Abstract
The full-field thickness distribution, three-dimensional surface model and general morphological data of six human tympanic membranes are presented. Cross-sectional images were taken perpendicular through the membranes using a high-resolution optical coherence tomography setup. Five normal membranes and one membrane containing a pathological site are included in this study. The thickness varies strongly across each membrane, and a great deal of inter-specimen variability can be seen in the measurement results, though all membranes show similar features in their respective relative thickness distributions. Mean thickness values across the pars tensa ranged between 79 and 97 μm; all membranes were thinnest in the central region between umbo and annular ring (50–70 μm), and thickness increased steeply over a small distance to approximately 100–120 μm when moving from the central region either towards the peripheral rim of the pars tensa or towards the manubrium. Furthermore, a local thickening was noticed in the antero–inferior quadrant of the membranes, and a strong linear correlation was observed between inferior–posterior length and mean thickness of the membrane. These features were combined into a single three-dimensional model to form an averaged representation of the human tympanic membrane. 3D reconstruction of the pathological tympanic membrane shows a structural atrophy with retraction pocket in the inferior portion of the pars tensa. The change of form at the pathological site of the membrane corresponds well with the decreased thickness values that can be measured there.
Keywords: eardrum, OCT, finite element modeling, pars tensa, 3D model
Introduction
Mechanical properties of the tympanic membrane (TM) play a crucial role in the functioning of the middle ear system. Various applications of finite element modeling in hearing research have expressed the necessity to know, with great precision, such properties as shape, thickness distribution and local elasticity of the tympanic membrane. The accurate determination of these parameters is indispensable in the creation of realistic mathematical models of dynamic eardrum behavior (Song and Lee 2012) that are employed in the construction of both active (Gan et al. 2010) and passive (Sun et al. 2002; Prendergast et al. 1999) middle ear prostheses. Although there is a general consensus on the inhomogeneous mass distribution in different locations of the TM, full-field quantitative data remain absent, causing authors to employ simplified thickness distributions in their finite element (FE) models. A summary of such models was composed by Volandri et al. (2011), who concluded that most authors adopt a single thickness value across the entire membrane, ranging between 30 and 150 μm. Wada et al. (1992) were the first to include thickness data refinement in their FE models by specifying ten different thickness regions, corresponding with the measurements by Uebo et al. (1988). The same data set was employed in the model by Koike et al. (2002), who interpolated between the data points to create a thickness data map of higher density. The diversity of numerical values employed in these models is due to the limited amount of thickness data on human TM available in literature. Furthermore, most of the thickness measurement studies were conducted on small localised parts of the membrane only and report large differences between them.
The first data on human TM thickness were gathered by Kojo (1954), who used conventional light microscopy and measured values between 30 and 120 μm at seven different points of human eardrum tissue. Lim (1970) employed electron microscopy and found thickness values ranging from 30 to 90 μm for the pars tensa (PT) and from 30 to 230 μm for the pars flaccida. In order to create uniformity in FE models of the human middle ear, Funnell and Laszlo (1982) proclaimed the need for ‘more detailed measurements on variations of overall thickness information across the surface of the TM’. To this end, Uebo et al. (1988) reported average thickness values in each of ten different regions of the TM, Ruah et al. (1991) performed three measurements on each of six different TM regions and Schmidt and Hellström (1991) and Kuypers et al. (2006) published the thickness distributions across several full-length TM cross-sections. However, to date, no full-field high-resolution thickness data of the human tympanic membrane are available.
Recently, several successful feasibility studies on optical coherence tomography (OCT) in middle ear research, both ex vivo (Pitris et al. 2001; Bibas et al. 2004) and in vivo (Djalilian et al. 2008; Just et al. 2009; Nguyen et al. 2012) have prompted us to conduct preliminary tests to measure full-field thickness variations of several rabbit and human TMs with OCT (Buytaert et al. 2013). Though successful, it was clear that additional hardware and software modifications to the measurement setup were required in order to measure full membranes. In the following, we describe the application of large-volume optical coherence tomography as a means to construct high-resolution and full-field thickness distribution maps of the human tympanic membrane. We will focus on the pars tensa in this study, as it is the main contributing part of the TM in sound transmission and biomechanical modeling.
Material and methods
Human tympanic membranes
The results presented in this study are based on OCT measurements of six human eardrums that were harvested from human cadavers by the Temporal Bone Foundation of Brussels, Belgium. In order to comply with the regulations of the National Health Service (NHS), UK, blinded samples were employed in this study. Five samples (TM1-5) from healthy donors that possessed normal otological middle ears were made available by the Temporal Bone Foundation-Brussels. Additionally, these were examined for irregularities under a microscope before commencement of the measurements. The temporal bones were originally gathered by the Foundation for use in allograft surgery of the middle ear. Hence, precautions were taken to select only samples free of any middle ear abnormalities, but these specifics are not transferred in detail to the end users. The sixth membrane (TMP) was deliberately chosen to contain a retraction pocket and was included in the study as an exemplary case. Three eardrums originated from right ears (TM1, TM3 and TM5) and three from left ears (TM2, TM4 and TMP). Ethical approval of this study was granted by the Research Ethics Committee (REC reference: 12/EM/0196, 4 May 2012) of the NHS, UK.
Specimen preparation
The temporal bones were received in preserved condition, each of them immersed in a buffered 4 % formaldehyde solution. The middle and inner ear parts of the temporal bones were kept intact, but the ear canal was carefully drilled away to completely expose the lateral side of the tympanic membrane to the OCT laser beam. The measured shape may deviate from the TM shape as it was in vivo, but the thickness calculation only requires taking into account the TM shape whilst the OCT measurements were done. Ventilation of the middle ear was ensured to eliminate possible pressure differences that could influence the shape of the tympanic membrane during the measurements. The samples were kept moist during preparation, using a compact ultrasonic humidifier (Bionaire, BU1300W) to avoid dehydration. The temporal bones were mounted onto the sample platform of the OCT setup using hard clay and a screw system, which was acceptably stable for an average measurement time of 100 s. Because the measurements were conducted in air, the temporal bone was wrapped in wet tissue paper to reduce dehydration. After completion of the scans, the samples were immediately returned to their respective containers for future measurements.
Optical coherence tomography measurements
The setup was developed at the Applied Optics Group at the University of Kent, Canterbury. Optical sections through the eardrum were made using a customised Fourier domain optical coherence tomography system, equipped with a wide-angle galvo-scanning unit. A broadband light source, based on an ytterbium-doped fiber (Multiwave Photonics, Porto, Portugal) identical to the one described by Trifanov et al. (2011) with a central wavelength at λ = 1,050 nm and bandwidth Δλ = 70 nm was used. The axial resolution of the OCT-setup was determined by measuring the full width at half maximum (FWHM) of the autocorrelation signal peak. For our setup, this FWHM varied between 9.45 and 9.77 μm, depending on the position within the sample and the local density, throughout the maximum sample depth of 2.36 mm. The transversal resolution and depth of focus of OCT-setups are decoupled from the axial resolution and are limited mainly by the focal spot size of the Gaussian profile light beam. The interface optics of the employed configuration are identical to the one described by Bradu et al. (2005) and are designed to maintain a lateral resolution of better than 10 μm in both X- and Y-directions across the sample with a depth of focus that covers at least the full axial scanning depth of 2.36 mm. Lateral field-of-view is measured in both X- and Y-direction by tracking a feature of the imaged object in the OCT image at the working distance of the imaging lens and by moving the object laterally using a translation stage so that this feature crosses the entire image from one side to the other. Our experimental setup produces a lateral field-of-view in the X- and Y-directions of 12.35 and 10.13 mm, respectively.
In summary, the employed setup is capable of maintaining a resolution of <10 μm within the sample, both axially and transversally, throughout an imaging volume of 12.35 × 10.13 × 2.36 mm3. A collection of axial images (i.e. images in a plane parallel to the imaging beam or B-scans) was gathered by scanning the laser beam along sections perpendicular to the plane defined by the annulus fibrosus of the TM. After correcting the data stack for fan distortion and refractive index mismatches in post-processing (Van der Jeught et al. 2013), the slice spacing is measured to be 9.8 μm and the axial pixel size is 4.6 μm. A full data-acquisition of a single 3D stack of 1,024 B-scans of 1,024 pixels wide and 1,024 pixels deep takes around 100 s on average to record without image processing and 300 s on average including image processing. No manual intervention or mechanical translation was needed during acquisition to acquire a complete data stack of cross-sectional OCT images.
Post-processing
The greyscale OCT images were segmented into binary image labels using a combination of automatic thresholding and manual segmentation in specialised visualisation software (Amira 5.3), and a 3D surface model of the tympanic membrane was constructed subsequently. Accurate 3D shape reconstruction was ensured by applying the distortion correction procedure described by Van der Jeught et al. (2012) to the recorded OCT volumes. As part of this reconstruction process, the perceived optical path distance is divided by the index of refraction of TM tissue (which was measured to be n = 1.44, see below) to obtain the actual physical distance.
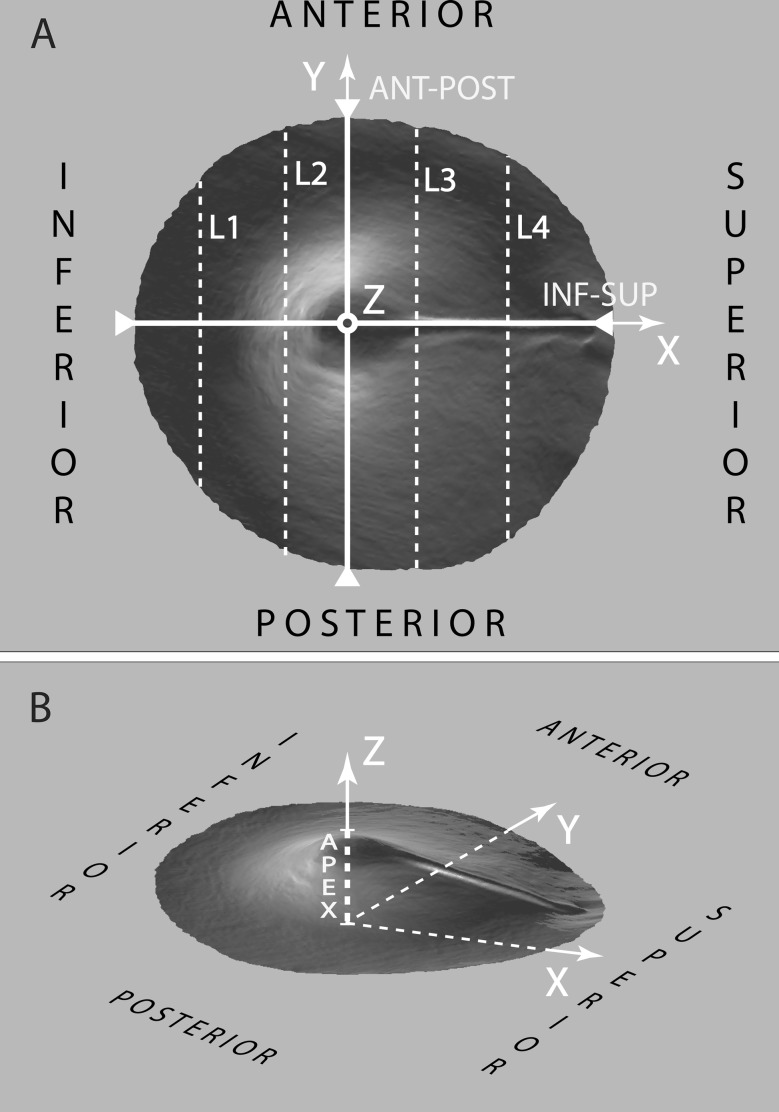
For the purpose of uniformity, the obtained models of TM1-5 were virtually aligned before numerical measurements were taken, in the coordinate system depicted in Fig. 1. This alignment procedure did not cause the absolute thickness values of the respective membranes to change at all, as it consists of rigid body rotation and translation operations exclusively. First, the best plane fit through the border of the pars tensa with the annular ring was defined as the XY-plane. Second, the origin of the axis system was determined, for each model, as the point on the XY-plane for which the Z-value (or height) of the respective surface model reaches its highest value. In this way, the positive Z-axis crosses the membrane surface model at the umbo. Third, the positive X-axis was aligned with the manubrium by rotating the model around the Z-axis. Finally, the Y-axis was defined so that 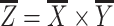 . After alignment, a series of quantitative data can be extracted from the models, including the anterior–posterior and inferior–superior length, the surface area and projected surface area, the apex height and the local and overall average thickness of the membrane.
. After alignment, a series of quantitative data can be extracted from the models, including the anterior–posterior and inferior–superior length, the surface area and projected surface area, the apex height and the local and overall average thickness of the membrane.
FIG. 1.
Axis setup illustrating membrane orientation. Part A shows the top view of the model after 3D alignment, from which the dimensional properties of the eardrum in the XY-plane can easily be obtained. Notice how the inferior–superior length of the eardrum is assessed as the length along the X-axis from the border of the pars tensa to the end of the lateral process. Lines L1–L4 indicate the locations at which virtual cross-sections will be taken to show membrane thickness variation in detail. Part B shows the 3D model from an arbitrary viewing angle, illustrating the orientation of the positive Z-axis along which the membrane’s apex height is defined. Note that the variation in pixel intensity is due to shading from the surface topography and rendering of the 3D model and does not represent varying pixel intensities in the actual data.
Local thickness evaluation
In order to measure the local thickness at different points on the eardrum, the height value Z(x,y) of both the upper (ZU) and lower (ZL) surface layers of the 3D model was evaluated on a Cartesian coordinate grid in the XY-plane. A first way to present the numerical thickness distribution TZ(x,y) of the TM is to calculate the height difference between upper and lower layers of the model, projected along the Z-direction onto the XY-plane:
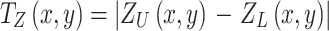 |
These values are easy to import into, e.g. finite element models, but they are of course dependent on the local orientation of the membrane. Another way to evaluate 3D thickness of arbitrary volumes was described by Hildebrand and Rüegsegger (2003) who proposed to define the local thickness 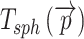 at a given point
at a given point 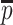 within an object by the diameter of the largest sphere that contains
within an object by the diameter of the largest sphere that contains 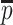 and that lies completely within the borders ZU and ZL of the object:
and that lies completely within the borders ZU and ZL of the object:
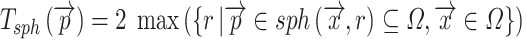 |
where Ωϵℝ3 represents the collection of points within the object and 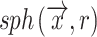 the set of points inside the sphere with center
the set of points inside the sphere with center 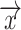 and radius r. This definition of local thickness can easily be adapted to one that renders the shortest Euclidian distance of point
and radius r. This definition of local thickness can easily be adapted to one that renders the shortest Euclidian distance of point 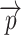 to either ZU or ZL by limiting the spheres containing
to either ZU or ZL by limiting the spheres containing 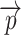 to the ones originating from
to the ones originating from 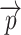 :
:
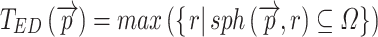 |
Both 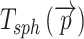 and
and 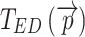 provide local thickness data in three dimensions, independent of local membrane orientation.
provide local thickness data in three dimensions, independent of local membrane orientation.
In order to compare them with each other and with TZ(x,y), they are evaluated at the points 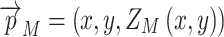 on the intermediate sheet ZM = (ZU + ZL)/2 between the upper and lower layers of the membrane. In this way, a two-dimensional representation of both thickness distributions is created on the same Cartesian grid (x,y) as the one on which TZ was defined:
on the intermediate sheet ZM = (ZU + ZL)/2 between the upper and lower layers of the membrane. In this way, a two-dimensional representation of both thickness distributions is created on the same Cartesian grid (x,y) as the one on which TZ was defined:
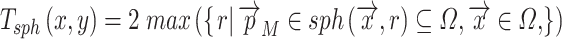 |
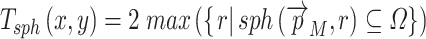 |
The Euclidian distance from the middle sheet to the outer layer of the object was doubled to create a 2D distribution TSD(x,y) which represents the shortest distance between the two layers of the membrane, as measured from the points 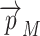 . In the following, whenever thickness results are reported, these are calculated using TSD(x,y) unless specified otherwise.
. In the following, whenever thickness results are reported, these are calculated using TSD(x,y) unless specified otherwise.
Results
Optical coherence tomography measurements of the human eardrum
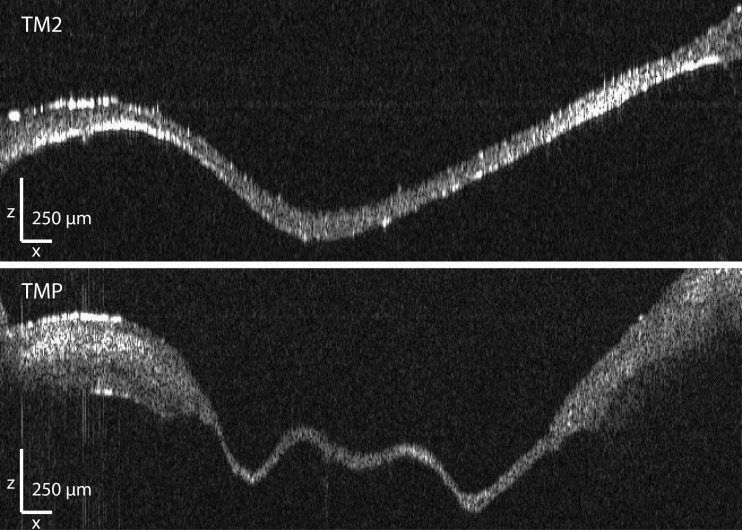
In Fig. 2, axial cross-sections of both a normal human eardrum (TM2, top) and a human eardrum showing pathology (TMP, bottom) are shown. A full stack of such B-scans was obtained for each membrane. After segmentation of the raw data into binary labels and geometric calibration, a 3D surface model of all six human tympanic membranes was constructed in post-processing. Several morphological and geometric properties were extracted from these models and are included in Table 1. Full models and two-dimensional thickness maps are available for download at the website of the Laboratory of Biomedical Physics, Antwerp (www.ua.ac.be/bimef, go to downloads > middle and inner ear models > human > tympanic membrane (OCT)).
FIG. 2.
Raw OCT data obtained along line L2 for both a normal tympanic membrane (top, TM2) and a pathological tympanic membrane showing a retraction pocket (bottom, TMP). The scale bars in both X-and Z-direction represent 250 μm.
TABLE 1.
Numerical values of several geometric features of the 3D human TM models that were constructed from OCT data
| Inf–sup (mm) | Ant–post (mm) | Proj. surf. area (mm2) | Surf. area (mm2) | Apex (mm) | <Tz (PT) > (μm) | <Tsph (PT) > (μm) | <TSD (PT) > (μm) | |
|---|---|---|---|---|---|---|---|---|
| TM1 | 8.86 | 8.11 | 60.58 | 67.95 | 1.72 | 99.18 | 97.01 | 91.76 |
| TM2 | 7.73 | 8.36 | 51.92 | 58.76 | 1.49 | 82.98 | 79.56 | 78.21 |
| TM3 | 7.96 | 7.55 | 43.57 | 49.84 | 1.51 | 85.91 | 84.28 | 78.55 |
| TM4 | 8.21 | 8.73 | 52.97 | 61.55 | 1.75 | 94.01 | 88.98 | 86.43 |
| TM5 | 8.05 | 8.47 | 53.13 | 60.44 | 1.53 | 83.70 | 83.55 | 77.93 |
| TMP | 7.91 | 8.50 | 55.06 | 68.51a | 1.58 | 88.75a | 87.01a | 80.73a |
Columns 1 and 2: inferior–superior and anterior–posterior length of the TM, respectively. Column 3: projected surface area of the membrane, measured perpendicularly to the Z-axis. Column 4: surface area of the lateral side of the membrane, taking into account any height variations in the Z-direction. Column 5: the apex of the membrane, determined as the shortest distance between the XY-plane and the umbo. Columns 6, 7 and 8: average values of the thickness distributions Tz, Tsph and TSD, measured at the pars tensa of the membrane, excluding the manubrium.
aValues in the last row may have been influenced by the local geometry at the pathological site of TMP
Statistical relations between geometric properties and average thickness values of the different tympanic membranes can be investigated using the presented numerical data. Correlating the average thickness < Tsph > of the pars tensa (column 7) with the inferior–superior length (column 1), the anterior–posterior length (column 2) and the projected (column 3) and total (column 4) surface area of the different tympanic membranes produces R-squared values of 0.97, 0.01, 0.45 and 0.44, respectively, indicating a strong linear relation between average thickness and inferior–posterior length of the TM and little correlation with the other parameters. The coefficients were calculated using the data derived from TM1-5 only, as the geometric properties of TMP may have been altered due to its pathology and would influence the correlation in a non-representative way.
Thickness distribution and thickness profiles along virtual cross-sections of the human eardrum
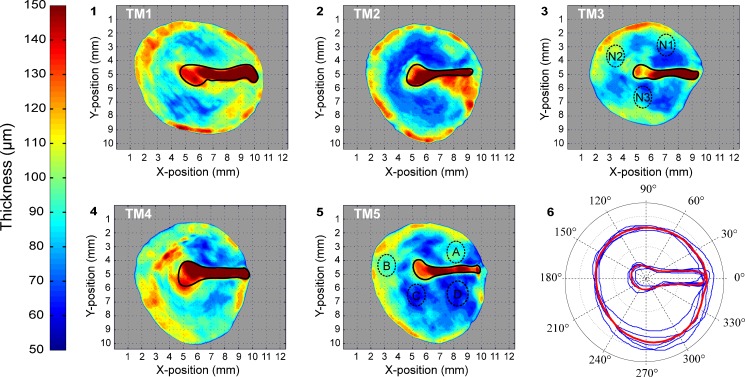
The local thickness data of TM1-5 can be computed for every point in the membrane volume and can be represented in 2D by plotting the resulting values of thickness distribution TSD using colour-coding. Figure 3 shows such thickness maps for the middle sheets of membranes TM1-5. The numerical thickness values extracted from the OCT models were verified against physical thickness measurements in several regions of TM5. Four distinct (small) regions of TM5 (Fig. 3, regions A through D on TM5) with high local thickness homogeneity were extracted from the tympanic membrane under a surgical microscope. Next, each of these regions was split in half by applying a perpendicular cut to the membrane surface. The physical thickness at multiple points along both sides of this cut was measured using an optical microscope, and the average obtained thickness per region is included in the top part of Table 2. The corresponding thickness values of the OCT model in these regions were extracted from TM5 using TSD and were averaged over the entire homogeneous region. In general, the optical microscopy (OM) measurements correlate well with the thickness values extracted from the OCT model. The slightly larger values of the OM measurements in comparison to the OCT measurements could be because the cuts were not perfectly perpendicular or could be due to a slight refractive index mismatch. The method described by Dirckx et al. (2005) to determine the refractive index of small tissue samples with confocal microscopy was applied to the tissue of TM3 across three distinct regions of the membrane (Fig. 3, regions N1, N2 and N3 on TM3). The results of these measurements are included in the bottom part of Table 2. As the standard deviation of the average refractive index at different regions of the TM is small (n = 1.4437 ± 0.0015), a uniform index of refraction of n = 1.4437 was adopted in the post-processing reconstruction process.
FIG. 3.
Subfigures 1–5 illustrate thickness maps of the individual membranes TM1-5. The manubrium border of each membrane is illustrated by a solid black line, delimiting the areas in which a greater thickness uncertainty exists due to limited penetration of the imaging beam. Subfigure 6 includes the contours of TM1-5 in blue and the resulting averaged contour of TMA in red.
TABLE 2.
Top: Averaged numerical thickness values of four regions of TM5 with relatively homogeneous local thickness distribution, both measured directly with optical microscopy (OM) and extracted from the optical coherence tomography (OCT) data model of TM5. Bottom: refractive index measurements of tympanic membrane tissue along three distinct regions of TM3. The ± error margins represent the standard error of the mean of the sample measurements.
| TM5 | OCT (μm) | OM (μm) |
| A | 83.0 ± 1.2 | 83.8 ± 2.7 |
| B | 87.2 ± 0.9 | 89.5 ± 3.3 |
| C | 60.6 ± 0.8 | 60.7 ± 1.2 |
| D | 62.7 ± 1.1 | 63.1 ± 1.7 |
| TM3 | Refractive index | |
| N1 | 1.443 ± 0.009 | |
| N2 | 1.451 ± 0.011 | |
| N3 | 1.437 ± 0.008 |
In order to perform a direct comparison of the local thickness values between the differently shaped membranes, the respective 2D thickness distributions of TM1-5 were remapped onto the same surface without scaling the thickness itself. For this purpose, the average contour of the five presented healthy membranes was calculated by selecting the umbo of each model as the origin of a polar coordinate grid onto which the averaged distances to both the peripheral rim of the pars tensa and the border of the pars tensa with the manubrium were plotted. The curve connecting these points represents the contour of the averaged, virtual tympanic membrane (in the following referred to as TMA) and is plotted (in red) in part 6 of Fig. 3.
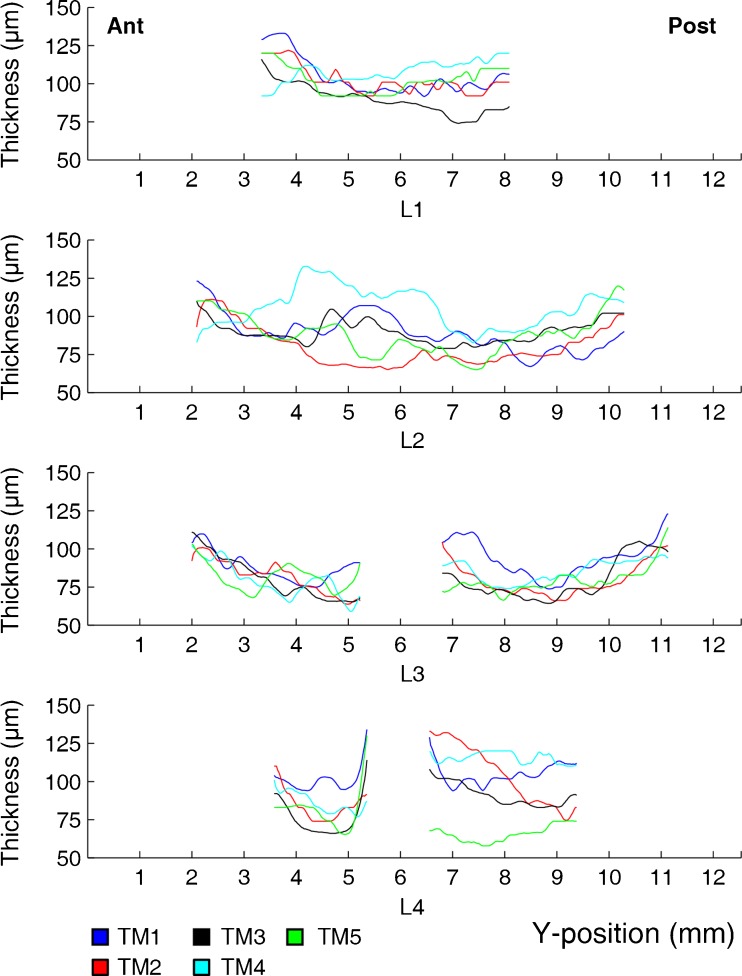
After projecting the thickness distributions of TM1-5 onto TMA, the thickness values along lines L1 through L4 were plotted as a function of the Y-position in Fig. 4. The presence of the malleus prohibited accurate determination of thickness locally; hence, no data for this region were included in the plots as we cannot guarantee their accuracy. All plots and colour maps indicate that the thickness of human tympanic membranes is far from uniformly distributed. Although considerable differences in absolute thickness can be observed between them, the relative thickness profiles do contain several similar features. In general, thickness values increase steeply from 40–75 μm in the central region between annulus and manubrium to 110–160 μm when moving over a small distance of 1–2 mm from the central region towards either the peripheral rim of the pars tensa or towards the manubrium. Notice also how all membranes, except for TM2, contain a local thickening in the antero–inferior quadrant, between the umbo and the edge of the pars tensa. This asymmetric distribution of mass is reflected in the thickness profiles along line L2, between 4 and 6 mm into the y-position. The largest inter-specimen spread between membranes TM1-5 can be seen in the postero–superior quadrant of the membranes (right part of the bottom plot in Fig. 4). Here, the average thickness values range between (60.1 ± 7.4) μm for TM5 and (122 ± 12) μm for TM4, where the error margins represent the standard deviation.
FIG. 4.
Thickness profiles of TM1-5 as a function of Y-position along lines L1–L4 after projection of the respective thickness distributions onto TMA. Ant, anterior; Post, posterior.
Average thickness distribution of the human eardrum
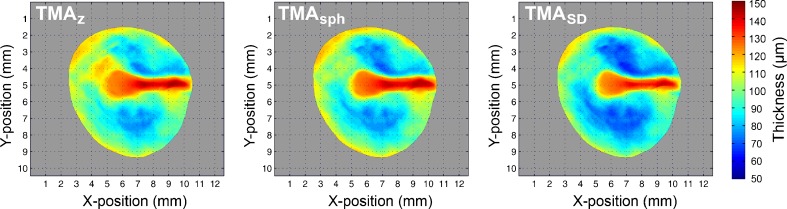
The full-field thickness data of TM1-5 can be combined into a single, averaged model of the human eardrum by projecting their respective thickness distributions onto the surface shape of TMA. It should be noted that the absolute thickness values of the membranes are not altered during this process. They are merely repositioned onto the same surface shape and resampled on the same grid by 2D interpolation. In this way, a virtual membrane can be constructed that represents the averaged thickness map of the five healthy eardrums that were included in this study. In Fig. 5, the averaged thickness distributions of TM1-5, as determined by the different thickness evaluation procedures defined by Tz, Tsph and TSD, are shown. The average thickness value of the pars tensa of TMA, as determined by Tz, Tsph and TSD, is (89.2 ± 3.8), (86.7 ± 3.2) and (82.6 ± 3.1) μm, respectively, where the error margins represent the standard error of the mean of the thickness maps of TM1-5. In general, the averaged version of our models has a thin central region of 50–70 μm, a local thickening in the antero–inferior quadrant of 80–100 μm and a steep increase in thickness near the peripheral rim of the pars tensa and towards the manubrium up to 120–140 μm. Notice also how, in general, Tz > Tsph > TSD.
FIG. 5.
Averaged thickness maps of TM1-5 as determined by Tz, Tsph and TSD, respectively.
The local thickening in the antero–inferior (AI) quadrant of membranes TM1, TM3, TM4 and TM5 is reflected in the relatively higher thickness value of the mean thickness of TMA in the AI quadrant (91.9 ± 4.1) μm when compared with that of the postero–inferior (83.2 ± 3.7), the postero–superior (80.5 ± 4.6) and the antero–superior (81.3 ± 3.9) quadrants. Student t test statistics on the five samples confirm that the mean AI quadrant is significantly thicker than the others (t8 = 3.9644, P = 0.0042). Note, however, that the thickness per quadrant is distributed unevenly and that a single mean thickness value does not suffice to describe the entire quadrant thickness distribution.
Thickness distribution of a pathological eardrum
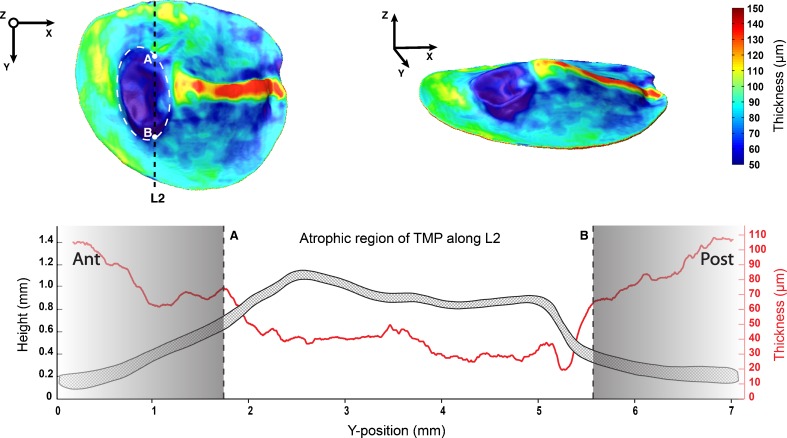
As a case example of possible future application of OCT imaging in diagnostics, the thickness map of a human tympanic membrane (TMP) containing a distinct pathological site is shown in the top part of Fig. 6.
FIG. 6.
Top part, left: thickness map of TMP with retraction pocket in region outlined by dashed white lines, as viewed from the Z-direction. Points A and B delimit the atrophic region of TMP across line L2. Top part, right: thickness map of TMP as viewed from an arbitrary angle. Bottom part: Cross-section of TMP along line L2 (dashed area) with corresponding local thickness values (solid red line). Notice the sudden drop in membrane thickness at the atrophic region of TMP. As the thickness distribution of TMP was added here as a stand-alone case study, its shape has not been projected onto the averaged membrane shape of TMA, and it should therefore be noted that the absolute position of line L2 differs from the one used earlier to describe the cross-sections of TM1-5.
It is suggested that this abnormality may be a structural atrophy with retraction pocket in the inferior portion of the pars tensa. The change of form at the pathological site of TMP corresponds well with the decreased thickness values that can be measured there. Although the thickness of the unaffected part of the pars tensa fluctuates between 60 and 100 μm, the atrophic region contains no thickness values higher than 50 μm. In fact, thickness values as low as 20 μm can be measured at the heart of the retraction pocket. A sudden decline in thickness of the membrane can be observed between points A and B in the cross-sectional graph of TMP along line L2, included in the bottom part of Fig. 6.
Discussion
Imaging the human tympanic membrane with optical coherence tomography
As the diameter of the average human TM is relatively large (∼10 mm) in comparison to its overall thickness range (∼50–150 μm), several important limitations are imposed onto possible imaging techniques. On the one hand, the depth resolution has to be sufficiently high to accurately detect the thickness variations. On the other hand, the system needs to be able to capture the entire TM in its field-of-view whilst at the same time maintaining a sufficiently high lateral resolution when scanning the drum from edge to edge. In order to obtain full-field thickness data of human eardrums, considerable effort has been made. Magnetic resonance imaging of the human middle ear (Lin et al. 2011) allows the orientation and geometry of the tympanic membrane to be observed clearly but is unable to resolve its subtle thickness variations. Due to the low X-ray absorption of soft tissue, general micro-scale X-ray computed tomography (μCT) images of the TM have limited contrast quality, preventing the technique from producing a detailed thickness distribution of the tympanic membrane (Buytaert et al. 2011). Applying phosphotungstic acid staining to the samples increases the X-ray absorption of soft tissue, which results in images with higher contrast (Metscher 2009), but the chemical treatment of the sample may cause thickness and shape aberrations in the TM. Furthermore, high-resolution μCT requires far more expensive equipment than OCT, requires the sample to fit within the limited dimensions of the scanning setup, requires heavy back-projection calculations and at the current state of the art, it is impossible to obtain soft tissue data in vivo with sufficiently high resolution to perform TM thickness measurements. A common disadvantage of ex vivo measurement techniques within international collaborations is that the sample requires formalin-based fixation for preservation during shipment. This may lead to possible sample shrinkage of 2.9 % up to 4.5 % with respect to unpreserved, fresh samples (Fox et al. 1985; Hopwood 1967; Jonmarker et al. 2006). Although these artifacts fall within the relative accuracy range of the employed OCT system and are small in comparison to the inter-specimen thickness variability of 15–25 %, future endoscopic setups capable of performing wide field-of view OCT measurements in vivo would eliminate the possibility of such artifacts entirely.
The optical sectioning ability of confocal microscopy (CM) was used by Kuypers et al. (2006) to produce a thickness map based on a set of high-resolution measurements along several lines of the TM. However, the typical height of the human TM (∼1.5–2 mm) greatly exceeds the working distance of confocal microscopes, requiring the sample to be flattened out in order to maintain depth resolution. In addition, the field of view of CM is several orders of magnitude smaller than the surface area of the typical human TM, inducing possible stitching errors in the construction of the full data map. In OCT images, the axial and lateral resolutions are decoupled from one another, which allows OCT setups to maintain high resolutions across the entire sample volume in all three dimensions. The added benefits of OCT imaging include its non-invasiveness, non-destructiveness and absence of ionising radiation and, in principal, the possibility to implement the probing head into a surgical endoscope. This combination of properties makes OCT a highly suitable imaging modality for in situ and in vivo applications in middle ear research. To date, the only reports of in vivo OCT measurements in middle ear research consist of preliminary tests that investigate its applicability in a clinical setup as a means to detect structural abnormalities in human TMs (Djalilian et al. 2008; Nguyen et al. 2012). These reports provide mainly qualitative descriptions of the measured TM thickness and include few quantitative measurements. Djalilian et al. report a single-point thickness measurement of 88 μm somewhere in the antero–superior quadrant without specification of the exact location; Nguyen et al. report a single-point thickness measurement of 97 μm at an unspecified location on the TM.
An important drawback of OCT imaging is its limited penetration depth of several millimeters into the sample, depending on the central wavelength of the employed light source. This introduces a choice between larger penetration depth (higher λ) and higher axial resolution (lower λ). In order to map the thickness distribution of the entire TM in situ, an axial scanning depth of at least 2 mm is needed. Therefore, in this study, we opted for a light source with a central wavelength at λ = 1,050 nm, yielding a maximum scanning depth of 2.36 mm whilst maintaining an axial and lateral resolution of <10 μm within the sample. Due to the SNR-falloff and the decreasing signal sensitivity at larger depths within the sample, we were not able to accurately image the entire manubrium, and therefore, we cannot vouch for the accuracy of the thickness values which we measured in this region. Likewise, the pars flaccida of the human eardrum was harder to image with the employed OCT setup, as it is typically much thicker than the pars tensa.
Local thickness distribution
Various algorithms can be used to extract local thickness information from 3D geometric shapes. In this study, the three local thickness definitions that were described in the introduction were applied to all membrane models. The first, Tz, straightforwardly calculates the difference between upper and lower sheets of the membrane and forms a 2D representation of the membrane thickness at every point in the XY-plane. Although quantitatively unambiguous and easy to import into FE models, Tz is highly influenced by the local geometry and orientation of the membrane which can provide a deceptive view of the model thickness in 2D. To this end, two other methods were included in the results that both allow better interpretation of the local thickness by taking into account the geometry of the model in all three dimensions. The first of these, Tsph, provides a volume-based and model-independent thickness definition that is often used in bone densitometry to predict the structural quality of human bone models. Whereas Tsph provides a measure for the local strength at a given point within the model, TSD rather detects the local weaknesses in the membranes by highlighting the regions where the thickness values of the models are lowest. Note that only Tz delivers the raw unmodified thickness data.
General trends in the thickness distribution of human tympanic membranes
Similarities and differences in local distribution of mass between the different healthy TMs were assessed by projecting their respective thickness maps onto the same geometric shape. By doing so, we found that the absolute thickness values at different positions differ considerably between membranes. Despite this relatively large variation in absolute thickness values across the different TMs, a great deal of similarity was found in the relative thickness distributions of the membranes. In accordance with the findings of Kuypers et al. (2006), we found the membranes to be thinnest in the central region of the pars tensa between manubrium and annular ring, with the thickness increasing steeply over a small distance when moving from the central region either towards the edge of the pars tensa or towards the manubrium. Also in accordance with Kuypers’ interpolated thickness map, we noticed a local thickening in the antero–inferior quadrant of membranes TM1, TM3, TM4 and TM5. TM2 did not possess such an asymmetry in its thickness distribution.
The mean thickness across the pars tensa of the measured eardrums ranges from 79.6 μm (TM2) to 97.0 μm (TM1), which corresponds well with the typical values of average membrane thickness adopted by modelers (74–100 μm). It should however be noted that our measurements clearly confirm the findings of previously mentioned authors about differing thickness values in different regions of the membrane. For example, in TM1, thickness values ranging between 50 and 150 μm were found across the pars tensa. After quantification, this non-uniform thickness distribution can be incorporated in finite element models using the presented thickness maps. Another trend we found in our measurements was the strong linear relation between inferior–superior length and average thickness value of the membrane. We are well aware of the limited sample size included in this study, and we acknowledge that further measurements are necessary to confirm this correlation, though if validated, this could prove to be an argument for scaling the average thickness of measured membranes with inferior–superior length rather than with surface area size or other geometric features of the TM.
Average thickness map of the human eardrum
Although the inter-specimen variability between different human tympanic membranes is large and there is no such thing as an “average human eardrum”, we found that the eardrums in our study do possess similar thickness trends across the membrane. Today, most authors adopt a uniform thickness value across the entire membrane, ranging from 30–150 μm for the human TM (Gaihede et al. 2007; Decraemer and Funnell 2008; Daphalapurkar et al. 2009; Aernouts et al. 2012). While most modelers agree to employ an average value of around 74 μm (Gan et al. 2004; Cheng et al. 2007; Huang et al. 2008; Luo et al. 2009; Zhao et al. 2009), some assume an average thickness value of 100 μm (Abel and Lord 2001; Wen et al. 2006; Lee et al. 2006; Le and Huynh 2008). Quantification of these trends could provide more insight into the fundamental anatomy of the human eardrum and enables modelers to employ more accurate and representative distributions of mass in their finite element models of the human TM.
To this end, we combined the distinct geometric features of TM1-5 into a single virtual membrane TMA.
By projecting the z- (or height) values of both the upper and lower planes of each membrane onto the same contour, an average height map and 3D model of TMA was created. As TMA incorporates the full-field geometric properties of multiple samples, it could provide a more representative model of the average human eardrum.
Conclusion
The accurate and full-field thickness distribution in tympanic membranes is a critical parameter in middle ear studies, as it describes the distribution of mass in the eardrum and influences the effective membrane stiffness. We measured the thickness distribution of six human tympanic membranes using a customised high-resolution and large-field optical coherence tomography setup. Large inter-specimen variability in absolute thickness was observed, as well as a non-uniform thickness distribution across the individual membranes. In general, thickness was found to be thinnest in the central region between umbo and manubrium (50–70 μm) and largest near the peripheral rim of the pars tensa and around the umbo (100–120 μm). In addition, local thickening of the membrane (60–80 μm) was noticed in the antero–inferior quadrant of the membrane, and a strong linear relation was found between inferior–superior length and mean thickness value of the membrane. Mean thickness values across the entire pars tensa, excluding the manubrium, ranged between approximately 80 and 100 μm.
The OCT measurements of an eardrum with an atrophic region and retraction pocket in the inferior part of the pars tensa showed a local thinning of the membrane in this zone. This shows that future combination of OCT with a surgical microscope or endoscopy to measure local tympanic membrane thickness can be of use in clinical diagnoses.
Acknowledgments
The authors thank Dr. M. von Unge for his much appreciated help in the diagnosis of the pathological membrane TMP. S. Van der Jeught and J. Buytaert acknowledge the support of the Research Foundation–Flanders (FWO), J. Aerts acknowledges the support of the Flemish Agency for Innovation by Science and Technology (IWT) and A. Bradu and A. Podoleanu acknowledge the support of the ERC grant COGATIMABIO, 249889.
Contributor Information
Sam Van der Jeught, Phone: +32-0-32653553, Email: sam.vanderjeught@ua.ac.be.
Joris J. J. Dirckx, Email: joris.dirckx@ua.ac.be
Johan R. M. Aerts, Email: johan.aerts@ua.ac.be
Adrian Bradu, Email: a.bradu@kent.ac.uk.
Adrian Gh Podoleanu, Email: a.g.h.podoleanu@kent.ac.uk.
Jan A. N. Buytaert, Email: jan.buytaert@ua.ac.be
References
- Abel EW, Lord RM. Eng Med Biol Soc. Proceedings of the 23rd Annual International Conference of the IEEE. 2001. A finite-element model for evaluation of middle ear mechanics; pp. 2110–2112. [Google Scholar]
- Aernouts J, Aerts JR, Dirckx JJ. Mechanical properties of human tympanic membrane in the quasi-static regime from in situ point indentation measurements. Hear Res. 2012;290:45–54. doi: 10.1016/j.heares.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Bibas AG, Podoleanu AGH, Cucu RG, Dobre GM, Odell E, Boxer AB, et al. Optical coherence tomography in otolaryngology: original results and review of the literature. Proc SPIE. 2004;5312:190–195. doi: 10.1117/12.539144. [DOI] [Google Scholar]
- Bradu A, Podoleanu AGH, Rosen RB. High-speed en-face optical coherence tomography system for the retina. Journal of Optoelectronics and Advanced Materials. 2005;7(6):2913–2918. [Google Scholar]
- Buytaert JA, Salih WH, Dierick M, Jacobs P, Dirckx JJ. Realistic 3D computer model of the gerbil middle ear, featuring accurate morphology of bone and soft tissue structures. J Assoc Res Otolaryngol. 2011;12:681–696. doi: 10.1007/s10162-011-0281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buytaert JA, Van der Jeught S, Ars B, Podoleanu AGH, Dirckx JJJ (2013) Preliminary human tympanic membrane thickness data from optical coherence tomography. Opt Meas Tech Struct Syst Shaker Publishing 75–84. ISBN 978-90-423-0419-2
- Cheng T, Dai C, Gan RZ. Viscoelastic properties of human tympanic membrane. Ann Biomed Eng. 2007;35:305–314. doi: 10.1007/s10439-006-9227-0. [DOI] [PubMed] [Google Scholar]
- Daphalapurkar NP, Dai C, Gan RZ, Lu H. Characterization of the linearly viscoelastic behavior of human tympanic membrane by nanoindentation. J Mech Behav Biomed Mater. 2009;2:82–92. doi: 10.1016/j.jmbbm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Decraemer WF, Funnell WRJ. Anatomical and mechanical properties of the tympanic membrane. In: Chronic otitis media. Pathogenesis-oriented therapeutic management. The Hague: Kugler; 2008. [Google Scholar]
- Dirckx JJ, Kuypers LC, Decraemer WF. Refractive index of tissue measured with confocal microscopy. J Biomed Optics. 2005;10(4):044014–044014. doi: 10.1117/1.1993487. [DOI] [PubMed] [Google Scholar]
- Djalilian HR, Ridgway J, Tam M, Sepehr A, Chen Z, Wong BJ. Imaging the human tympanic membrane using optical coherence tomography in vivo. Otol Neurotol. 2008;29:10911094. doi: 10.1097/MAO.0b013e31818a08ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. 1985;33(8):845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- Funnell WRJ, Laszlo CA. A critical review of experimental observations on ear-drum structure and function. J Otorhinolaryngol Relat Spec. 1982;44:181–205. doi: 10.1159/000275593. [DOI] [PubMed] [Google Scholar]
- Gaihede M, Liao D, Gregersen H. In vivo areal modulus of elasticity estimation of the human tympanic membrane system: modeling of middle ear mechanical function in normal young and aged ears. Phys Med Biol. 2007;52:803–814. doi: 10.1088/0031-9155/52/3/019. [DOI] [PubMed] [Google Scholar]
- Gan RZ, Feng B, Sun Q. Three-dimensional finite element modeling of human ear for sound transmission. Ann Biomed Eng. 2004;32:847–859. doi: 10.1023/B:ABME.0000030260.22737.53. [DOI] [PubMed] [Google Scholar]
- Gan RZ, Dai C, Wang X, Nakmali D, Wood MW. A totally implantable hearing system—design and function characterization in 3D computational model and temporal bones. Hearing Research. 2010;263(1):138–144. doi: 10.1016/j.heares.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Hildebrand T, Rüegsegger P. A new method for the model‐independent assessment of thickness in three‐dimensional images. J Microsc. 2003;185:67–75. doi: 10.1046/j.1365-2818.1997.1340694.x. [DOI] [Google Scholar]
- Hopwood D. Some aspects of fixation with glutaraldehyde. J Anatomy. 1967;101(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- Huang G, Daphalapurkar NP, Gan RZ, Lu H. A method for measuring linearly viscoelastic properties of human tympanic membrane using nanoindentation. J Biomech Eng. 2008;130:014501. doi: 10.1115/1.2838034. [DOI] [PubMed] [Google Scholar]
- Jonmarker S, Valdman A, Lindberg A, Hellström M, Egevad L. Tissue shrinkage after fixation with formalin injection of prostatectomy specimens. Virchows Archiv An Int J Pathol. 2006;449(3):297–301. doi: 10.1007/s00428-006-0259-5. [DOI] [PubMed] [Google Scholar]
- Just T, Lankenau E, Hüttmann G, Pau HW. Optical coherence tomography of the oval window niche. J Laryngol Otol. 2009;123:603–608. doi: 10.1017/S0022215109004381. [DOI] [PubMed] [Google Scholar]
- Koike T, Wada H, Kobayashi T. Modeling of the human middle ear using the finite-element method. J Acoust Soc Am. 2002;111:1306–1317. doi: 10.1121/1.1451073. [DOI] [PubMed] [Google Scholar]
- Kojo Y. Morphological studies of the human tympanic membrane. J Otolaryngol Jpn. 1954;57:115–126. [Google Scholar]
- Kuypers LC, Decraemer WF, Dirckx JJ. Thickness distribution of fresh and preserved human eardrums measured with confocal microscopy. Otol Neurotol. 2006;27:256–264. doi: 10.1097/01.mao.0000187044.73791.92. [DOI] [PubMed] [Google Scholar]
- Le CD, Huynh QL (2008) Mathematical models of human middle ear in chronic otitis media. Proc Inf Technol Appl Biomed:426–429
- Lee CF, Chen PR, Lee WJ, Chen JH, Liu TC. Computer aided three-dimensional reconstruction and modeling of middle ear biomechanics by high-resolution computed tomography and finite element analysis. Biomed Eng Appl Basis Commun. 2006;18:214. doi: 10.4015/S1016237206000348. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Human tympanic membrane: an ultrastructural observation. Acta Otolaryngol. 1970;70:176–186. doi: 10.3109/00016487009181875. [DOI] [PubMed] [Google Scholar]
- Lin TY, Yu JF, Chen CK. Magnetic resonance imaging of the in vivo human tympanic membrane. Chang Gung Med J. 2011;34:166–71. [PubMed] [Google Scholar]
- Luo H, Dai C, Gan RZ, Lu H. Measurement of young's modulus of human tympanic membrane at high strain rates. J Biomech Eng. 2009;131:064501. doi: 10.1115/1.3118770. [DOI] [PubMed] [Google Scholar]
- Metscher BD. Micro-CT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. Biomed Centr Physiol. 2009;9:11. doi: 10.1186/1472-6793-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CT, Jung W, Kim J, Chaney EJ, Novak M, Stewart CN, Boppart SA. Noninvasive in vivo optical detection of biofilm in the human middle ear. Proc Nat Acad Sci. 2012;109(24):9529–9534. doi: 10.1073/pnas.1201592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitris C, Saunders KT, Fujimoto JG, Brezinski ME. High-resolution imaging of the middle ear with optical coherence tomography: a feasibility study. Arch Otolaryngol Head Neck Surg. 2001;127:637642. doi: 10.1001/archotol.127.6.637. [DOI] [PubMed] [Google Scholar]
- Prendergast PJ, Ferris P, Rice HJ, Blayney AW. Vibro-acoustic modelling of the outer and middle ear using the finite-element method. Audiol Neurootol. 1999;4:185–191. doi: 10.1159/000013839. [DOI] [PubMed] [Google Scholar]
- Ruah CB, Schachern PA, Zelterman D, Paparella MM, Yoon TH. Age-related morphologic changes in the human tympanic membrane. A light and electron microscopic study. Arch Otolaryngol Head Neck Surg. 1991;117:627–634. doi: 10.1001/archotol.1991.01870180063013. [DOI] [PubMed] [Google Scholar]
- Schmidt SH, Hellström S. Tympanic-membrane structure—new views. J Otorhinolaryngol Relat Spec. 1991;53:32–36. doi: 10.1159/000276181. [DOI] [PubMed] [Google Scholar]
- Song YL, Lee CF. Computer-aided modeling of sound transmission of the human middle ear and its otological applications using finite element analysis. Tzu Chi Med J. 2012;24:178–180. doi: 10.1016/j.tcmj.2012.08.004. [DOI] [Google Scholar]
- Sun Q, Gan RZ, Chang KH, Dormer KJ. Computer-integrated finite element modeling of human middle ear. Biomechanics Modeling Mechanobiol. 2002;1(2):109–122. doi: 10.1007/s10237-002-0014-z. [DOI] [PubMed] [Google Scholar]
- Trifanov I, Caldas P, Neagu L, Romero R, Berendt MO, Salcedo JAR, Ribeiro ABL. Combined neodymium–ytterbium-doped ASE fiber-optic source for optical coherence tomography applications. Photonics Technology Letters IEEE. 2011;23(1):21–23. doi: 10.1109/LPT.2010.2090039. [DOI] [Google Scholar]
- Uebo K, Kodama A, Oka Y, Ishii T. Thickness of normal human tympanic membrane. Ear Res Jpn. 1988;19:70–73. [Google Scholar]
- Van der Jeught S, Buytaert J, Bradu A, Podoleanu AGH, Dirckx JJJ (2013) Real-time correction of geometric distortion artifacts in large-volume optical coherence tomography. Meas Sci Technol 24. doi:10.1088/0957-0233/24/5/057001
- Van der Jeught S, Buytaert J, Bradu A, Podoleanu AGH, Dirckx JJJ (2012) Large-volume optical coherence tomography with real-time correction of geometric distortion artifacts. Recent Advances in Topography. Publ. Nova Science Publishers, New York. http://arxiv.org/abs/1212.1595
- Volandri G, Di Puccio F, Forte P, Carmignani C. Biomechanics of the tympanic membrane. J Biomech. 2011;44:1219–1236. doi: 10.1016/j.jbiomech.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Wada H, Metoki T, Kobayashi T. Analysis of dynamic behavior of human middle ear using a finite‐element method. J Acoust Soc Am. 1992;92:3157–3168. doi: 10.1121/1.404211. [DOI] [PubMed] [Google Scholar]
- Wen YH, Hsu LP, Chen PR, Lee CF. Design optimization of cartilage myringoplasty using finite element analysis. Tzu Chi Med J. 2006;18:370–377. doi: 10.1159/000095900. [DOI] [PubMed] [Google Scholar]
- Zhao F, Koike T, Wang J, Sienz H, Meredith R. Finite element analysis of the middle ear transfer functions and related pathologies. Med Eng Phys. 2009;31:907–916. doi: 10.1016/j.medengphy.2009.06.009. [DOI] [PubMed] [Google Scholar]