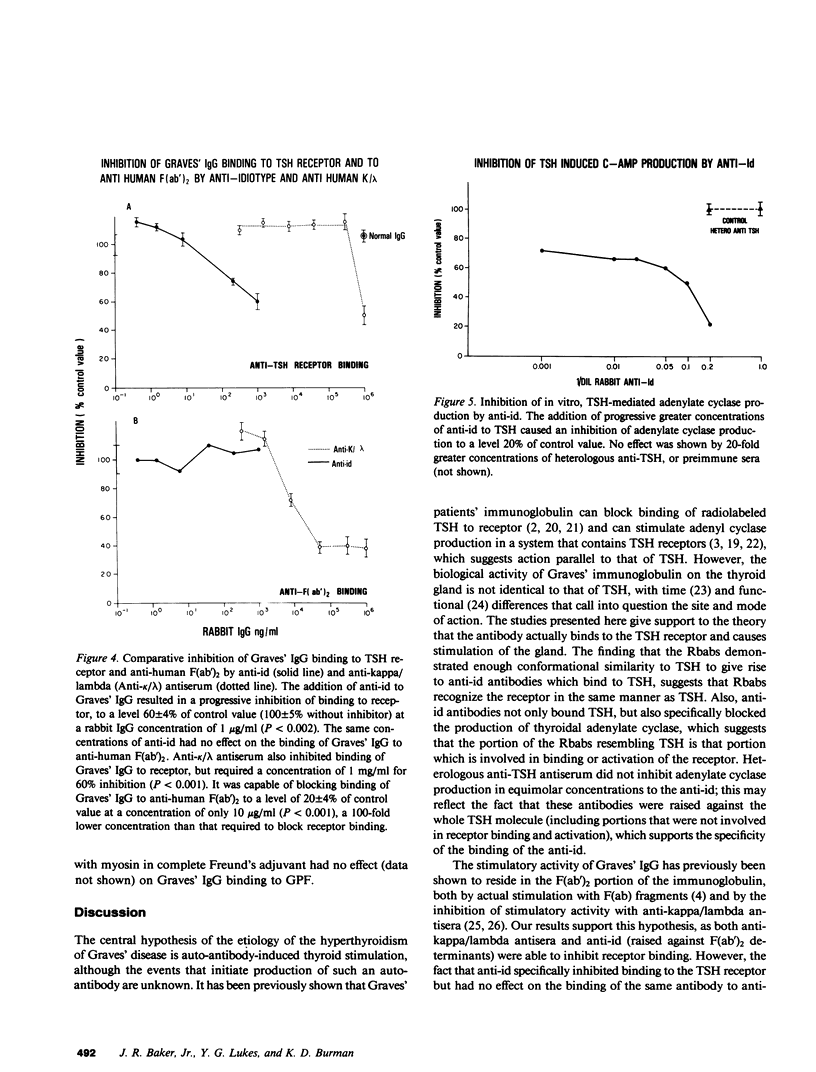
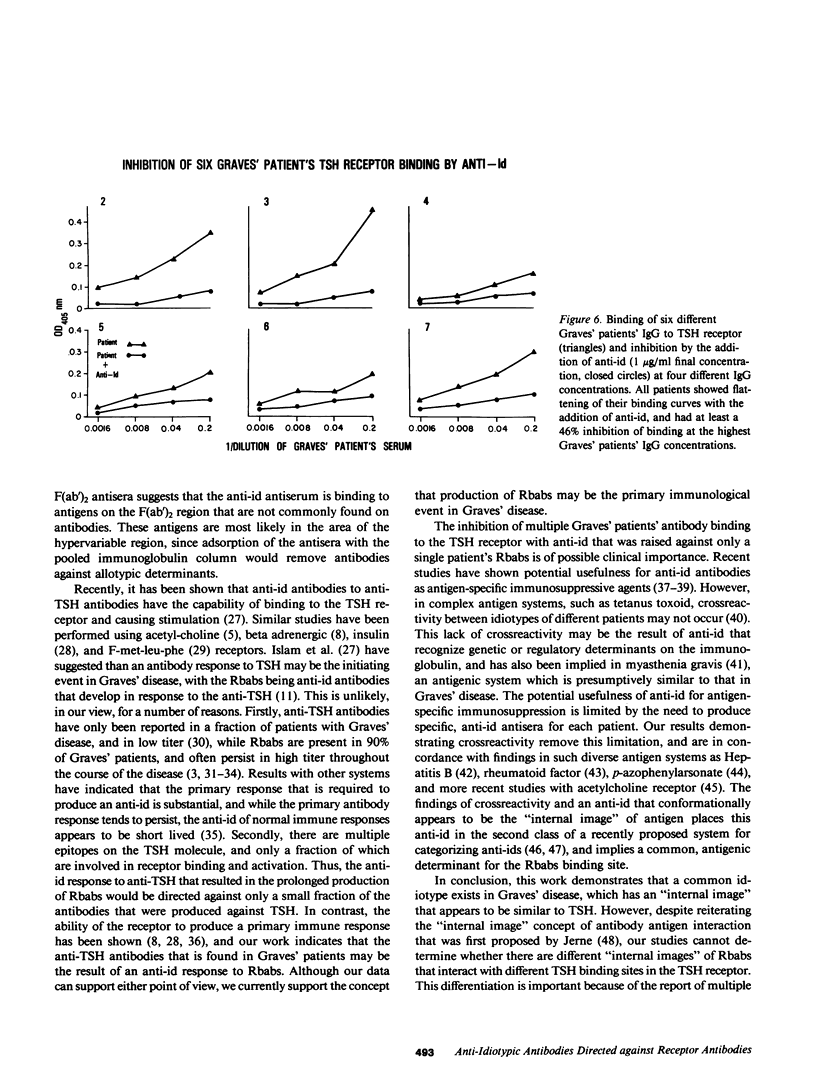
Previous studies have shown that anti-idiotypic antibodies can be developed in vivo through animal immunization with idiotype, and that these antibodies can be isolated from other anti-immunoglobulin antibodies by affinity purification. These techniques have relied on large amounts of idiotype, which were produced either by hyperimmunization or by monoclonal antibodies, to serve as the affinity adsorbent. In the present study, we produced anti-idiotypic antibodies to human anti-thyroid-stimulating hormone (TSH) receptor antibodies by first injecting rabbits with (TSH receptor purified) IgG from Graves' patients. The resulting antiserum was then adsorbed with Sepharose-coupled TSH in an attempt to specifically bind and isolate the anti-idiotype. The antibody obtained from this process was shown to bind specifically to TSH receptor-binding antibodies from Graves' patients, and this binding could be inhibited by 56% with the addition of 10(-4) M TSH but not by HCG (10(-2) M). The anti-idiotype also bound to TSH, and this binding could be specifically inhibited by receptor-purified Graves' IgG (60% inhibition at 10 micrograms/ml IgG), but not by IgG from normal subjects (no inhibition at 50 micrograms/ml IgG). In a TSH receptor binding assay, the anti-idiotype could inhibit TSH receptor binding in Graves' sera at a 1,000-fold lower concentration than could anti-kappa/lambda antiserum; the anti-idiotypic antiserum also inhibited in vitro TSH-mediated adenylate cyclase stimulation at an IgG concentration of 5 micrograms/ml, while heterologous anti-TSH antisera and normal IgG at similar concentrations had no effect. Finally, despite being generated against a single patient's TSH receptor binding antibody, the anti-idiotype was able to block TSH receptor binding in the serum of six other Graves' patients, thus suggesting that there may be conformational conservation in the antigen that is recognized by different individuals' TSH receptor-binding immunoglobulins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Wall H., Lindsley H. B., Halsey J. F., Suzuki T. Network theory in autoimmunity. In vitro suppression of serum anti-DNA antibody binding to DNA by anti-idiotypic antibody in systemic lupus erythematosus. J Clin Invest. 1981 May;67(5):1297–1304. doi: 10.1172/JCI110158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. R., Jr, Lukes Y. G., Smallridge R. C., Berger M., Burman K. D. Partial characterization and clinical correlation of circulating human immunoglobulins directed against thyrotrophin binding sites in guinea pig fat cell membranes. Development of a direct enzyme immunoassay. J Clin Invest. 1983 Oct;72(4):1487–1497. doi: 10.1172/JCI111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkas T., Simpson J. A. Lack of inter-animal cross-reaction of anti-acetylcholine receptor antibodies at the receptor-binding site as demonstrated by heterologous anti-idiotype antisera: implications for immunotherapy of myasthenia gravis. Clin Exp Immunol. 1982 Jan;47(1):119–126. [PMC free article] [PubMed] [Google Scholar]
- Beall G. N., Kruger S. R. Binding of 125I-human TSH by gamma globulins of sera containing thyroid-stimulating immunoglobulin (TSI). Life Sci. 1983 Jan 3;32(1-2):77–83. doi: 10.1016/0024-3205(83)90175-3. [DOI] [PubMed] [Google Scholar]
- Bech K., Nistrup Madsen S. Thyroid adenylate cyclase stimulating immunoglobulins in thyroid diseases. Clin Endocrinol (Oxf) 1979 Jul;11(1):47–58. doi: 10.1111/j.1365-2265.1979.tb03045.x. [DOI] [PubMed] [Google Scholar]
- Bona C. A., Finley S., Waters S., Kunkel H. G. Anti-immunoglobulin antibodies. III. Properties of sequential anti-idiotypic antibodies to heterologous anti-gamma globulins. Detection of reactivity of anti-idiotype antibodies with epitopes of Fc fragments (homobodies) and with epitopes and idiotopes (epibodies). J Exp Med. 1982 Oct 1;156(4):986–999. doi: 10.1084/jem.156.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bíró J. Thyroid-stimulating antibodies in Grave's disease and the effect of thyrotrophin-binding globulins on their determination. J Endocrinol. 1982 Feb;92(2):175–184. doi: 10.1677/joe.0.0920175. [DOI] [PubMed] [Google Scholar]
- DORRINGTON K. J., MUNRO D. S. IMMUNOLOGICAL STUDIES ON THE LONG-ACTING THYROID STIMULATOR. Clin Sci. 1965 Feb;28:165–174. [PubMed] [Google Scholar]
- Endo K., Amir S. M., Ingbar S. H. Development and evaluation of a method for the partial purification of immunoglobulins specific for Graves' disease. J Clin Endocrinol Metab. 1981 Jun;52(6):1113–1123. doi: 10.1210/jcem-52-6-1113. [DOI] [PubMed] [Google Scholar]
- Fenzi G., Hashizume K., Roudebush C. P., DeGroot L. J. Changes in thyroid-stimulating immunoglobulins during antithyroid therapy. J Clin Endocrinol Metab. 1979 Apr;48(4):572–576. doi: 10.1210/jcem-48-4-572. [DOI] [PubMed] [Google Scholar]
- Fong S., Gilbertson T. A., Carson D. A. The internal image of IgG in cross-reactive anti-idiotypic antibodies against human rheumatoid factors. J Immunol. 1983 Aug;131(2):719–724. [PubMed] [Google Scholar]
- Geha R. S. Presence of auto-anti-idiotypic antibody during the normal human immune response to tetanus toxoid antigen. J Immunol. 1982 Jul;129(1):139–144. [PubMed] [Google Scholar]
- Geha R. S., Weinberg R. P. Anti-idiotypic antisera in man. I. Production and immunochemical characterization of anti-idiotypic antisera to human antitetanus antibodies. J Immunol. 1978 Oct;121(4):1518–1523. [PubMed] [Google Scholar]
- Homcy C. J., Rockson S. G., Haber E. An antiidiotypic antibody that recognizes the beta-adrenergic receptor. J Clin Invest. 1982 May;69(5):1147–1154. doi: 10.1172/JCI110550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P. V., Lewis G. K. Idiotype connectance in the immune system. I. Expression of a cross-reactive idiotype on induced anti-p-azophenylarsonate antibodies and on endogenous antibodies not specific for arsonate. J Exp Med. 1983 Apr 1;157(4):1116–1136. doi: 10.1084/jem.157.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. N., Pepper B. M., Briones-Urbina R., Farid N. R. Biological activity of anti-thyrotropin anti-idiotypic antibody. Eur J Immunol. 1983 Jan;13(1):57–63. doi: 10.1002/eji.1830130113. [DOI] [PubMed] [Google Scholar]
- Iverson G. M., Eardley D. D., Janeway C. A., Gershon R. K. Use of anti-idiotype immunosorbents to isolate circulating antigen-specific T cell-derived molecules from hyperimmune sera. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1435–1439. doi: 10.1073/pnas.80.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kennedy R. C., Dreesman G. R. Common idiotypic determinant associated with human antibodies to hepatitis B surface antigen. J Immunol. 1983 Jan;130(1):385–389. [PubMed] [Google Scholar]
- Kleinmann R. E., Braverman L. E., Vagenakis A. G., Butcher R. W., Clark R. B. A new method for measurement of human thyroid-stimulating immunoglobulins. J Lab Clin Med. 1980 Apr;95(4):581–589. [PubMed] [Google Scholar]
- Kriss J. P. Inactivation of long-acting thyroid stimulator (LATS) by anti-kappa and anti-lambda antisera. J Clin Endocrinol Metab. 1968 Oct;28(10):1440–1444. doi: 10.1210/jcem-28-10-1440. [DOI] [PubMed] [Google Scholar]
- Lefvert A. K., James R. W., Alliod C., Fulpius B. W. A monoclonal anti-idiotypic antibody against anti-receptor antibodies from myasthenic sera. Eur J Immunol. 1982 Sep;12(9):790–792. doi: 10.1002/eji.1830120917. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Becker E. L. Anti-idiotype as antibody against the formyl peptide chemotaxis receptor of the neutrophil. J Immunol. 1982 Feb;128(2):963–968. [PubMed] [Google Scholar]
- Miller G. G., Nadler P. I., Hodes R. J., Sachs D. H. Modification of T cell antinuclease idiotype expression by in vivo administration of anti-idiotype. J Exp Med. 1982 Jan 1;155(1):190–200. doi: 10.1084/jem.155.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson E. G., Phillips M. Suppression of interstitial nephritis by auto-anti-idiotypic immunity. J Exp Med. 1982 Jan 1;155(1):179–189. doi: 10.1084/jem.155.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisonoff A., Lamoyi E. Implications of the presence of an internal image of the antigen in anti-idiotypic antibodies: possible application to vaccine production. Clin Immunol Immunopathol. 1981 Dec;21(3):397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Olson J. C., Wagner C. R., Leslie G. A. The assessment of anti-idiopathic antibodies as effective immunoregulatory probes in vivo. Clin Exp Immunol. 1982 May;48(2):458–468. [PMC free article] [PubMed] [Google Scholar]
- Piatier-Tonneau D., Boyer B., Debre P., Charron D. J. Syngeneic anti-idiotypes against monoclonal anti-HLA-DR antibodies: characterization of recurrent idiotopes. J Immunol. 1983 Sep;131(3):1339–1343. [PubMed] [Google Scholar]
- Schleusener H., Kotulla P., Finke R., Sörje H., Meinhold H., Adlkofer F., Wenzel K. W. Relationship between thyroid status and Graves' disease-specific immunoglobulins. J Clin Endocrinol Metab. 1978 Aug;47(2):379–384. doi: 10.1210/jcem-47-2-379. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Couraud P. O., Andre C., Vray B., Strosberg A. D. Anti-alprenolol anti-idiotypic antibodies bind to beta-adrenergic receptors and modulate catecholamine-sensitive adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7385–7389. doi: 10.1073/pnas.77.12.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Maron R., Elias D., Cohen I. R. Autoantibodies to insulin receptor spontaneously develop as anti-idiotypes in mice immunized with insulin. Science. 1982 Apr 30;216(4545):542–545. doi: 10.1126/science.7041258. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Munro D. S., Dorrington K. J. The distribution of the long-acting thyroid stimulator among gamma G-immunoglobulins. Biochim Biophys Acta. 1969 Aug 12;188(1):89–100. doi: 10.1016/0005-2795(69)90048-8. [DOI] [PubMed] [Google Scholar]
- Strakosch C. R., Wenzel B. E., Row V. V., Volpé R. Immunology of autoimmune thyroid diseases. N Engl J Med. 1982 Dec 9;307(24):1499–1507. doi: 10.1056/NEJM198212093072407. [DOI] [PubMed] [Google Scholar]
- Sugenoya A., Kidd A., Row V. V., Volpé R. Correlation between thyrotropin-displacing activity and human thyroid-stimulating activity by immunoglobulins from patients with Graves' disease and other thyroid disorders. J Clin Endocrinol Metab. 1979 Mar;48(3):398–402. doi: 10.1210/jcem-48-3-398. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Smith B. R., Clayton B., Evered D. C., Clark F., Hall R. Thyroid-stimulating immunoglobulins in ophthalmic Graves' disease. Clin Endocrinol (Oxf) 1977 Mar;6(3):207–211. doi: 10.1111/j.1365-2265.1977.tb03316.x. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Yeung R. T. Changes in thyroid-stimulating antibody activity in Graves' disease treated with antithyroid drug and its relationship to relapse: a prospective study. J Clin Endocrinol Metab. 1980 Jan;50(1):144–147. doi: 10.1210/jcem-50-1-144. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Yeung R. T., Khoo R. K., Alagaratnam T. T. A prospective study of the changes in thyrotropin binding inhibitory immunoglobulins in Graves' disease treated by subtotal thyroidectomy or radioactive iodine. J Clin Endocrinol Metab. 1980 Jun;50(6):1005–1010. doi: 10.1210/jcem-50-6-1005. [DOI] [PubMed] [Google Scholar]
- Valente W. A., Vitti P., Yavin Z., Yavin E., Rotella C. M., Grollman E. F., Toccafondi R. S., Kohn L. D. Monoclonal antibodies to the thyrotropin receptor: stimulating and blocking antibodies derived from the lymphocytes of patients with Graves disease. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6680–6684. doi: 10.1073/pnas.79.21.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman D. J., Lane H. C., Fauci A. S. Antigen-induced in vitro antibody production in humans: a model for B cell activation and immunoregulation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2528–2531. doi: 10.1073/pnas.78.4.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann N. H., Penn A. S., Freimuth P. I., Treptow N., Wentzel S., Cleveland W. L., Erlanger B. F. Anti-idiotypic route to anti-acetylcholine receptor antibodies and experimental myasthenia gravis. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4810–4814. doi: 10.1073/pnas.79.15.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L. C., Ingbar S. H. Hypothyroidism as a late sequela in patient with Graves' disease treated with antithyroid agents. J Clin Invest. 1979 Nov;64(5):1429–1436. doi: 10.1172/JCI109601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M., Banovac K. Clinical significance of assay of thyroid-stimulating antibody in Graves' disease. Ann Intern Med. 1980 Jul;93(1):28–32. doi: 10.7326/0003-4819-93-1-28. [DOI] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M., Munro D. S. Immunoglobulin G inhibitor of thyroid-stimulating antibody is a cause of delay in the onset of neonatal Graves' disease. J Clin Invest. 1983 Oct;72(4):1352–1356. doi: 10.1172/JCI111091. [DOI] [PMC free article] [PubMed] [Google Scholar]


