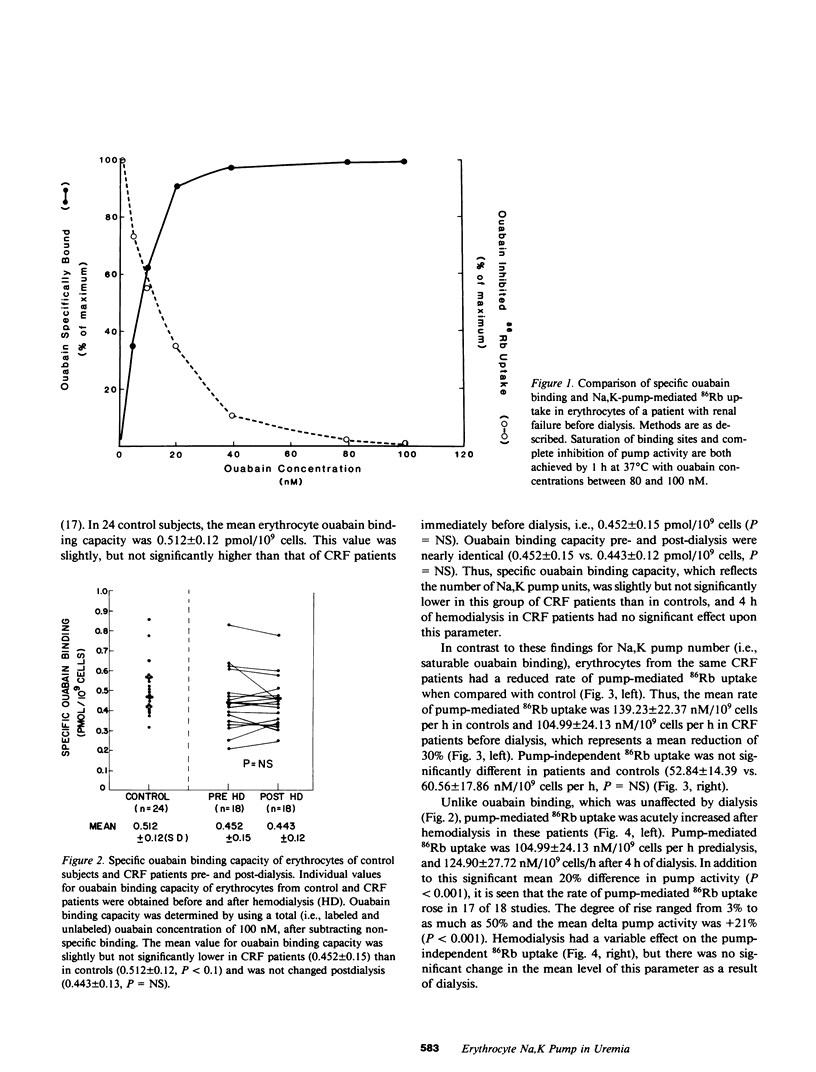
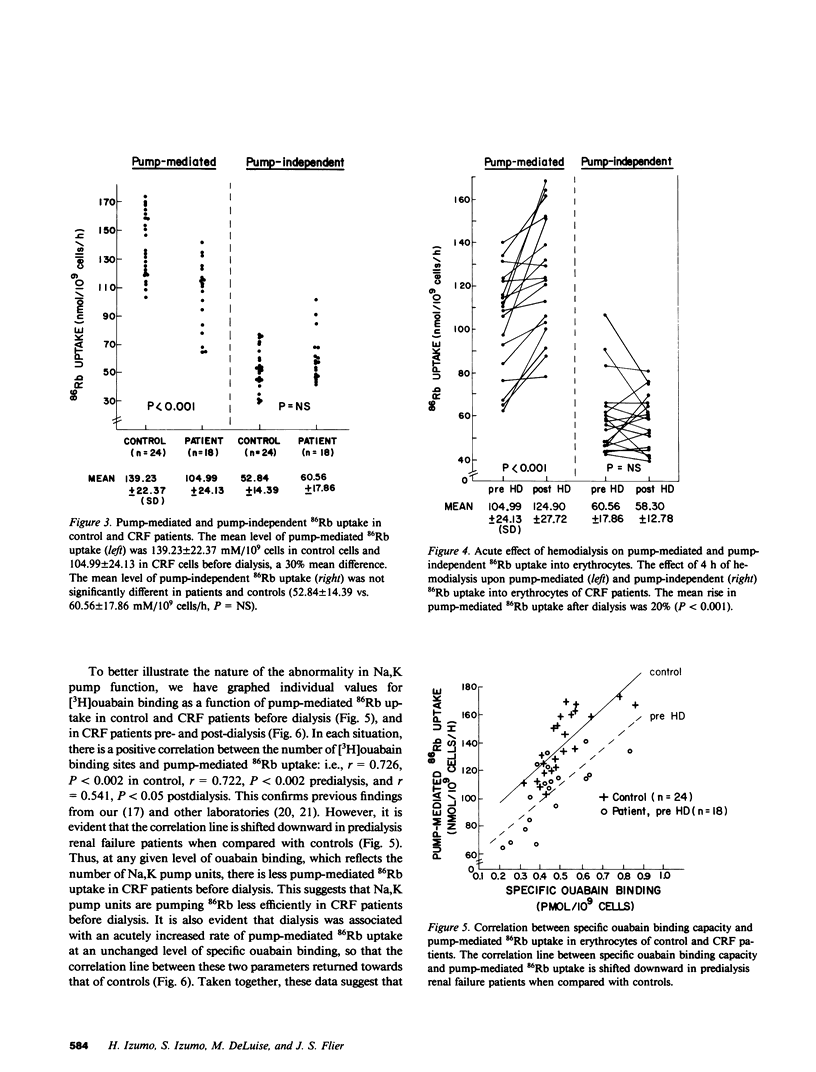
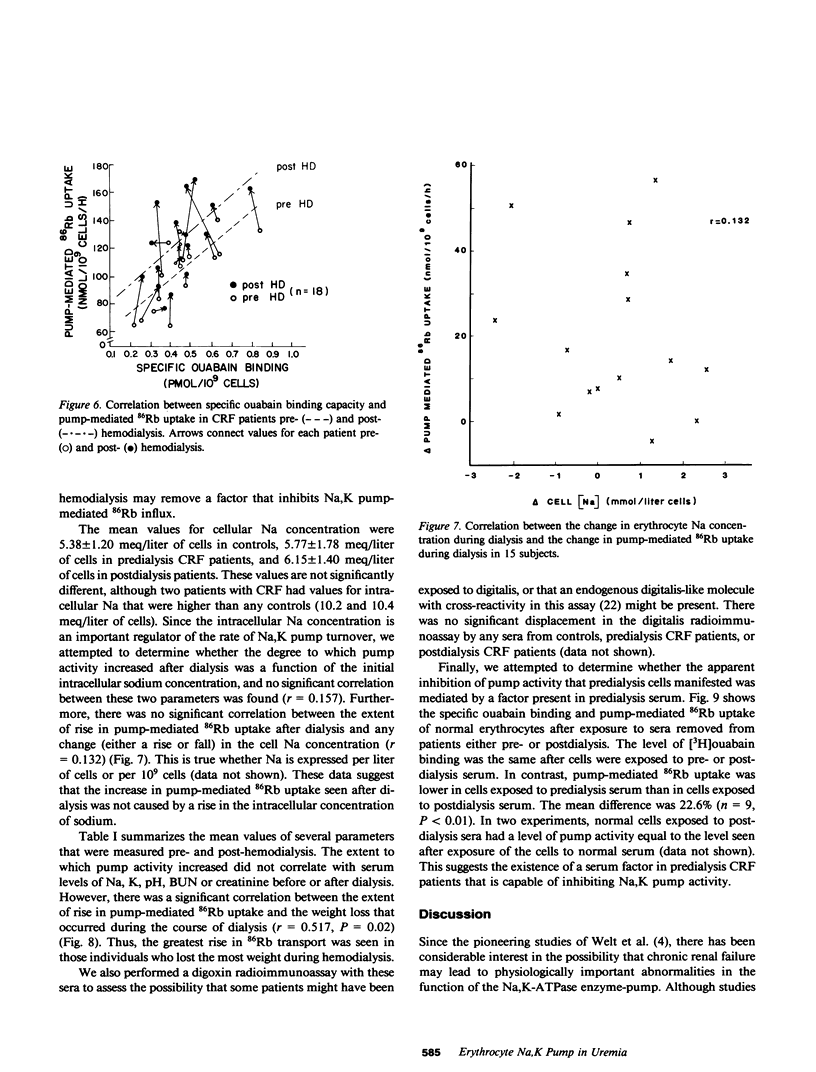
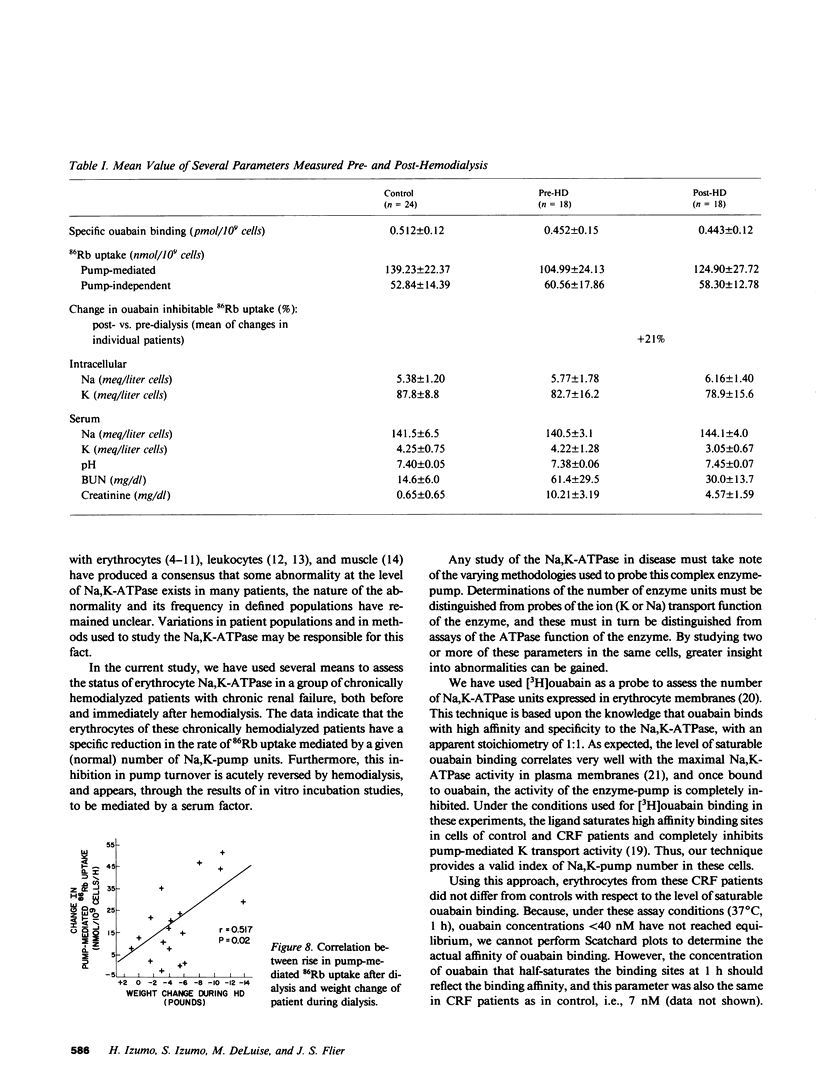
Abstract
We studied the erythrocyte Na,K-pump in chronically hemodialyzed uremic patients, immediately before and after a 4-h period of hemodialysis. Using [3H]ouabain as a probe, the number of Na,K-pump units per erythrocyte did not differ in uremic and control subjects, and hemodialysis had no acute effect on this parameter. In contrast, in these same cells the mean level of Na,K-pump-mediated 86Rb transport was 30% lower in predialysis uremic patients than in controls, and this diminution in the rate of 86Rb transport per pump unit was improved after 4 h of hemodialysis in 17 of 18 subjects. The results of in vitro incubation of normal cells with pre- and post-dialysis sera from uremic patients suggested that a serum factor is responsible for the observed inhibition of Na,K-pump activity. Changes in cell Na concentration during dialysis did not appear to be responsible for the increased rate of Na,K-pump turnover after hemodialysis. However, there was a significant correlation between the extent of rise in pump-mediated 86Rb uptake and the weight loss that occurred during dialysis. We conclude that the ion transport turnover rate of the erythrocyte Na,K-pump is impaired in uremia by a nonouabain like circulating factor. This factor, whose activity is diminished acutely by hemodialysis, may play an important role in the systemic manifestations of the uremic syndrome, and could be an important endogenous regulator of the Na,K-ATPase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole C. H., Balfe J. W., Welt L. G. Induction of a ouabain-sensitive ATPase defect by uremic plasma. Trans Assoc Am Physicians. 1968;81:213–220. [PubMed] [Google Scholar]
- Cole C. H. Decreased ouabain-sensitive adenosine triphosphatase activity in the erythrocyte membrame of patients with chronic renal disease. Clin Sci Mol Med. 1973 Dec;45(6):775–784. doi: 10.1042/cs0450775. [DOI] [PubMed] [Google Scholar]
- Cotton J. R., Woodard T., Carter N. W., Knochel J. P. Resting skeletal muscle membrane potential as an index of uremic toxicity. A proposed new method to assess adequacy of hemodialysis. J Clin Invest. 1979 Mar;63(3):501–506. doi: 10.1172/JCI109328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M., Morgan D. B. Relations between sodium transport and sodium concentration in human erythrocytes in health and disease. Clin Sci (Lond) 1981 May;60(5):555–564. doi: 10.1042/cs0600555. [DOI] [PubMed] [Google Scholar]
- De Luise M., Blackburn G. L., Flier J. S. Reduced activity of the red-cell sodium-potassium pump in human obesity. N Engl J Med. 1980 Oct 30;303(18):1017–1022. doi: 10.1056/NEJM198010303031801. [DOI] [PubMed] [Google Scholar]
- DeLuise M., Izumo H., Grace E. E., Flier J. S. Effect of diet upon the erythrocyte Na,K pump. J Clin Endocrinol Metab. 1983 Apr;56(4):739–743. doi: 10.1210/jcem-56-4-739. [DOI] [PubMed] [Google Scholar]
- Edmondson R. P., Hilton P. J., Jones N. F., Patrick J., Thomas R. D. Leucocyte sodium transport in uraemia. Clin Sci Mol Med. 1975 Sep;49(3):213–216. doi: 10.1042/cs0490213. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P. The effects of sodium and potassium on ouabain binding by human erythrocytes. J Gen Physiol. 1972 Nov;60(5):609–629. doi: 10.1085/jgp.60.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Kiino D. R. Ouabain binding and cation transport in human erythrocytes. J Clin Invest. 1973 Aug;52(8):1845–1851. doi: 10.1172/JCI107367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- Gruber K. A., Whitaker J. M., Buckalew V. M., Jr Endogenous digitalis-like substance in plasma of volume-expanded dogs. Nature. 1980 Oct 23;287(5784):743–745. doi: 10.1038/287743a0. [DOI] [PubMed] [Google Scholar]
- Kramer H. J., Bäcker A., Krück F. Inhibition of intestinal (Na+--K+)-ATPase in experimental uremia. Clin Chim Acta. 1974 Jan 19;50(1):13–18. doi: 10.1016/0009-8981(74)90072-2. [DOI] [PubMed] [Google Scholar]
- Kramer H. J., Gospodinov D., Krück F. Functional and metabolic studies on red blood cell sodium transport in chronic uremia. Nephron. 1976;16(5):344–358. doi: 10.1159/000180621. [DOI] [PubMed] [Google Scholar]
- Minkoff L., Gaertner G., Darab M., Mercier C., Levin M. L. Inhibition of brain sodium-potassium ATPase in uremic rats. J Lab Clin Med. 1972 Jul;80(1):71–78. [PubMed] [Google Scholar]
- Patrick J., Jones N. F. Cell sodium, potassium and water in uraemia and the effects of regular dialysis as studied in the leucocyte. Clin Sci Mol Med. 1974 May;46(5):583–590. doi: 10.1042/cs0460583. [DOI] [PubMed] [Google Scholar]
- Schmalzing G., Pfaff E., Breyer-Pfaff U. Red cell ouabain binding sites, Na+K+-ATPase, and intracellular Na+ as individual characteristics. Life Sci. 1981 Jul 27;29(4):371–381. doi: 10.1016/0024-3205(81)90330-1. [DOI] [PubMed] [Google Scholar]
- Skou J. C. The relationship of the (Na + + K + )-activated enzyme system to transport of sodium and potassium across the cell membrane.. J Bioenerg. 1973 Jan;4(1):1–30. doi: 10.1007/BF01516049. [DOI] [PubMed] [Google Scholar]
- Sodium-lithium countertransport in red cells. N Engl J Med. 1983 Oct 20;309(16):987–989. doi: 10.1056/NEJM198310203091618. [DOI] [PubMed] [Google Scholar]
- Swaminathan R., Clegg G., Cumberbatch M., Zareian Z., McKenna F. Erythrocyte sodium transport in chronic renal failure. Clin Sci (Lond) 1982 May;62(5):489–494. doi: 10.1042/cs0620489. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J., Goldin S. M. Active transport of sodium and potassium ions: mechanism, function, and regulation. N Engl J Med. 1980 Apr 3;302(14):777–783. doi: 10.1056/NEJM198004033021404. [DOI] [PubMed] [Google Scholar]
- Villamil M. F., Rettori V., Kleeman C. R. Sodium transport by red blood cells in uremia. J Lab Clin Med. 1968 Aug;72(2):308–317. [PubMed] [Google Scholar]
- WELT L. G., SACHS J. R., MCMANUS T. J. AN ION TRANSPORT DEFECT IN ERYTHROCYTES FROM UREMIC PATIENTS. Trans Assoc Am Physicians. 1964;77:169–181. [PubMed] [Google Scholar]
- Welt L. G. Membrane transport defect: the sick cell. Trans Assoc Am Physicians. 1967;80:217–226. [PubMed] [Google Scholar]
- Woods J. W., Parker J. C., Watson B. S. Perturbation of sodium-lithium countertransport in red cells. N Engl J Med. 1983 May 26;308(21):1258–1261. doi: 10.1056/NEJM198305263082103. [DOI] [PubMed] [Google Scholar]


