Abstract
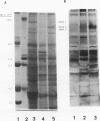
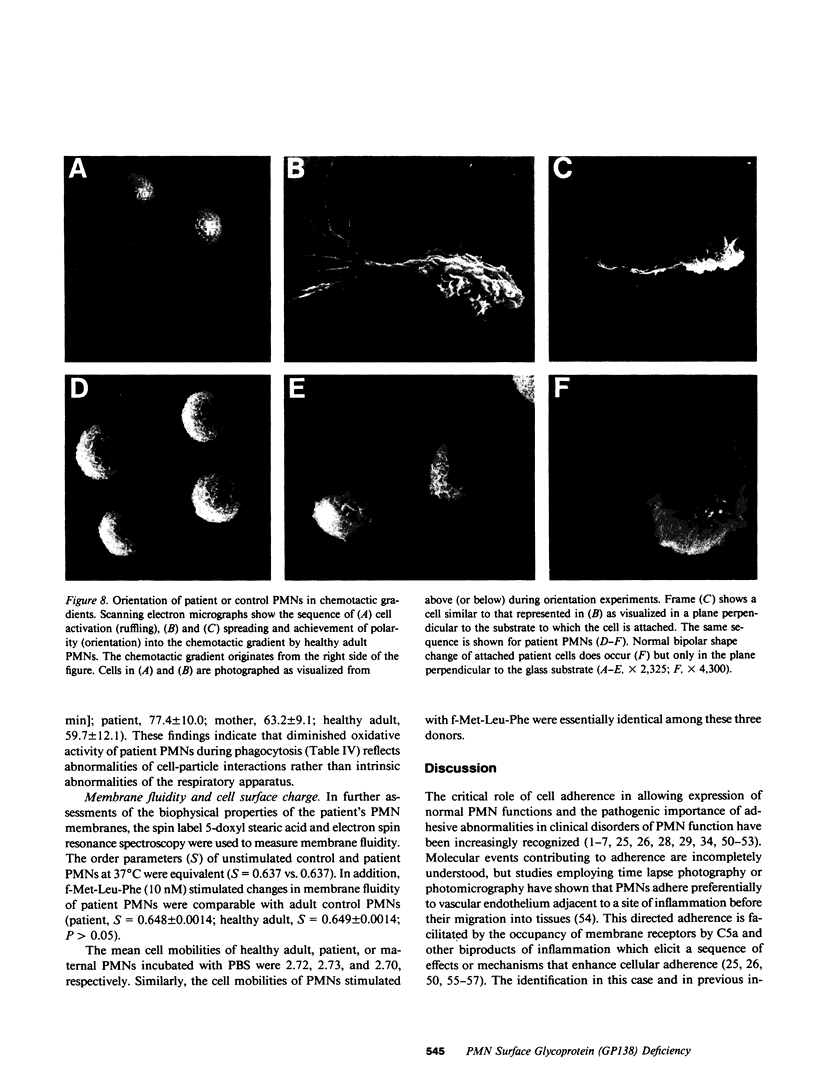
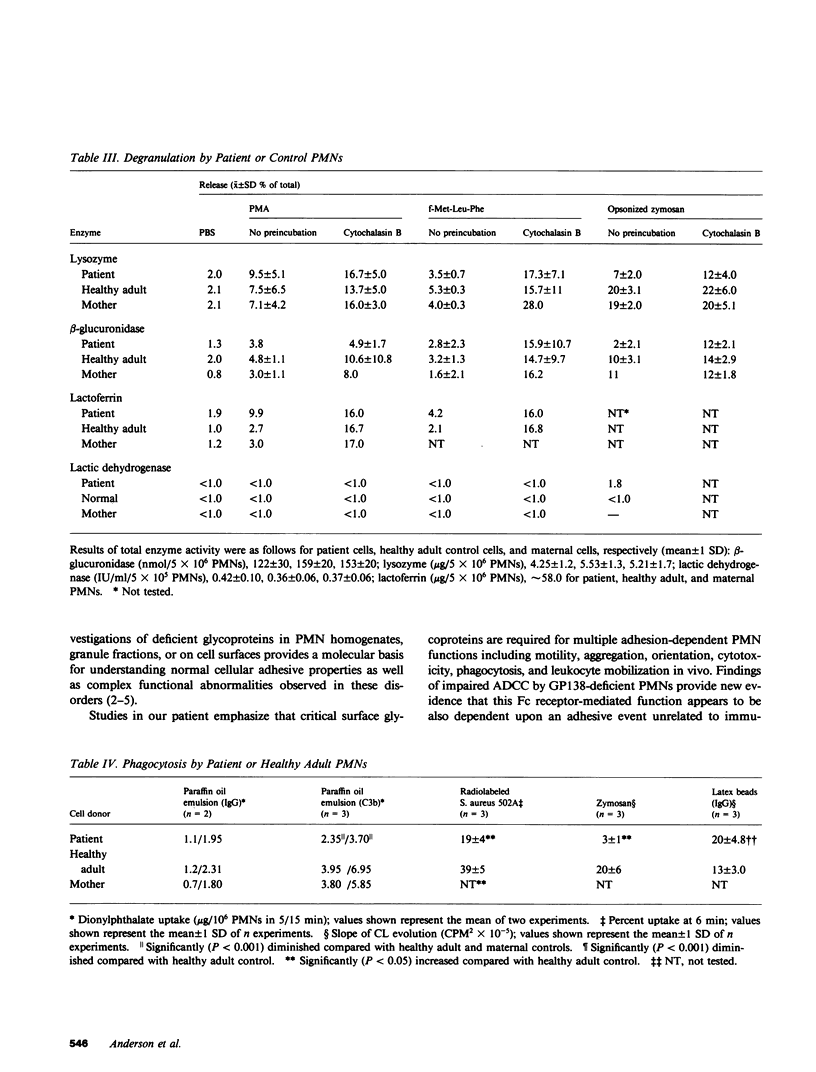
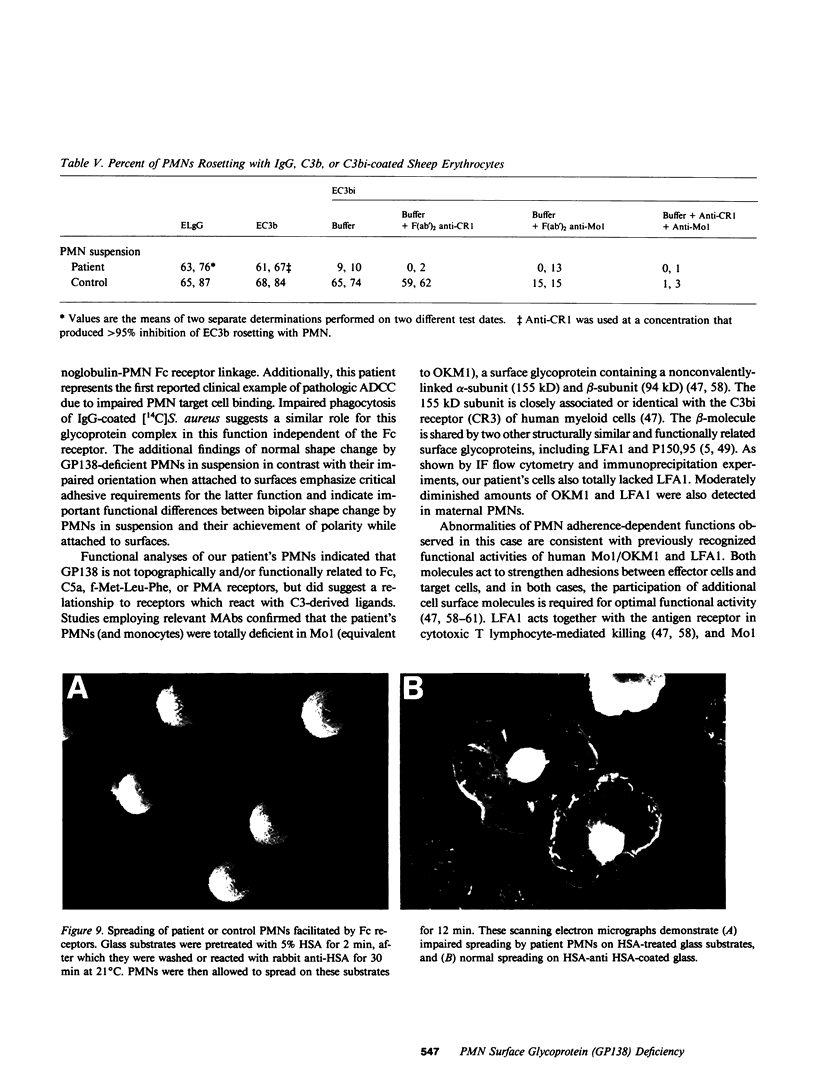
Investigations of polymorphonuclear leukocyte (PMN) function were performed in a 5-yr-old white female with delayed umbilical cord separation, impaired pus formation, and a severe defect of PMN chemotaxis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis demonstrated an almost total deficiency of a high molecular weight glycoprotein(s) (GP138) in the granule and membrane fractions of the patient's cells, and NaB3H4-galactose oxidase labeling demonstrated the absence of a major glycoprotein complex on the surface of her PMNs. Monoclonal antibodies (MAb) were employed in flow cytometry experiments to demonstrate that two previously characterized glycoproteins (Mo1 and LFA1) were undetectable on the surface of the patient's PMNs and monocytes. Immunoprecipitation of 125I-labeled patient cells with subunit specific MAbs confirmed that the alpha-subunits of Mo1 (155 kD) and LFA1 (177 kD) and their common beta-subunit (94 kD) were totally deficient. Functional analyses of patient PMNs demonstrated severe impairment of adherence- and adhesion-dependent cell functions including spreading, aggregation, orientation in chemotactic gradients, antibody-dependent cellular cytotoxicity, and phagocytosis of particles (Oil-Red-0-paraffin, zymosan) selectively opsonized with C3-derived ligands. Patient PMNs demonstrated a normal capacity to rosette with IgG or C3b-coated sheep erythrocytes, but rosette formation with C3bi-coated erythrocytes was profoundly diminished. Adhesion-independent functions including shape change, N-formyl-methionyl-leucyl-3H-phenylalanine binding, and O-2 generation or secretion elicited by soluble stimuli were normal. Membrane fluidity, surface charge, and microtubule assembly were also normal. These findings provide new evidence that critical PMN surface glycoproteins are required to facilitate multiple adhesion-dependent cellular functions of the inflammatory response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. S., Mills E. L., Sawyer M. K., Regelmann W. R., Nelson J. D., Quie P. G. Recurrent infections and delayed separation of the umbilical cord in an infant with abnormal phagocytic cell locomotion and oxidative response during particle phagocytosis. J Pediatr. 1981 Dec;99(6):887–894. doi: 10.1016/s0022-3476(81)80011-x. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Hughes B. J., Smith C. W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981 Oct;68(4):863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Mace M. L., Brinkley B. R., Martin R. R., Smith C. W. Recurrent infection in glycogenosis type Ib: abnormal neutrophil motility related to impaired redistribution of adhesion sites. J Infect Dis. 1981 Mar;143(3):447–459. doi: 10.1093/infdis/143.3.447. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Wible L. J., Hughes B. J., Smith C. W., Brinkley B. R. Cytoplasmic microtubules in polymorphonuclear leukocytes: effects of chemotactic stimulation and colchicine. Cell. 1982 Dec;31(3 Pt 2):719–729. doi: 10.1016/0092-8674(82)90326-9. [DOI] [PubMed] [Google Scholar]
- Andersson L. C., Gahmberg C. G. Surface glycoproteins of human white blood cells. Analysis by surface labeling. Blood. 1978 Jul;52(1):57–67. [PubMed] [Google Scholar]
- Arnaout M. A., Pitt J., Cohen H. J., Melamed J., Rosen F. S., Colten H. R. Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982 Mar 25;306(12):693–699. doi: 10.1056/NEJM198203253061201. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Bass D. A., Grover W. H., Lewis J. C., Szejda P., DeChatelet L. R., McCall C. E. Comparison of human eosinophils from normals and patients with eosinophilia. J Clin Invest. 1980 Dec;66(6):1265–1273. doi: 10.1172/JCI109978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Nussenzweig V. Complement receptors. Contemp Top Mol Immunol. 1977;6:145–176. doi: 10.1007/978-1-4684-2841-4_5. [DOI] [PubMed] [Google Scholar]
- Bissenden J. G., Haeney M. R., Tarlow M. J., Thompson R. A. Delayed separation of the umbilical cord, severe widespread infections, and immunodeficiency. Arch Dis Child. 1981 May;56(5):397–399. doi: 10.1136/adc.56.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen T. J., Ochs H. D., Altman L. C., Price T. H., Van Epps D. E., Brautigan D. L., Rosin R. E., Perkins W. D., Babior B. M., Klebanoff S. J. Severe recurrent bacterial infections associated with defective adherence and chemotaxis in two patients with neutrophils deficient in a cell-associated glycoprotein. J Pediatr. 1982 Dec;101(6):932–940. doi: 10.1016/s0022-3476(82)80013-9. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C. A., Curnutte J. T., Rosin R. E., André-Schwartz J., Gallin J. I., Klempner M., Snyderman R., Southwick F. S., Stossel T. P., Babior B. M. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980 May 22;302(21):1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- Davies E. G., Isaacs D., Levinsky R. J. Defective immune interferon production and natural killer activity associated with poor neutrophil mobility and delayed umbilical cord separation. Clin Exp Immunol. 1982 Nov;50(2):454–460. [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Fischer A., Trung P. H., Descamps-Latscha B., Lisowska-Grospierre B., Gerota I., Perez N., Scheinmetzler C., Durandy A., Virelizier J. L., Griscelli C. Bone-marrow transplantation for inborn error of phagocytic cells associated with defective adherence, chemotaxis, and oxidative response during opsonised particle phagocytosis. Lancet. 1983 Aug 27;2(8348):473–476. doi: 10.1016/s0140-6736(83)90509-3. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gahmberg C. G. Tritium labeling of cell-surface glycoproteins and glycolipids using galactose oxidase. Methods Enzymol. 1978;50:204–206. doi: 10.1016/0076-6879(78)50020-7. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Abnormal phagocyte chemotaxis: pathophysiology, clinical manifestations, and management of patients. Rev Infect Dis. 1981 Nov-Dec;3(6):1196–1220. doi: 10.1093/clinids/3.6.1196. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Fletcher M. P., Seligmann B. E., Hoffstein S., Cehrs K., Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982 Jun;59(6):1317–1329. [PubMed] [Google Scholar]
- Gallin J. I., Malech H. L., Wright D. G., Whisnant J. K., Kirkpatrick C. H. Recurrent severe infections in a child with abnormal leukocyte function: possible relationship to increased microtubule assembly. Blood. 1978 May;51(5):919–933. [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak R. A., Ingraham L. M., Baehner R. L., Boxer L. A. Membrane fluidity in human and mouse Chediak-Higashi leukocytes. J Clin Invest. 1979 Jul;64(1):138–144. doi: 10.1172/JCI109432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Bowers T. K., Lammi-Keefe C. J., Jacob H. S., Craddock P. R. Granulocyte aggregometry: a sensitive technique for the detection of C5a and complement activation. Blood. 1980 Jun;55(6):898–902. [PubMed] [Google Scholar]
- Hoover R. L., Briggs R. T., Karnovsky M. J. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978 Jun;14(2):423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Defects of neutrophil function. N Engl J Med. 1982 Aug 12;307(7):434–436. doi: 10.1056/NEJM198208123070709. [DOI] [PubMed] [Google Scholar]
- Kohl S., Frazier J. P., Pickering L. K., Loo L. S. Normal function of neonatal polymorphonuclear leukocytes in antibody-dependent cellular-cytotoxicity to herpes simplex virus-infected cells. J Pediatr. 1981 May;98(5):783–785. doi: 10.1016/s0022-3476(81)80847-5. [DOI] [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo A., Caimi L., Marchesini S., Goi G. C., Tettamanti G. Enzymes of lysosomal origin in human plasma and serum: assay conditions and parameters influencing the assay. Clin Chim Acta. 1980 Dec 22;108(3):337–346. doi: 10.1016/0009-8981(80)90339-3. [DOI] [PubMed] [Google Scholar]
- Maderazo E. G., Woronick C. L. Micropore filter assay of human granulocyte locomotion: problems and solutions. Clin Immunol Immunopathol. 1978 Oct;11(2):196–211. doi: 10.1016/0090-1229(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Martz E. Mechanism of specific tumor-cell lysis by alloimmune T lymphocytes: resolution and characterization of discrete steps in the cellular interaction. Contemp Top Immunobiol. 1977;7:301–361. doi: 10.1007/978-1-4684-3054-7_9. [DOI] [PubMed] [Google Scholar]
- Mease A. D., Fischer G. W., Hunter K. W., Ruymann F. B. Decreased phytohemagglutinin-induced aggregation and C5a-induced chemotaxis of human newborn neutrophils. Pediatr Res. 1980 Feb;14(2):142–146. doi: 10.1203/00006450-198002000-00015. [DOI] [PubMed] [Google Scholar]
- Oliver J. M. Concanavalin A cap formation on human polymorphonuclear leukocytes induced by R17934, a new antitumor drug that interferes with microtubule assembly. J Reticuloendothel Soc. 1976 Jun;19(6):389–395. [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Schreiber R. D., Müller-Eberhard H. J. Interaction of target cell-bound C3bi and C3d with human lymphocyte receptors. Enhancement of antibody-mediated cellular cytotoxicity. J Exp Med. 1981 Jun 1;153(6):1592–1603. doi: 10.1084/jem.153.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBUCK J. W., CROWLEY J. H. A method of studying leukocytic functions in vivo. Ann N Y Acad Sci. 1955 Mar 24;59(5):757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin S. B., Stossel T. P. Complement (C3)-activated phagocytosis by lung macrophages. J Immunol. 1978 Apr;120(4):1305–1312. [PubMed] [Google Scholar]
- Silva A., Bonavida B., Targan S. Mode of action of interferon-mediated modulation of natural killer cytotoxic activity: recruitment of pre-NK cells and enhanced kinetics of lysis. J Immunol. 1980 Aug;125(2):479–484. [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. Motility and adhesiveness in human neutrophils. Redistribution of chemotactic factor-induced adhesion sites. J Clin Invest. 1980 Apr;65(4):804–812. doi: 10.1172/JCI109731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977 Nov;75(2 Pt 1):606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]