Abstract
Background
A periprosthetic joint infection is one of the most challenging complications associated with THA and TKA. In the diagnostic process for detecting a periprosthetic joint infection, one of the most important steps is analysis of laboratory infection biomarkers.
Questions/purposes
We investigated the sensitivity and specificity of the biomarkers procalcitonin, interleukin 6 (IL-6), and interferon α (IFN-α) as compared with conventional biomarkers (C-reactive protein [CRP], leukocyte level) for a periprosthetic joint infection associated with revision arthroplasties.
Methods
We prospectively included and analyzed 84 patients (124 revision arthroplasties). The blood parameters of interest were procalcitonin, IL-6, IFN-α, leukocyte level, and CRP. Samples were taken preoperatively and on the first, third, and seventh postoperative days. The sensitivity and specificity of these biomarkers then were calculated.
Results
Considering the preoperative values of 84 patients (124 operations), procalcitonin, IL-6, CRP, and leukocyte level correlated with periprosthetic joint infection, whereas IFN-α did not. A procalcitonin cut-off level of 0.35 ng/mL revealed a sensitivity of 80% and specificity of 37%. An IL-6 cut-off level of 2.55 pg/mL had a sensitivity of 92% and specificity of 59%.
Conclusions
In this study procalcitonin and IL-6 were helpful for detecting periprosthetic joint infections in revision arthroplasties, although CRP generally was superior. Procalcitonin and IL-6 may be considered adjuvant tests when the diagnosis of a periprosthetic joint infection is in doubt. This study showed, in addition to conventional biomarkers such as CRP and leukocyte level, procalcitonin and IL-6 were helpful for detecting infections associated with revision arthroplasties.
Level of Evidence
Level II, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic joint infections occur commonly; in some series, infection complicates revision arthroplasty in as many as 10% to 20% of patients [9, 15, 17, 36]. The clinical picture often is ambiguous [9], and although biomarkers like C-reactive protein (CRP) or leukocyte levels [4, 7, 9–11, 28, 34] are helpful, they can be misleading in patients with chronic inflammatory diseases, obesity, metabolic syndrome, and insulin resistance, and in smokers [3, 4, 27] and patients with postoperative hematomas [11, 22].
It is important to identify periprosthetic joint infections as early as possible [6, 9, 19, 35, 39]. Inflammatory biomarkers play a pivotal role in this diagnostic process. In the case of aseptic inflammation, however, reliance on elevated laboratory infection biomarkers may lead to unnecessary and inappropriate surgery (implants unnecessarily removed or antibiotic cement spacers needlessly implanted) [12, 16, 35]. To optimize this diagnostic process, infection biomarkers with a fast response and high sensitivity and specificity for infection are needed [4, 8, 11, 15].
Procalcitonin, the 116-amino acid prohormone of calcitonin, is synthesized in the C cells of the thyroid gland and was first described in the 1970s [25, 32]. Numerous studies have shown procalcitonin is elevated in cases of bacterial infection or sepsis and levels are considerably higher in bacterial infections than in viral infections [2, 5, 11, 14, 21, 26, 29, 30, 33]. Procalcitonin increases 2 to 4 hours after the onset of sepsis, which is later than proinflammatory cytokines, such as interleukin 6 (IL-6), but considerably earlier than CRP [31]. It reaches its peak after 6 hours and has a half-life of 25 to 30 hours [13, 23]. Some studies have shown the course of procalcitonin serum levels reflects the success of antibiotic treatment of bacterial infection rapidly, closely, and more reliably than CRP level [13, 18, 29, 31, 37].
IL-6 has been reported to be a sensitive marker for bacterial infection after total joint arthroplasty [4, 40, 41]. As IL-6 triggers the release of CRP in liver cells, it reacts much faster to infection than CRP [38, 40]. Different cells, such as monocytes, macrophages, fibroblasts, and T2 lymphocytes, also produce IL-6 after trauma [40]. The IL-6 level increases rapidly after surgery, with a peak after 3 to 6 hours. IL-6 has a mean half-life of 15 hours and decreases rapidly to normal concentrations [40].
In addition, interferon cytokines play a crucial part in the function of the innate and the adaptive immune systems [1, 42]. Interferon α (IFN-α) is important for modulation and regulation of different cytokines (eg, it boosts the signaling effect of IL-6 and thus participates in the proinflammatory cascade after viral infection) [1]. This suggests it may be a useful laboratory marker [24, 26].
We investigated the sensitivity and specificity of serum levels of procalcitonin, IL-6, and IFN-α in detecting bacterial infection in cases of surgical revision of TKAs and THAs and compared these new parameters with the conventional infection parameters CRP and leukocyte level. We also analyzed the sensitivity and specificity of combinations of these parameters.
Patients and Methods
The protocol for this prospective study was approved by the Ethics Committee of the Medical University of Graz (Number 19-279 ex 07/08), and written, informed consent was obtained from all participants included in the study.
The inclusion criteria were patients scheduled to have revision surgery after an arthroplasty of the hip or knee. We specified the following exclusion criteria: patients with inflammation other than an orthopaedic infection (eg, autoimmunity, intercurrent febrile infection) (to avoid interference with other inflammatory processes); patients with other possible preconditions for elevated inflammatory markers, such as chronic inflammatory diseases, obesity (BMI > 30), viral infections, malignancies, or heavy smoking; and patients with renal, hepatic failure, or deficiencies of the immune system. Patients were recruited between March 2008 and June 2010 in the Department for Orthopaedic Surgery, Medical University of Graz (Graz, Austria).
Blood was taken from the cubital vein the day before surgery and on the first, third, and seventh postoperative days to assess the biomarkers of interest (procalcitonin, IL-6, IFN-α, CRP, leukocyte level). Procalcitonin and IL-6 were measured with commercially available kits (Elecsys BRAHMS PCT and IL-6 Kit; Roche Diagnostics, Mannheim, Germany). The detection limit was 0.02 ng/mL (normal < 0.5 ng/mL) for procalcitonin and 1.5 pg/mL (normal < 10 pg/mL) for IL-6. IFN-α was measured with a commercially available ELISA assay (Bender MedSystems GmbH, Vienna, Austria). The detection limit was 1 pg/mL (normal < 260 pg/mL). CRP was analyzed by immune turbidimetry and required lithium-heparin blood (normal < 5.0 mg/L). Leukocyte level was analyzed by flow cytometry with EDTA plasma (normal range, 4.4–11.3 G/L).
Samples of the synovial membrane and pseudocapsule were taken during surgery and examined histologically. The specimens were obtained consistently from a defined area: in hip prostheses from the part of the membrane in contact with the neck and in knee prostheses from the medial parapatellar membrane. The surfaces of pseudocapsules that face the joint cavity were identified and sections were taken perpendicular to the cavity. The specimens for paraffin histology sections were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin. When more than five neutrophils per high-power field (x400) in five high-power fields were found in at least five separate microscopic fields, the histologic specimen was considered positive for bacterial infection, as described by Parvizi et al. [28]. Only paraffin sections (ie, no frozen sections) were used to avoid technical histologic bias.
Microbiologic samples were collected before surgery by aspiration of the joint and during the operation and/or postoperatively from drainage fluid. Parts of the synovial membrane were cultured for a minimum of 10 days in bovine bouillon. A blinded researcher collected all clinical and laboratory data and divided the patients into two groups: patients with and without bacterial infection of the orthopaedic implant. Parameters for the definition of a periprosthetic joint infection were according to Parvizi et al. [28]. The parameters were either fistulation of the prosthesis, a pathogen isolated by culture from at least two separate samples obtained from the affected prosthetic joint, or four of these criteria: elevated CRP, elevated leukocyte count, presence of purulence in the affected joint, elevated synovial leukocytes, elevated synovial neutrophils, isolated microorganism in one culture, or more than five neutrophils per high-power field in five high-power fields.
One hundred forty-six surgical revision procedures of knee or hip prostheses (104 patients) were performed at the Medical University of Graz between March 2008 and June 2010. During this time, 16 patients were scheduled for revision of an arthroplasty but were not included in the study because of an emergency operation or impossibility of analyzing the parameters of interest. Therefore, 88 patients (130 operative procedures) were included in the study. Four patients (six operations) were excluded: three of whom (five operations) had either no histologic diagnosis or a missing bacterial culture and one patient (one operation) who initially was included in the study but then was excluded because preoperative measurements were missing. This left 84 patients, 38 men (45%) and 46 women (55%) (124 operations) with acute or chronic infection of a knee and hip endoprosthesis or aseptic loosening of an implant and with preoperative and postoperative markers for analysis. The analysis included 54 patients with one operation, 22 with two operations, six with three operations, and two with four operations.
Based on the data for the first operation only (one operation per patient), 55 patients (66%) had sepsis (infection group), and 29 (35%) had no signs of infection (noninfection group). Seventy-eight (63%) revision operations were defined as septic (infection group), and 46 (37%) had no signs of infection (no infection group). The median age of patients was 66 (± 16) years in the infection group and 65 (± 15) years in the no infection group. For patients in the infection group, the knee was affected in 47 cases (60%) and the hip in 31 cases (40%). In the no infection group, 45% of the patients had knee surgery, and 55% had hip surgery. All patients were treated with antibiotic therapy starting on the day of the operation and lasting for a minimum of 2 weeks (all measurements after the operation were made during antibiotic therapy).
The operation performed in the infection group was explantation of the prosthesis and spacer implantation in 33 cases; lavage, débridement, and exchange of all mobile parts in 32 cases; exchange of the spacer owing to ongoing infection in five cases; and hip resection arthroplasty in three cases. In three cases where preoperative signs of infection were negative and therefore reimplantation of an endoprosthesis was planned, the histologic specimen was surprisingly positive for infection. In addition, after one exchange of the stem and one exchange of the inlay, the bacteriologic analysis was positive for infection. In the no infection group, an exchange of the prosthesis (or parts of it) was performed in 26 cases, and reimplantation of the prosthesis after spacer implantation was done in 18 cases owing to aseptic loosening.
Bacteria were identified in 39 (71%) of 55 patients of the infection group. Staphylococci were found in 30 (55%), Streptococci in eight (15%) and Enterococci and Propionibacterium acnes were found in one (2%). In 16 patients (29%) in the infection group with positive histologic specimens for infection, no bacteria were isolated after 14 days’ incubation.
In the noninfection group, the isolated bacteria (all of which were obtained postoperatively from the Redon drain) were a Staphylococcus species in three (10%), Enterobacter cloacae in one (3%), and Proteus mirabilis in one (3%) of 29 patients. There were no clinical or laboratory signs of bacterial infection, and the 6-month followup indicated no infection. These five cases (17%) were defined as noninfected and interpreted as being caused by contamination.
The biomarkers of interest were procalcitonin, IL-6, IFN-α, CRP, and leukocytes. Univariate logistic regressions were performed to verify if these data differed preoperatively in terms of histology. To ensure all available measurements of all times were taken into account, nonlinear mixed models with a first-order autoregressive covariance structure also were calculated to detect differences between the two groups (infection versus no infection) and changes to the preoperative value for each group. Receiver operating characteristic (ROC) curves were plotted for the parameters. For combining different parameters we first calculated an optimal threshold for each parameter. Therefore we calculated the distance between each point of the ROC curve and the point sensitivity: 1, and 1-specificity: 0. We performed statistical analyses using PASW® Version 18 software (SPSS Inc, Chicago, IL, USA).
Results
Based on the data for the first operation (one operation per patient), the preoperative procalcitonin value was a significant predictor of infection (p = 0.038). For procalcitonin, the cut-off point of 0.055 ng/mL had a sensitivity of 0.81 and a specificity of 0.54. The cut-off point of 0.35 ng/mL had a sensitivity of 0.90 and a specificity of 0.33 (Fig. 1). The groups had different preoperative procalcitonin values (p = 0.003) but comparable postoperative values (Fig. 2). Based on the data for all operations, the preoperative procalcitonin value also was significant for periprosthetic joint infection (p = 0.015). The procalcitonin value of 0.055 ng/mL had a sensitivity of 0.77 and specificity of 0.58. The value of 0.35 ng/mL had a sensitivity of 0.89 and specificity of 0.37.
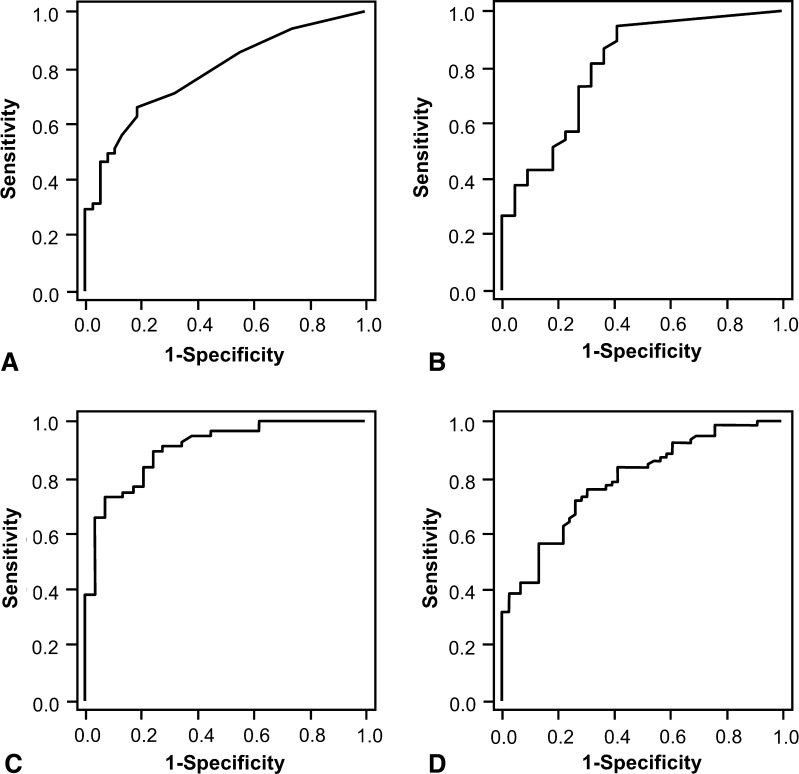
Fig. 1A–D.
ROC curves show the predictive performance of preoperative values of (A) procalcitonin, (B) IL-6, (C) CRP, and (D) leukocyte level for infection, including all operations in the analysis. The ROC curve is a graphic plot of the positive rate (sensitivity) versus the false-positive rate (specificity) for detection of infection. Procalcitonin values of 0.055 and 0.035 ng/mL had sensitivities of 0.77 and 0.89, respectively, and specificities of 0.58 and 0.37, respectively. IL-6 values of 4.7 and 2.55 pg/mL had sensitivities of 0.86 and 0.94, respectively, and specificities of 0.67 and 0.53, respectively. CRP values of 21.95 and 11.00 mg/L had sensitivities of 0.81 and 0.90, respectively, and specificities of 0.80 and 0.74, respectively. Leukocyte levels of 6.58 and 5.68 giga (G) per liter had sensitivities of 0.81 and 0.90, respectively, and specificities of 0.59 and 0.39, respectively. INF-α was a predictor of infection so ROC was not calculated.
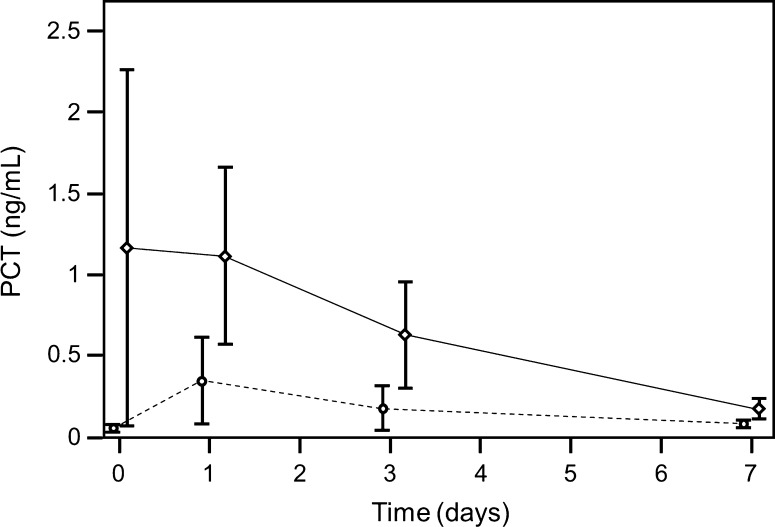
Fig. 2.
A graph shows time-dependent procalcitonin (PCT) levels for the first operation of every patient (broken line non infection group, solid line infection group; first operation, preoperative = Day 0; operation = Day 1). In the infected group changes from preoperative values to postoperative Day 3 (p = 0.029) and Day 7 (p = 0.001) were significant. After Day 1, procalcitonin decreased rapidly until it reached normal levels at Day 7. In the group without infection there were no significant changes.
Based on the data from the first operation, preoperative IL-6 was not a significant predictor for infection (p = 0.056) (Fig. 1). Group differences between groups were found preoperatively (p = 0.004) and on Day 1 (p = 0.039), with higher values in the infected group (Fig. 3). With the data for all operations, IL-6 was significant (p = 0.012) for detecting infection. The IL-6 value of 4.7 pg/mL had a sensitivity of 0.86 and specificity of 0.67, and the value of 2.55 pg/mL had a sensitivity of 0.94 and specificity of 0.53.
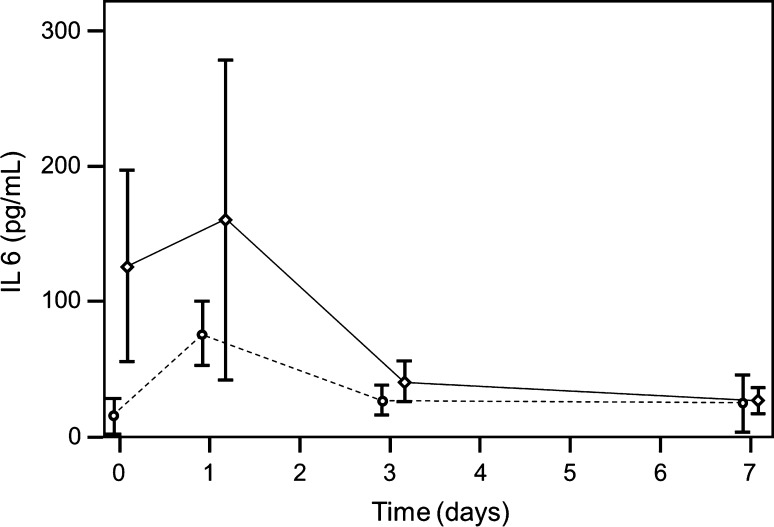
Fig. 3.
A graph shows time-dependent IL-6 levels (broken line non infection group, solid line infection group; first operation, preoperative = Day 0; operation = Day 1). In the group with infection at postoperative Days 3 (p = 0.003) and 7 (p = 0.001), IL-6 values differ from preoperative values. IL-6 was elevated preoperatively with a peak at Day 1.
When only the first operation was considered, preoperative IFN-α was not a significant predictor for infection (p = 0.402), nor when all operations were considered (p = 0.432). In both groups, the curves neither decreased nor increased (p = 0.394) (Fig. 4). Changes from preoperative values in both groups were not significant at any time, and the differences between the times were never significant.
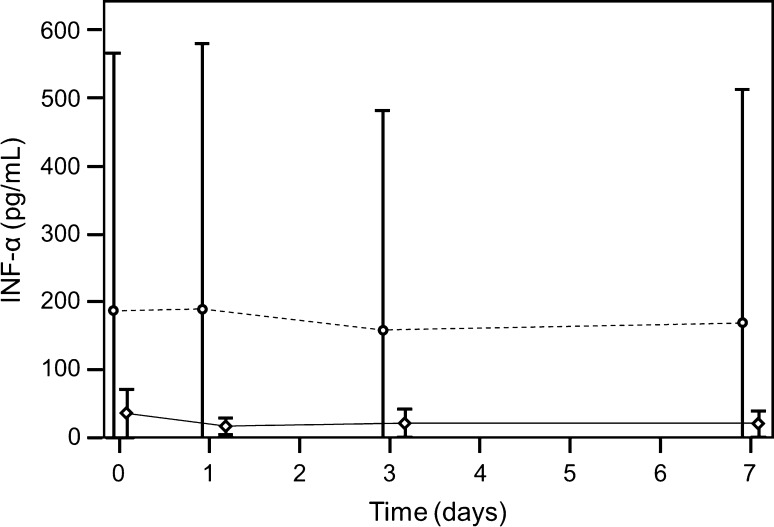
Fig. 4.
A graph shows time-dependent IFN-α levels (broken line non infection group, solid line infection group; first operation, preoperative = Day 0; operation = Day 1). The curve of IFN-α does not change with time in either group.
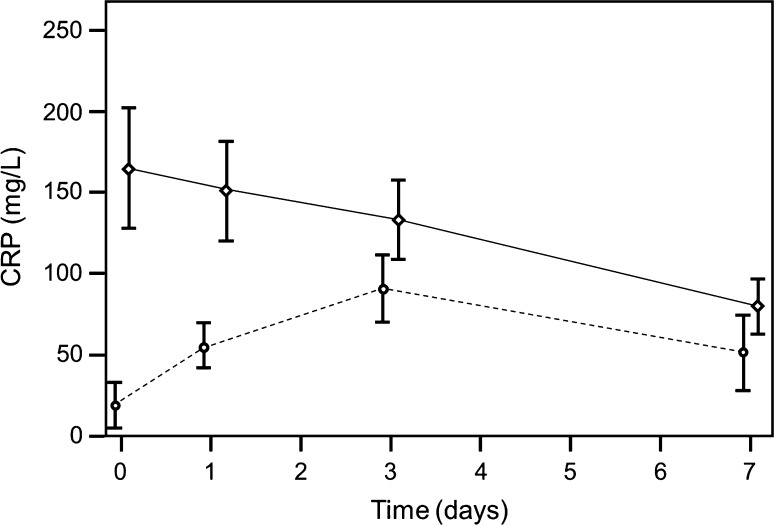
When only the first operation was evaluated, preoperative CRP was a significant predictor for bacterial infection (p < 0.001). For CRP, the cut-off point of 23.65 had a sensitivity of 0.80 and specificity of 0.79, and the cut-off point of 10.25 had a sensitivity of 0.91 and specificity of 0.72 (Fig. 1). In the infection group, values were higher preoperatively and decreased postoperatively; in the group without infection, the values increased postoperatively and decreased after postoperative Day 3 (Fig. 5). Taking all 124 operations into account, CRP was predictive (p < 0.001). The value of 21.95 mg/L had a sensitivity of 0.81 and specificity of 0.80, and the value of 11.00 mg/L had a sensitivity of 0.90 and specificity of 0.74 (Fig. 1). In the infection group, values were higher preoperatively and decreased postoperatively; in the no infection group, the values increased postoperatively and decreased after Postoperative Day 3. The progressions of the two curves differed with time (p < 0.001) (Fig. 5).
Fig. 5.
A graph shows time-dependent CRP levels (broken line non infection group, solid line infection group; first operation, preoperative = Day 0; operation = Day 1). CRP was highly elevated in the infection group and decreased continuously after surgery until Day 7, whereas in the no infection group, the CRP levels increased after surgery, with a peak at Day 3. The progressions of the two curves differ in a statistically significant manner with time (p < 0.001).
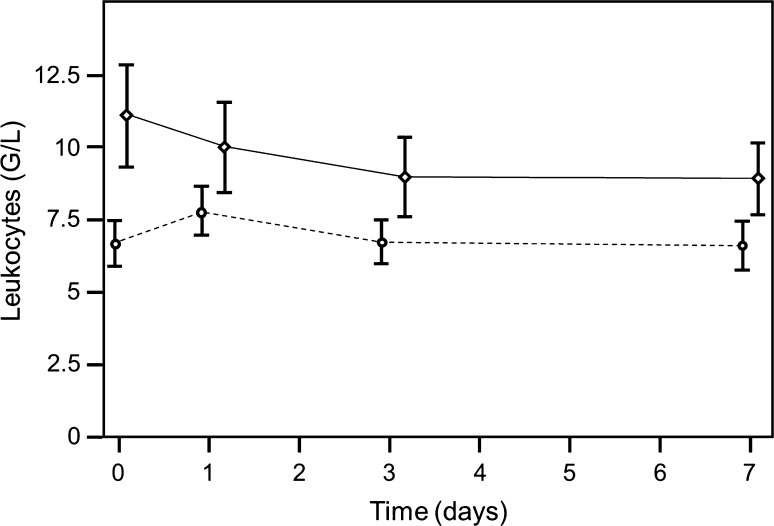
Taking into account the first operation, the preoperative level of leukocytes also was a statistically significant predictor for infection (p = 0.001). The value of 6.27 had a sensitivity of 0.8 and specificity of 0.48, and the value of 5.48 had a sensitivity of 0.91 and specificity of 0.34 (Fig. 1). The progressions of the two curves were statistically significantly different with time (p = 0.009) (Fig. 6). Taking all operations into account, the leukocyte level predicted periprosthetic joint infection (p < 0.001). The value of 6.58 giga (G) per liter had a sensitivity of 0.81 and specificity of 0.59, and the value of 5.68 G/L had a sensitivity of 0.90 and specificity of 0.39 (Fig. 1). The progressions of the two curves were different with time (p = 0.012) (Fig. 6).
Fig. 6.
A graph shows time-dependent leukocyte levels (broken line non infection group, solid line infection group; first operation, preoperative = Day 0; operation = Day 1). Although differences from the baseline value were significant in both groups for Day 1, this change stabilized in the group without infection after the first day. In the infection group, however, there was a significant change between preoperative values and values on Days 3 and 7.
To compare the capability of the four significant biomarkers to distinguish patients without infection from patients with infection, the area under the curve was calculated. The highest area under the curve was 0.90 (95% CI, 0.84–0.97) observed for CRP. The other three biomarkers had comparable areas under the curve. For procalcitonin, the area under the curve was 0.81 (95% CI, 0.71–0.91), for IL-6 the area under the curve was 0.80 (95% CI, 0.69–0.92), and for leukocytes the area under the curve was 0.77 (95% CI, 0.67–0.87).
For combining different parameters, the point with the minimal distance on the ROC curve was considered to be the optimal threshold. These optimal thresholds were 0.75 for procalcitonin, 4.7 for IL-6, 17.05 for CRP, and 7.355 for leukocytes (Table 1). A combination of two of these parameters leads to only minor improvement of sensitivity, specificity, positive predictive value (PPV), or negative predictive value (NPV) (Table 2). CRP alone has the highest sensitivity value and the second highest specificity value compared with the other parameters. Adding IL-6 does not change sensitivity but decreases specificity, adding procalcitonin slightly decreases sensitivity and slightly increases specificity, and adding leukocytes increases sensitivity (+ 9%) but decreases specificity (−9%) (Table 2).
Table 1.
Sensitivity and specificity of optimal thresholds for each parameter
| Parameter | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| CRP | 84% | 79% | 88% | 72% |
| IL-6 | 81% | 68% | 81% | 68% |
| Procalcitonin | 48% | 100% | 100% | 59% |
| Leukocytes | 73% | 72% | 83% | 58% |
CRP = C-reactive protein; IL-6 = interleukin-6.
Table 2.
Combinations of parameters leading to minor improvement
| Parameter | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| CRP + IL-6 | 84% | 68% | 82% | 71% |
| CRP + procalcitonin | 83% | 83% | 89% | 74% |
| CRP + leukocytes | 93% | 59% | 81% | 81% |
| IL-6 + procalcitonin | 83% | 68% | 81% | 71% |
| IL-6 + leukocytes | 89% | 50% | 75% | 73% |
| Procalcitonin + leukocytes | 73% | 79% | 86% | 63% |
CRP = C-reactive protein; IL-6 = interleukin-6.
Discussion
A periprosthetic joint infection is one of the most challenging complications associated with THAs and TKAs. In the diagnostic process for detecting a periprosthetic joint infection, one of the most important steps is analysis of laboratory infection biomarkers. In this study, we analyzed the plasma concentrations of different biomarkers with respect to their sensitivity and specificity for detecting bacterial joint infections associated with revision arthroplasties.
The limitations of our study include the heterogeneity of the study population and the extent of the surgical procedures differed. In addition, unknown reasons for elevated infection parameters could have influenced the outcome. Patients with a risk for elevated infection parameters were not included. We could not evaluate the new infection parameters for these patients. In the analyses where more than one operation is counted for each patient, the different baseline values of each patient could introduce a bias.
We found procalcitonin was a helpful parameter for detecting an infection, with a sensitivity of 90% and specificity of 67% (procalcitonin value, 0.35 ng/mL). Others have found sensitivity and specificity values for procalcitonin in similar ranges (Table 3) [2, 5, 11, 14, 21, 26, 29, 30, 33]. However, the results reported by Worthington et al. were quite different: serum procalcitonin did not help distinguish between infectious and noninfectious causes of prosthetic hip loosening [41].
Table 3.
Procalcitonin and interleukin-6: overview of sensitivity and specificity values in different studies
| Study | Cut-off procalcitonin | Sensitivity procalcitonin | Specificity procalcitonin | Cut-off IL-6 | Sensitivity IL-6 | Specificity IL-6 |
|---|---|---|---|---|---|---|
| Martinot et al. [23] | 0.5 ng/mL | 55 % | 94 % | – | – | – |
| Bottner et al. [4] | 0.3 ng/mL | 33 % | 98 % | 12.0 pg/mL | 95% | 87% |
| Hugle et al. [20] | 0.1 ng/mL | 100 % | 46 % | – | – | – |
| 0.25 ng/mL | 93 % | 75 % | – | – | – | |
| Current study | 0.35 ng/mL | 90 % | 33 % | 2.6 pg/mL | 94 % | 53 % |
| 0.06 ng/mL | 81 % | 54 % | 4.7 pg/mL | 86 % | 67 % |
IL-6 = interleukin-6.
Procalcitonin may prove useful for distinguishing between bacterial infections of the joint and other causes of postoperative inflammation (eg, patients with chronic inflammatory diseases, increased BMI, or other reasons for elevated CRP levels). It also could provide some indication regarding whether an antimicrobial therapy might be effective and therefore could reduce the duration of medication and length of hospitalization [18]. In our study, the biomarker IL-6 was helpful for detecting bacterial infection. Considering IL-6 increases with infection earlier than CRP, IL-6 seems useful for early detection of a septic process and for selecting antibiotic therapy.
Although IFN-α is important for modulation and regulation of different cytokines [1], this parameter remained at a baseline level in both groups and was not useful for detecting bacterial infection in our patients. We are not aware of any other trial in which INF-α was helpful in detecting bacterial periprosthetic infections. This might be because IFN-α has an important role in antiviral immunity but not in antimicrobial immunity [1].
Comparing the four significant biomarkers, in our study CRP showed the highest capability to determine a periprosthetic joint infection. The other three biomarkers had similar sensitivities and specificities. Bottner et al. [4] reported CRP and IL-6 had the highest sensitivity but IL-6 was less specific than CRP. Procalcitonin was specific for a periprosthetic joint infection but had a low sensitivity. Hugle et al. [20] reported that procalcitonin had higher sensitivity and specificity for diagnosing septic arthritis than CRP. Leukocyte count was not helpful for detecting septic arthritis. Wirtz et al. [40] found IL-6 concentration correlates with a high degree of inflammatory activity, with a more rapid increase and quicker return to normal values than CRP, suggesting IL-6 measurements give a better indication of postoperative inflammatory response than CRP measurements after TKA and THA. Worthington et al. [41] reported CRP and leukocyte level were significantly higher in patients with septic loosening of a hip prosthesis, IL-6 was seen only as additional information, and serum procalcitonin did not help in distinguishing infectious from noninfectious causes.
To our knowledge, our study is the first to evaluate a combination of conventional and new infection parameters. The combination led to only minor improvement in sensibility and specificity.
Our study suggested that procalcitonin and IL-6 are helpful biomarkers for detecting periprosthetic joint infections, although generally the performance of CRP was better than that of the novel biomarkers. We believe use of procalcitonin and IL-6 may be worthwhile as an adjuvant in patients when the diagnosis of periprosthetic joint infection remains unclear.
Acknowledgments
We thank Alexandra Ujvari MD for excellent assistance in data acquisition and literature research, Bodo Koppany MD from the Department of Pathology, Medical University Graz, for histologic analyses, Magdalena Kapitan PhD for statistical advice and analysis, and Eugenia Lamont for language editing.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Medical University of Graz, Graz, Austria.
References
- 1.Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010;77:848–854. doi: 10.1038/ki.2010.71. [DOI] [PubMed] [Google Scholar]
- 2.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bo M, Raspo S, Morra F, Isaia G, Cassader M, Fabris F, Poli L. Body fat and C-reactive protein levels in healthy non-obese men. Nutr Metab Cardiovasc Dis. 2004;14:66–72. doi: 10.1016/S0939-4753(04)80012-7. [DOI] [PubMed] [Google Scholar]
- 4.Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Gotze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89:94–99. doi: 10.1302/0301-620X.89B1.17485. [DOI] [PubMed] [Google Scholar]
- 5.Carrol ED, Thomson AP, Hart CA. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents. 2002;20:1–9. doi: 10.1016/S0924-8579(02)00047-X. [DOI] [PubMed] [Google Scholar]
- 6.Costerton JW, Post JC, Ehrlich GD, Hu FZ, Kreft R, Nistico L, Kathju S, Stoodley P, Hall-Stoodley L, Maale G, James G, Sotereanos N, DeMeo P. New methods for the detection of orthopedic and other biofilm infection. FEMS Immunol Med Microbiol. 2011;61:133–140. doi: 10.1111/j.1574-695X.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 7.Coventry MB. Treatment of infections occurring in total hip surgery. Orthop Clin North Am. 1975;6:991–1003. [PubMed] [Google Scholar]
- 8.Drago L, Vassena C, Dozio E, Corsi MM, De Vecchi E, Mattina R, Romano C. Procalcitonin, C-reactive protein, interleukin-6, and soluble intercellular adhesion molecule-1 as markers of postoperative orthopaedic joint prosthesis infections. Int J Immunopathol Pharmacol. 2011;24:433–440. doi: 10.1177/039463201102400216. [DOI] [PubMed] [Google Scholar]
- 9.Esposito S, Leone S. Prosthetic joint infections: microbiology, diagnosis, management and prevention. Int J Antimicrob Agents. 2008;32:287–293. doi: 10.1016/j.ijantimicag.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald RH, Jr, Nolan DR, Ilstrup DM, Van Scoy RE, Washington JA, 2nd, Coventry MB. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847–855. [PubMed] [Google Scholar]
- 11.Fottner A, Birkenmaier C, von Schulze Pellengahr C, Wegener B, Jansson V. Can serum procalcitonin help to differentiate between septic and nonseptic arthritis? Arthroscopy. 2008;24:229–233. [DOI] [PubMed]
- 12.Galbraith JG, Butler JS, Browne TJ, Mulcahy D, Harty JA. Infection or metal hypersensitivity? The diagnostic challenge of failure in metal-on-metal bearings. Acta Orthop Belg. 2011;77:145–151. [PubMed] [Google Scholar]
- 13.Gendrel D, Bohuon C. Procalcitonin, a marker of bacterial infection. Infection. 1997;25:133–134. doi: 10.1007/BF02113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P, Bohuon C. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997;24:1240–1242. doi: 10.1086/513633. [DOI] [PubMed] [Google Scholar]
- 15.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez MH, Mekhail AO. The failed total knee arthroplasty: evaluation and etiology. J Am Acad Orthop Surg. 2004;12:436–446. doi: 10.5435/00124635-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 18.Hatzistilianou M. Diagnostic and prognostic role of procalcitonin in infections. ScientificWorldJournal. 2010;10:1941–1946. doi: 10.1100/tsw.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogdall D, Hvolris JJ, Christensen L. Improved detection methods for infected hip joint prostheses. APMIS. 2010;118:815–823. doi: 10.1111/j.1600-0463.2010.02671.x. [DOI] [PubMed] [Google Scholar]
- 20.Hugle T, Schuetz P, Mueller B, Laifer G, Tyndall A, Regenass S, Daikeler T. Serum procalcitonin for discrimination between septic and non-septic arthritis. Clin Exp Rheumatol. 2008;26:453–456. [PubMed] [Google Scholar]
- 21.Hunziker S, Hugle T, Schuchardt K, Groeschl I, Schuetz P, Mueller B, Dick W, Eriksson U, Trampuz A. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am. 2010;92:138–148. doi: 10.2106/JBJS.H.01600. [DOI] [PubMed] [Google Scholar]
- 22.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Martinot M, Sordet C, Soubrier M, Puechal X, Saraux A, Liote F, Guggenbuhl P, Legre V, Jaulhac B, Maillefert JF, Zeisel M, Coumaros G, Sibilia J. Diagnostic value of serum and synovial procalcitonin in acute arthritis: a prospective study of 42 patients. Clin Exp Rheumatol. 2005;23:303–310. [PubMed] [Google Scholar]
- 24.Moulin F, Raymond J, Iniguez JL, Ravilly S, Lebon P, Gendrel D. Serum alpha-interferon in lower respiratory tract infections of children. Pediatr Infect Dis J. 1996;15:883–886. doi: 10.1097/00006454-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Moya F, Nieto A, R-Candela JL. Calcitonin biosynthesis: evidence for a precursor. Eur J Biochem. 1975;55:407–413. doi: 10.1111/j.1432-1033.1975.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 26.Nascimento-Carvalho CM, Cardoso MR, Barral A, Araujo-Neto CA, Guerin S, Saukkoriipi A, Paldanius M, Vainionpaa R, Lebon P, Leinonen M, Ruuskanen O, Gendrel D. Procalcitonin is useful in identifying bacteraemia among children with pneumonia. Scand J Infect Dis. 2010;42:644–649. doi: 10.3109/00365541003796775. [DOI] [PubMed] [Google Scholar]
- 27.Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 2003;17:286–295. doi: 10.1016/S0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 28.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. [DOI] [PMC free article] [PubMed]
- 29.Pramod J, Singh A. Sepsis biomarkers. Am J Med. 2008;121:e11; author reply e13. [DOI] [PubMed]
- 30.Prat C, Dominguez J, Rodrigo C, Gimenez M, Azuara M, Jimenez O, Gali N, Ausina V. Procalcitonin, C-reactive protein and leukocyte count in children with lower respiratory tract infection. Pediatr Infect Dis J. 2003;22:963–968. doi: 10.1097/01.inf.0000095197.72976.4f. [DOI] [PubMed] [Google Scholar]
- 31.Rau B, Steinbach G, Gansauge F, Mayer JM, Grunert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832–840. doi: 10.1136/gut.41.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roos BA, Okano K, Deftos LJ. Evidence for a pro-calcitonin. Biochem Biophys Res Commun. 1974;60:1134–1140. doi: 10.1016/0006-291X(74)90430-6. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Alvarez MJ, Garcia-Valdecasas S, De Pablo R, Sanchez Garcia M, Coca C, Groeneveld TW, Roos A, Daha MR, Arribas I. Diagnostic efficacy and prognostic value of serum procalcitonin concentration in patients with suspected sepsis. J Intensive Care Med. 2009;24:63–71. [DOI] [PubMed]
- 34.Sanzen L, Sundberg M. Periprosthetic low-grade hip infections: erythrocyte sedimentation rate and C-reactive protein in 23 cases. Acta Orthop Scand. 1997;68:461–465. doi: 10.3109/17453679708996263. [DOI] [PubMed] [Google Scholar]
- 35.Savarino L, Baldini N, Tarabusi C, Pellacani A, Giunti A. Diagnosis of infection after total hip replacement. J Biomed Mater Res B Appl Biomater. 2004;70:139–145. doi: 10.1002/jbm.b.30030. [DOI] [PubMed] [Google Scholar]
- 36.Savarino L, Tigani D, Baldini N, Bochicchio V, Giunti A. Pre-operative diagnosis of infection in total knee arthroplasty: an algorithm. Knee Surg Sports Traumatol Arthrosc. 2009;17:667–675. doi: 10.1007/s00167-009-0759-3. [DOI] [PubMed] [Google Scholar]
- 37.Schuetz P, Batschwaroff M, Dusemund F, Albrich W, Burgi U, Maurer M, Brutsche M, Huber AR, Muller B. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post-study survey. Eur J Clin Microbiol Infect Dis. 2010;29:269–277. doi: 10.1007/s10096-009-0851-0. [DOI] [PubMed] [Google Scholar]
- 38.Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Kohl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med. 2000;28:2793–2798. doi: 10.1097/00003246-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Van Kleunen JP, Knox D, Garino JP, Lee GC. Irrigation and debridement and prosthesis retention for treating acute periprosthetic infections. Clin Orthop Relat Res. 2010;468:2024–2028. doi: 10.1007/s11999-010-1291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–196. doi: 10.1007/s002640000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worthington T, Dunlop D, Casey A, Lambert R, Luscombe J, Elliott T. Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to short-chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci. 2010;67:71–76. doi: 10.1080/09674845.2010.11730294. [DOI] [PubMed] [Google Scholar]
- 42.Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]