Abstract
Background
Biologic glenoid resurfacing is a treatment option for young patients with glenohumeral arthritis. An optimal synthetic graft for glenoid resurfacing should allow repopulation with host cells, be durable enough to tolerate suture fixation and forces across the joint, and present no host inflammatory response. We report two cases of giant cell reaction to GraftJacket® after biologic glenoid resurfacing.
Case Description
Two patients who underwent hemiarthroplasty and biologic glenoid resurfacing using GraftJacket® had a foreign body giant cell reaction that required revision surgery. Intraoperatively, both patients were observed to have a well-fixed humeral component and a dense, erythematous, synovitic membrane overlying the glenoid. Pathology specimens showed a benign reactive synovium, chronic inflammation, and foreign body giant cell reaction. After débridement and conversion to total shoulder arthroplasty, both patients continued to be pain-free at greater than 1-year followup.
Literature Review
Multinucleated giant cell and mononuclear cell responses have been observed in an animal model after use of GraftJacket®. Although the use of acellular matrix-based scaffold for biologic glenoid resurfacing is not new, the possibility of foreign body reaction as a source of persistent symptoms has not been described.
Clinical Relevance
Given the lack of data to indicate an advantage to biologic resurfacing of the glenoid over hemiarthroplasty alone, resurfacing should not introduce significant additional surgical complications. We suggest foreign body reaction be considered in the differential diagnosis for a persistently painful shoulder after biologic glenoid resurfacing using an acellular allograft patch.
Introduction
Because of concerns regarding the longevity of the glenoid component in a total shoulder arthroplasty (TSA), biologic glenoid resurfacing has been described as an alternative to other materials such as polyethylene for treatment for young patients with glenohumeral joint arthritis. In a systematic review of outcomes of biologic glenoid resurfacing, a complication rate of 13.3% and a reoperation rate of 26% at a mean followup of approximately 2 years were reported [12]. Techniques have been described using autografts and allografts [3–5, 7, 10, 11, 13].
Commercially available acellular, matrix-based scaffolds have been used in shoulder surgery with FDA approval for use in rotator cuff repair augmentation [15]. The off-label use of these scaffolds for glenoid resurfacing also has been described [6, 14]. No study has evaluated durability of different graft materials, and there is no gold standard. An optimal graft for glenoid resurfacing should be a material that allows repopulation with host cells, is durable enough to tolerate suture fixation and forces across the glenohumeral joint, has a low coefficient of friction, and presents little to no host inflammatory response.
We report two cases of giant cell reaction and graft rejection after biologic glenoid resurfacing with GraftJacket® (Wright Medical Technology, Inc, Arlington, TN, USA).
Case Reports
A 28-year-old woman presented with complaints of left shoulder pain and stiffness. Previously, she had a work-related injury and underwent arthroscopic labral repair at an outside hospital. She was treated with an intraarticular analgesic pain pump postoperatively. She subsequently had stiffness and pain develop and underwent an arthroscopic procedure during which she was diagnosed with severe chondrolysis of the glenoid and humeral surfaces. She underwent two additional arthroscopic débridements at outside facilities. When she presented to our clinic, she had 1 year of failed nonoperative treatment.
On examination, she had painful active forward elevation to 90°, abduction in the scapular plane to 60°, and external rotation with the arm at the side to 30°. She had limited internal rotation to the sacrum. On review of radiographs and prior arthroscopic images, the diagnosis of diffuse glenohumeral chondrolysis was confirmed. After completely discussing the risks and benefits, the patient elected to undergo hemiarthroplasty using a resurfacing cap (DePuy Orthopaedics, Inc, Warsaw, IN, USA) and glenoid resurfacing using a GraftJacket® Maxforce graft secured with polyetheretherketone (PEEK) anchors (FASTak®; Arthrex, Inc, Naples, FL, USA) circumferentially around the glenoid. There were no complications and intraoperative cultures were negative.
She did not experience any postoperative complications, but she did not achieve a satisfactory level of pain relief. Two years after the index arthroplasty, she continued to report significant pain at night and with motion. Radiographs were normal, and a bone scan did not suggest infection, glenoid wear, or humeral component loosening. At 3.5 years after the index arthroplasty, her pain had worsened. She underwent diagnostic arthroscopy to evaluate the joint, perform a synovectomy, and obtain tissue for culture to exclude subclinical infection as a source of her symptoms.
In the operating room, diagnostic arthroscopy showed a well-fixed humeral component and a membrane overlying the glenoid that grossly appeared inflamed (Fig. 1). Microbiologic and histopathologic analyses were performed on several specimens from this membrane. A synovectomy was performed and the membrane was débrided from the glenoid surface. A capsular release also was performed. Cultures from the surgery, including anaerobic cultures saved for 14 days, revealed no growth and pathology specimens showed a reactive synovium with chronic inflammation. Additionally, there were foci of foreign body giant cell reaction, diffuse foci of histiocytic reaction to brown granular pigmented particles, and foci of hemosiderin-laden macrophages.
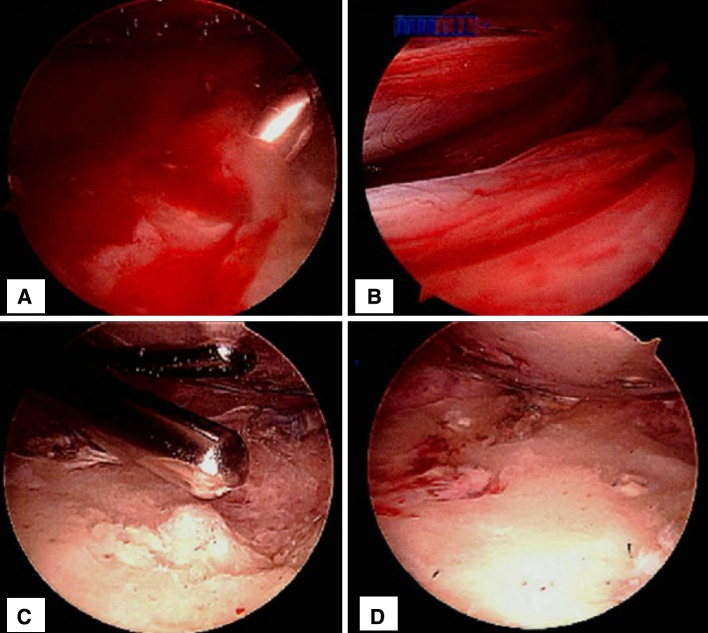
Fig. 1A–D.
(A) The glenoid surface covered by an erythematous membrane, (B) a view of the glenoid rim and overlying erythematous membrane, (C) débridement of the membrane and glenoid face using an arthroscopic shaver, and (D) the glenoid surface after débridement of the membrane and remnant graft material are shown.
The patient continued to have significant shoulder pain after the arthroscopy and ultimately underwent a TSA 4 years after the index arthroplasty. Three weeks after surgery, the patient reported nearly complete pain relief. Two years after revision of the hemiarthroplasty to a TSA, she had active forward elevation to 150°, active external rotation to 65°, and a painless shoulder.
Patient 2 was a 38-year-old right-hand-dominant woman who presented to our clinic with right shoulder pain. She had a history of a traumatic shoulder dislocation 3 years ago. Additionally, she had undergone an arthroscopic shoulder débridement 18 months previously. On examination, forward elevation was 150°, abduction in the scapular plane was 60°, external rotation was 45°, and internal rotation was limited to the lower lumbar spine. Radiographs showed progressive glenohumeral arthrosis with joint space narrowing. MRI confirmed severe glenohumeral arthrosis, no eccentric glenoid wear, and an intact rotator cuff. An infection workup was negative. After 6 months of nonoperative treatment, she had no symptom resolution. After a discussion of the potential risks of the procedure, the patient underwent a hemiarthroplasty using a resurfacing cap and glenoid resurfacing using a GraftJacket® Maxforce graft secured with PEEK anchors (FASTak®) circumferentially around the glenoid. There were no complications and all intraoperative cultures were negative.
The patient had an uneventful postoperative course; however, she continued to experience pain throughout the postoperative setting. Two years after biologic glenoid resurfacing, the patient elected to undergo a TSA. On exposing the glenohumeral joint, a dense, erythematous, membrane was seen covering the glenoid. Cultures and a pathology specimen were obtained from the glenoid surface before débridement. All cultures from surgery, including those held for 14 days, revealed no growth. Histopathology assessment of the GraftJacket® membrane showed fragments of benign reactive synovium with chronic inflammation. Additionally, there were foci of foreign body giant cell reaction (Fig. 2). The patient reported nearly immediate pain relief after conversion of the hemiarthroplasty with biologic glenoid resurfacing to a TSA. At the 1-year followup, she was pain-free with near-normal motion.
Fig. 2.
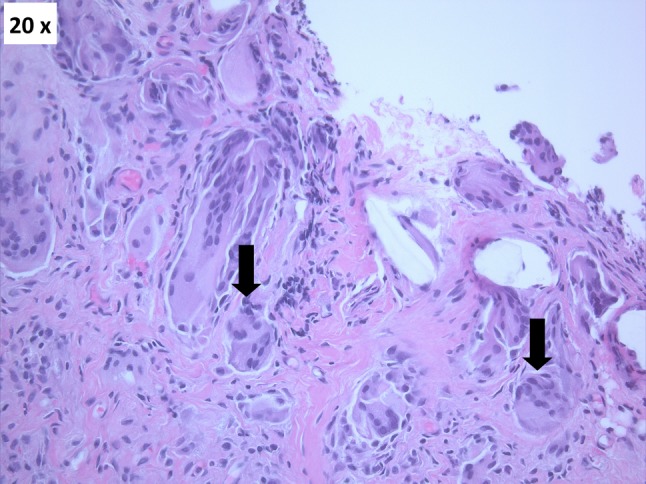
Hematoxylin and eosin staining of inflamed membrane covering the glenoid surface shows multiple giant cell (black arrows) reactions (Original magnification, ×20).
Discussion
Various allograft and autograft options have been used for biologic glenoid resurfacing with widely variable success [7, 9]. Given uncertainties regarding allografts and autografts, some have used acellular, matrix-based scaffolds for glenoid resurfacing. Savoie et al. [14] used the Restore® patch (DePuy Orthopaedics, Inc), an implant made of porcine small intestine submucosa cells that was postulated to have pluripotent properties in the hope of regenerating viable chondrocytes on the articular surface of the glenoid [14]. In the discussion of that paper, since the completion of their study, the authors noted an allergic-type reaction in a patient in whom this patch was used to supplement a rotator cuff repair. This is in keeping with several other investigators who had noted a similar type of reaction with use of the Restore® patch [8, 17]. GraftJacket® is an acellular human dermal matrix allograft that is believed to be less immunogenic. Studies of GraftJacket® use in the rotator cuff support its potential for revascularization and repopulation, and its graft strength for suture retention without complications of inflammation seen with other extracellular matrices [1, 15]. Despite this, its off-label use in biologic glenoid resurfacing has not been extensively studied [2, 6]. In one report of 32 patients with biologic glenoid resurfacings using Graftjacket®, the authors noted one foreign body reaction to graft material, although this case was not extensively described [6]. In a histologic study [16] that evaluated the host-tissue morphologic response to five commercially available extracellular matrix-derived biologic scaffolds used for orthopaedic soft tissue repair in a rodent model, GraftJacket® and Restore® devices were associated with the most intense cell response, including multinucleated giant cells and a mononuclear cell response.
We have used GraftJacket® for biologic resurfacing of the glenoid in 11 patients during a 4-year span, including the two patients discussed in the current report. In addition to the two described here who underwent conversion of their hemiarthroplasties to TSAs, a third has had an arthroscopic débridement without additional surgery. In the third case, acellular fibrous tissue with no histopathologic evidence of inflammation or foreign body giant cell reaction was noted. As only three patients have undergone any repeat surgery and since these 11 patients have not been prospectively followed in a study-specific fashion, we cannot comment on the true incidence of foreign body reaction to GraftJacket® in biologic glenoid resurfacing.
There are no strong clinical data that favor biologic resurfacing of the glenoid over hemiarthroplasty or débridement alone. Therefore, if resurfacing is performed, it should not introduce significant additional surgical complications. Because of the absence of a clear benefit of biologic resurfacing [12], and in light of our observation of a foreign body reaction in these patients, we no longer use this technique. Additionally, we suggest foreign body reaction be considered in the differential diagnosis for a persistently painful shoulder after biologic glenoid resurfacing using an acellular allograft patch.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the reporting of these cases and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Penn Presbyterian Medical Center, Philadelphia, PA, USA.
References
- 1.Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534–538. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Arthroscopic technique of interposition arthroplasty of the glenohumeral joint. Arthroscopy. 2006;22:570e1–570e5. doi: 10.1016/j.arthro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Burkhead WZ, Jr, Hutton KS. Biologic resurfacing of the glenoid with hemiarthroplasty of the shoulder. J Shoulder Elbow Surg. 1995;4:263–270. doi: 10.1016/S1058-2746(05)80019-9. [DOI] [PubMed] [Google Scholar]
- 4.Burkhead WZ, Jr, Krishnan SG, Lin KC. Biologic resurfacing of the arthritic glenohumeral joint: historical review and current applications. J Shoulder Elbow Surg. 2007;16(5 suppl):S248–S253. doi: 10.1016/j.jse.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Creighton RA, Cole BJ, Nicholson GP, Romeo AA, Lorenz EP. Effect of lateral meniscus allograft on shoulder articular contact areas and pressures. J Shoulder Elbow Surg. 2007;16:367–372. doi: 10.1016/j.jse.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 6.de Beer JF, Bhatia DN, van Rooyen KS, Du Toit DF. Arthroscopic debridement and biological resurfacing of the glenoid in glenohumeral arthritis. Knee Surg Sports Traumatol Arthrosc. 2010;18:1767–1773. doi: 10.1007/s00167-010-1155-8. [DOI] [PubMed] [Google Scholar]
- 7.Elhassan B, Ozbaydar M, Diller D, Higgins LD, Warner JP. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am. 2009;91:419–424. doi: 10.2106/JBJS.H.00318. [DOI] [PubMed] [Google Scholar]
- 8.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan SG, Nowinski RJ, Harrison D, Burkhead WZ. Humeral hemiarthroplasty with biologic resurfacing of the glenoid for glenohumeral arthritis: two to fifteen-year outcomes. J Bone Joint Surg Am. 2007;89:727–734. doi: 10.2106/JBJS.E.01291. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan SG, Reineck JR, Nowinski RJ, Harrison D, Burkhead WZ. Humeral hemiarthroplasty with biologic resurfacing of the glenoid for glenohumeral arthritis: surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 1):9–19. doi: 10.2106/JBJS.G.01220. [DOI] [PubMed] [Google Scholar]
- 11.Lee KT, Bell S, Salmon J. Cementless surface replacement arthroplasty of the shoulder with biologic resurfacing of the glenoid. J Shoulder Elbow Surg. 2009;18:915–919. doi: 10.1016/j.jse.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Namdari S, Alosh H, Baldwin K, Glaser D, Kelly JD. Biological glenoid resurfacing for glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg. 2011;20:1184–1190. doi: 10.1016/j.jse.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson GP, Goldstein JL, Romeo AA, Cole BJ, Hayden JK, Twigg SL, McCarty LP, Detterline AJ. Lateral meniscus allograft biologic glenoid arthroplasty in total shoulder arthroplasty for young shoulders with degenerative joint disease. J Shoulder Elbow Surg. 2007;16(5 suppl):S261–S266. doi: 10.1016/j.jse.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Savoie FH, 3rd, Brislin KJ, Argo D. Arthroscopic glenoid resurfacing as a surgical treatment for glenohumeral arthritis in the young patient: midterm results. Arthroscopy. 2009;25:864–871. doi: 10.1016/j.arthro.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Snyder SJ, Arnoczky SP, Bond JL, Dopirak R. Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket Matrix. Arthroscopy. 2009;25:329–333. doi: 10.1016/j.arthro.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications: a comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 17.Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89:786–791. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]