Abstract
Previous studies indicate aging results in significantly decreased cardiac function and increased myocardial apoptosis after myocardial ischemia/reperfusion (MI/R) in humans or rats. The underlying mechanisms of aging-exacerbated effects remain unknown. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are known to play vital roles in aging-related MI/R injury. Heretofore, the effects of aging upon ROS and RNS formation were not investigated in humans, which is the focus of the current study. Due to experimental limitations with clinical trials, an additional animal experiment was performed. All enrolled acute myocardial infarction (AMI) patients received percutaneous coronary intervention (PCI) therapy. AMI patients were assigned into two groups: adult (age <65, n = 34) and elderly (age ≥65, n = 45) AMI patients. Blood samples were obtained from all study participants at 24 h and 3 days post-PCI. Plasma/white blood cell (WBC) ROS and RNS markers (malondialdehyde (MDA), myeloperoxidase (MPO), reduced glutathione (GSH), inducible nitric oxide synthase (iNOS) activity, NOx, and nitrotyrosine) were determined. The same markers were determined in rat cardiac tissue after 24 h MI/R. Compared to the adult group, elderly patients manifested increased plasma MDA and MPO and decreased plasma GSH concentrations. No significant differences in plasma NOx or nitrotyrosine concentration existed between adult and elderly patients. Furthermore, WBC iNOS activity in elderly patients was significantly decreased compared to the adult group. The measurement of ROS markers in the rat experiments was consistent and supported human study data. Surprisingly, RNS markers (NOx and nitrotyrosine) in blood and heart tissue increased from young to middle-aged rats but decreased from middle age to old age. Aging augments ROS, which might exacerbate MI/R injury. Additionally, our data support aging-induced changes of RNS levels in humans and rats in vivo.
Keywords: Aging, Acute myocardial infarction (AMI), Percutaneous coronary intervention (PCI), Reactive oxygen species, iNOS
Introduction
Rapid growth of the world’s geriatric population has heightened awareness of age-related cardiovascular diseases. Cardiovascular diseases are the leading causes of death in the elderly; those 65 years of age and older account for greater than 80 % of patients with ischemic heart disease (Rosamond et al. 2007). Additionally, the exponential increase in mortality rate related to cardiovascular diseases in the geriatric population implies that cardiac aging itself may be a major risk factor for cardiovascular pathology, such as ischemic heart disease (Curtis et al. 2008).
In our previous study, we tested the effects of aging upon post-ischemic cardiac function. The results demonstrated aging significantly depressed post-ischemic cardiac function. Furthermore, we determined aging resulted in augmented reperfusion-induced myocardial apoptosis. Therefore, we hypothesized a close relationship between pro-apoptotic effects and age-related cardiac function attenuation (Liu et al. 2011). To date, the underlying mechanisms of these effects remain unknown.
Substantial evidence exists indicating that oxidative stress may be one of the major etiologies of ischemia-induced myocardial apoptosis. Numerous experiments have demonstrated markedly increased superoxide (O2−) generation from ischemic/reperfused endothelial cells and activated neutrophils (PMNs) in post-ischemic myocardial tissue. ·O2− further dismutates to H2O2 and ·OH, the latter being highly toxic to biological tissues, causative of significant myocardial necrosis and apoptosis.
Several recent studies report that aging increases reactive oxygen species (ROS) (Adler et al. 2003; Jacob et al. 2010a; Jacob et al. 2010b; Judge et al. 2005). Leeuwenburgh and colleagues demonstrate increased mitochondrial ROS formation with aging (Judge et al. 2005). Additionally, accumulating evidence indicates reactive nitrogen series (RNS), such as peroxynitrite (ONOO−), play vital roles in reperfusion-induced myocardial apoptosis. Inducible nitric oxide synthase (iNOS) activated during I/R produces toxic RNS, inducing myocardial apoptosis. The deleterious effects of RNS are further exacerbated with increased ambient ROS. However, nearly all studies concerning aging, myocardial ischemia/reperfusion (MI/R) injury, ROS, and RNS exacerbating MI/R injury (Rebrin et al. 2007; Zhang et al. 2007) utilize animal experiments. Aging-related effects with clinical applicability to humans are unknown.
In our previous study, we determined that aging increased post-ischemic myocardial apoptosis (Liu et al. 2011). In the current study, we further investigated this phenomenon in clinical patients. We designed a clinical trial involving patients with acute myocardial infarction and receiving percutaneous coronary intervention (PCI) in parallel with animal MI/R experiments to determine: (1) whether aging results in exacerbated myocardial ROS after I/R and (2) whether aging results in exacerbated myocardial RNS after I/R; if so, (3) we seek to clarify the involved underlying mechanisms of this phenomenon.
Materials and methods
The clinical trial was carried out in accordance with the Declaration of the World Medical Association. The study protocol was approved by the institutional ethics committee in Beijing Chaoyang Hospital-Affiliate of Beijing Capital Medical University. After full disclosure of the study’s purpose, nature, and inherent risks of participation, all subjects gave written informed consent prior to study inclusion.
Inclusion and exclusion criteria of acute myocardial infarction patients
Patients undergoing acute myocardial infarction (AMI) met inclusion criteria if two or more of the following conditions were present: (1) presence of chest pain; (2) presence of electrocardiographic changes with Q waves/elevated ST segment; or (3) elevated serum creatine kinase–myoglobin fraction/troponin I (cTnI) levels. Nontransmural infarction was diagnosed by ST segment or T wave changes accompanied by increased creatine kinase–myoglobin fraction/troponin I (cTnI) concentrations. Exclusion criteria for this study included: (1) cardiogenic shock; (2) left main coronary artery occlusion or severe stenosis; (3) blood flow in the infarct-related artery ≥ thrombolysis in myocardial infarction grade 1; (4) obvious coronary collaterals to the risk region evidenced by Rentrop grade ≥1; (5) previous myocardial infarction; or (6) major infection or surgery within the past 2 weeks prior to presentation (Liu et al. 2011; Fan et al. 2011).
Coronary angiography and clinical experimental design
All AMI patients were examined at admission and pre-medicated with clopidogrel (300–600 mg) and aspirin before catheterization. Clinical PCI was completed as described (Fan et al. 2011). AMI patients were divided into two groups: (1) adult AMI patients (<65 years) and (2) elderly AMI patients (≥65 years).
Animal experiment protocol
The study was approved by the institutional ethics committee and was in accordance with the United States National Institutes of Health guidelines. Male Sprague-Dawley rats were anesthetized with sodium pentobarbital. Myocardial ischemia was produced by exteriorizing the heart via a left thoracic incision and occlusion of the left coronary artery (LCA) with a silk slipknot. After 30 min of ischemia, the slipknot was released, and myocardium reperfusion commenced for 24 h (Gao E 2999). Sham-operated control rats (Sham) underwent the same surgical procedure, with no LCA occlusion. Male Sprague-Dawley rats (8 weeks, 10, and 24 months) were randomized to receive either sham or I/R and were divided into the following groups (n = 12 each): (1) young sham; (2) middle-aged sham; (3) old sham; (4) young I/R; (5) middle-aged I/R; and (6) old I/R.
Quantitative evaluation of reactive oxygen species level in humans and rats
In order to detect the effects of aging upon post-ischemic ROS in vivo, malondialdehyde (MDA), myeloperoxidase (MPO), and reduced glutathione (GSH) were utilized as oxidative stress markers. Blood samples were drawn from AMI patients after 24 h and 3 days reperfusion. Blood samples were immediately centrifuged at 3,000 rpm for 10 min at 4°C. Supernatant was collected and stored at −80°C until measurement. Plasma MDA, MPO, and reduced GSH concentrations were detected via commercially available kits as reported previously (Fan et al. 2011).
Plasma MDA, MPO, and reduced GSH concentrations from rats were measured; however, plasma markers cannot completely reflect oxidative stress in cardiac tissue. Therefore, MDA, MPO, and reduced GSH concentrations in rat cardiac tissue were measured. After 24 h reperfusion, rat cardiac tissue samples in the ischemic area were homogenized and centrifuged for 30 min at 12,000 × g at 4°C. Protein concentrations in the supernatants were measured by the bicinchoninic acid method. MDA, MPO, and reduced GSH concentrations were determined.
Quantitative evaluation of reactive nitrogen species levels in humans and rats
WBCs were isolated in patient blood samples via red blood cell lysis solution kit (Genmed Scientifics Inc, USA). iNOS activity of both rat heart tissue supernatants and isolated WBCs were measured via commercial assay kit (Jiancheng CO, Nanjing, China). Specific iNOS enzymatic activity is reported as picomoles of L-[3 H]citrulline produced per milligram of protein per minute. NOx (nitrite and nitrate, stable metabolites of NO) quantity in supernatants was determined by the Griess reaction and assayed utilizing an NOx concentration assay kit (R&D Systems Inc., Minneapolis, MN). Rat ischemic cardiac tissues and blood samples were harvested and similarly processed via the above method.
Nitrotyrosine is the accepted footprint of in vivo ONOO− formation. The concentration of nitrotyrosine in both rat cardiac tissue homogenate and patient plasma was determined via ELISA kit (Cell Sciences Inc., Canton, MA, USA), as previously described (Fan et al. 2005). The results are reported as nanomoles of nitrotyrosine per gram of protein in tissue homogenate and nanomoles of nitrotyrosine per liter in the plasma of human and rat samples.
Statistical analysis
All values are presented as mean ± SEM. All biochemical assays were performed in duplicate and averaged. Data were subjected to ANOVA, followed by Bonferroni correction for post hoc Student’s t tests. All statistics were calculated utilizing Graphpad Prism 5.0. P values <0.05 were considered statistically significant.
Results
Study patient population demographics and characteristics
Forty-two adult AMI patients and 56 elderly AMI patients were enrolled in the clinical trial. Primary PTCA with stent placement was successful in 100 % of patients. Of 98 AMI patients, 8 adult and 9 elderly patients refused study participation after enrollment, and 2 elderly patients died during the study (Fig. 1). Demographic data, baseline statistics, cardiovascular risk profile, and medication profile of all enrolled subjects are shown in Table 1. The major difference between the adult and elderly patient groups concerns the latter group having significantly more infarction-related arteries.
Fig. 1.
The grouping of clinical trial
Table 1.
Baseline characteristics of the study population (mean ± SEM)
| MI adult | MI elderly | P value | |
|---|---|---|---|
| (n = 34) | (n = 45) | ||
| Age, years | 54.6 ± 3.5 | 75.0 ± 4.3 | <0.01 |
| Sex, M/F | 20/14 | 27/18 | NS |
| HBP/total | 22/34 | 39/45 | <0.05 |
| Dyslipidemia/total | 21/34 | 34/45 | NS |
| Diabetes/total | 17/34 | 34/45 | <0.05 |
| Smoker/total | 23/34 | 32/45 | NS |
| Culprit arteries number | 1.34 ± 0.12 | 2.81 ± 0.21 | <0.01 |
| Past drug treatment (n/total) | |||
| Statins | 13/34 | 32/45 | <0.01 |
| Calcium channel blocker | 6/34 | 15/45 | NS |
| ACEI | 10/34 | 25/45 | <0.05 |
| β-Adrenoceptor blocker | 13/34 | 22/45 | NS |
| Drug treatment during the study (n/total) | |||
| Statins | 34/34 | 45/45 | NS |
| Calcium channel blocker | 10/34 | 12/45 | NS |
| ACEI | 32/34 | 36/45 | NS |
| β-Adrenoceptor blocker | 30/34 | 41/45 | NS |
| Nitrates | 34/34 | 45/45 | NS |
| Mean dosage of nitrates (μg/min) | 52.05 | 55.76 | NS |
HBP high blood pressure, ACEI angiotensin-converting enzyme inhibitor, M male, F female
Aging increased oxidative stress in AMI patients
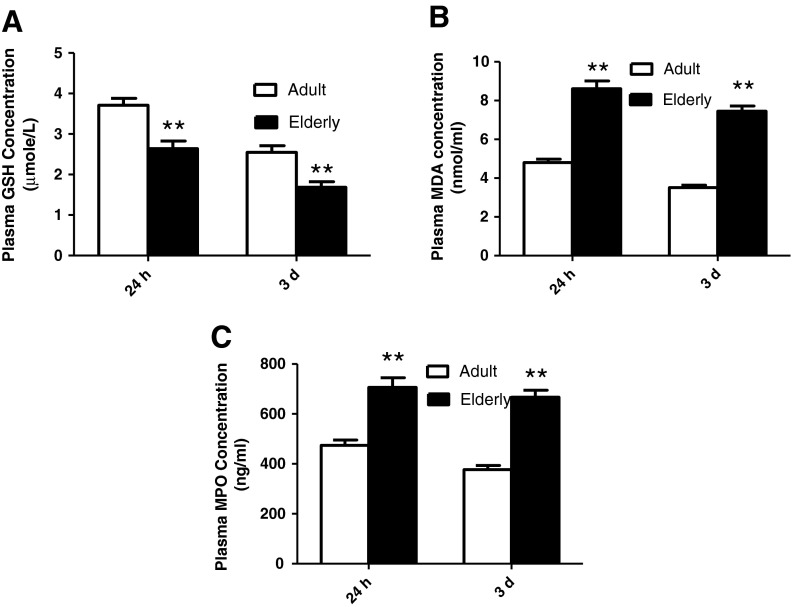
To determine the effects of aging upon post-ischemic myocardial ROS levels, plasma levels of MDA, MPO, and reduced GSH (markers reflecting myocardial oxidative stress) at two distinct time points, 24 h and 3 days after PCI, were determined. Plasma MDA and MPO concentration in elderly AMI patients was significantly greater in comparison to the adult group (Fig. 2).
Fig. 2.
Aging was associated with increased ROS (MDA and MPO) and decreased reduced glutathione (GSH) in AMI patient plasma post-PCI. a Plasma-reduced glutathione (GSH) concentration was lower in old patients than in adult patients; b Plasma malondialdehyde (MDA) concentration was higher in old patients than in adult patients; and c plasma myeloperoxidase (MPO) concentration was greater in elderly than adult patients. Adult indicates adult group, and elderly indicates elderly group. Totals for the following groups: 34 adult patients; 45 elderly patients. Data expressed as mean ± SEM. **P < 0.01 vs. adult group
Glutathione is regarded as the body’s most important self-generated antioxidant. To test its protective ability against oxidative stress in vivo, reduced form GSH concentration was determined in AMI patient plasma. Consistent with plasma MDA and MPO data, reduced GSH concentrations in elderly patients were significantly decreased compared to the adult group (Fig. 2).
Aging increased oxidative stress in rats
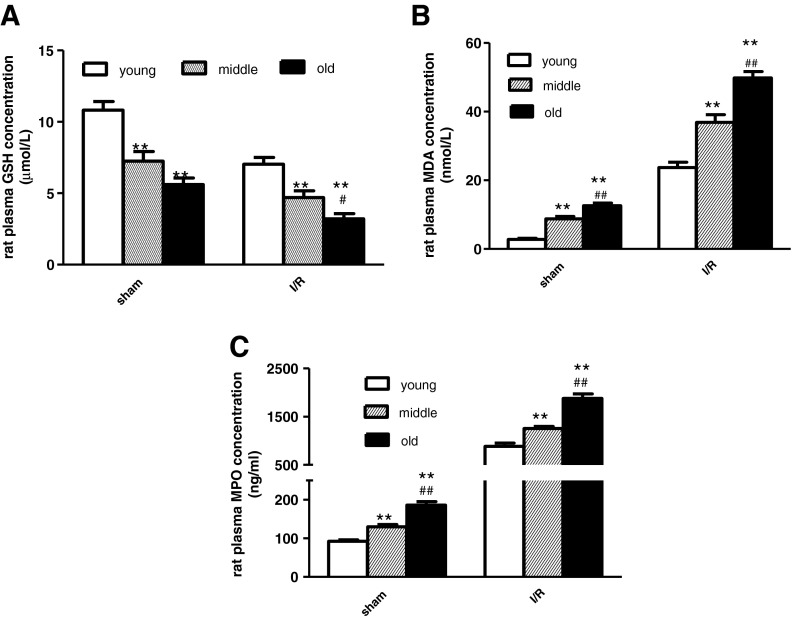
With data trends similar to those from the clinical trial, myocardial ischemia/reperfusion significantly increased rat heart myocardial/plasma MDA and MPO concentrations, with decreased myocardial-reduced GSH level compared to sham-operated rats (Fig. 3). In sham rats, myocardial MDA and MPO concentrations were greater, and myocardial-reduced GSH was significantly decreased in the middle-aged group than in the young group. The same trends appeared between middle-aged- and old rats. Consistent with sham, aging increased myocardial MDA and MPO concentrations and decreased reduced GSH levels after 24 h reperfusion (Fig. 3). Consistent with the trends of myocardial ROS markers, aging increased plasma MDA and MPO concentrations and decreased plasma GSH concentration (Fig. 4).
Fig. 3.
Aging was associated with increased ROS (MDA and MPO) and decreased reduced GSH in rat ischemic cardiac tissue after 24 h reperfusion. a Effects of aging upon cardiac-reduced GSH; b MDA; and c MPO. Sham indicates sham group and I/R indicates I/R group. n = 12 per group. Data expressed as mean ± SEM. *P < 0.05; **P < 0.01 vs. young group. #P < 0.05 vs. middle-aged group
Fig. 4.
The plasma GSH, MDA, and MPO concentration in young, middle-aged, and old rats after 24 h reperfusion. Sham indicates sham group and I/R indicates I/R group. Young indicates young rats, middle middle age rats, and old old rats. n = 12 per group. Data expressed as mean ± SEM. **P < 0.01 vs. young group. #P < 0.05, ##P < 0.01 vs. middle-aged group
Aging decreased iNOS enzymatic activity and did not alter plasma NOx or nitrotyrosine concentrations in humans
Growing evidence demonstrates that RNS play a critical pathogenic role in mediating myocardial apoptosis after I/R. During MI/R, iNOS is a major source of RNS. After ischemia, iNOS is activated and releases NO, which may react with superoxide (whose production is increased during post-ischemic reperfusion) to form the toxic molecule peroxynitrite (ONOO−), a formidable nitrating and oxidizing agent. ONOO− substantially induces myocardial apoptosis. Presently, iNOS activity in WBCs, plasma NOx, and nitrotyrosine concentration was determined. There were no significant differences in plasma NOx and nitrotyrosine concentration between adult and elderly AMI patients after 24 h and 3 days of reperfusion (Fig. 5). Elderly patients had significantly decreased iNOS activity in WBCs in comparison to the adult group (Fig. 5). To confirm the effects of aging upon RNS formation, we repeated the experiment utilizing a rat ischemia model.
Fig. 5.
In AMI patients’ status post-PCI treatment, aging decreased iNOS activity a in WBC slightly, but failed to alter b plasma RNS; c NOx; or d nitrotyrosine concentrations. Totals for the following groups: 34 adult patients; 45 elderly patients. Data expressed as mean ± SEM. *P < 0.05 vs. adult group
Aging increased iNOS enzymatic activity, NOx, and nitrotyrosine concentrations from young to middle age but decreased these RNS markers from middle age to the oldest in a rat I/R model
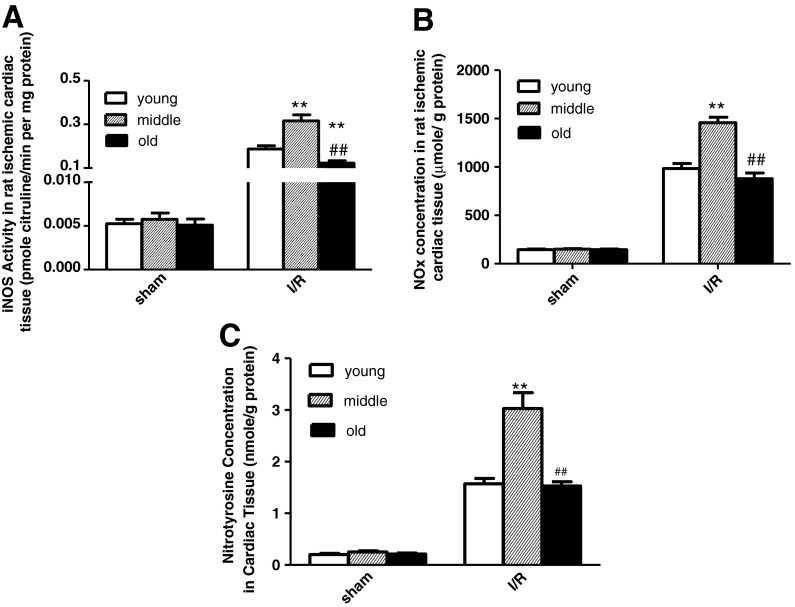
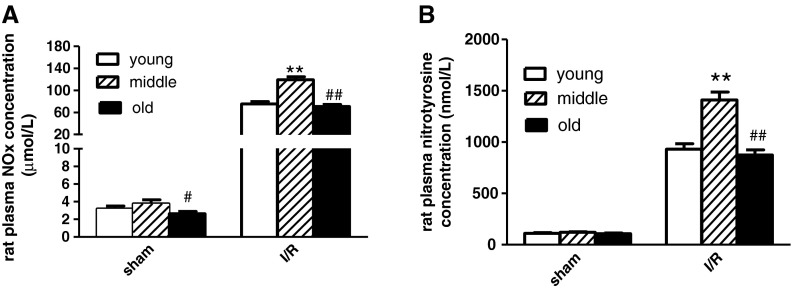
MI/R resulted in significantly elevated myocardial iNOS activity and increased NOx and nitrotyrosine concentrations (Fig. 6). As shown in Fig. 6, aging significantly increased iNOS enzymatic activity and NOx and nitrotyrosine concentrations from young to middle age. However, surprisingly, from middle age to the oldest, the trends of the above RNS markers were completely different. Compared with the middle-aged group, old rat myocardial iNOS enzymatic activity and NOx and nitrotyrosine concentrations were lower. Furthermore, we tested NOx and nitrotyrosine concentrations in rat plasma. The trends of plasma NOx and nitrotyrosine concentrations were consistent with the measurements in heart tissues (Fig. 7). Taken together, the present study demonstrates that although increasing ROS may promote RNS formation, aging did not increase in vivo RNS levels in the oldest and actually decreased iNOS activity.
Fig. 6.
Consistent with the clinical trial, aging decreased iNOS activity slightly in a rat ischemic cardiac tissue, but did not exert any effect upon b cardiac NOx; or c nitrotyrosine concentrations. n = 12 per group. Data expressed as mean ± SEM. **P < 0.01 vs. young group. ##P < 0.01 vs. middle-aged group
Fig. 7.
Rat plasma NOx (a) and nitrotyrosine (b) concentration in young, middle-aged rats, and old rats after 24 h reperfusion. n = 12 per group. Data expressed as mean ± SEM. **P < 0.01 vs. young group. #P < 0.05, ##P < 0.01 vs. middle-aged group
Discussion
Several novel observations have been made in the present study. For one, we demonstrate for the first time with clinical trial data that aging increases oxidative stress post-reperfusion. To our knowledge, previous investigations demonstrating aging-associated ROS increase post-I/R were all animal experiments and had not been confirmed in human studies. Secondly, although myocardial ROS increased significantly post-reperfusion, the clinical trial did not support that advancing age exerts effects upon RNS formation. Finally and most interestingly, our rat study demonstrated that aging had adverse effects on the formation of RNS in vivo. Aging increased iNOS activity post-reperfusion from young to middle age. However, iNOS activity and RNS concentration in vivo decreased from middle age to oldest.
Our previous study reported aging increased I/R-induced myocardial apoptosis, retarding cardiac functional recovery post-reperfusion. However, the etiological factors underlying effects of aging are unknown. Yacoub and colleagues utilized HSP72-transfected rats and demonstrated that increased Mn-SOD (a reduced protein) might attenuate mitochondria-related apoptosis post-reperfusion (Suzuki et al. 2002). Patel and colleagues utilized the rabbit myocardial infarction model and demonstrated that inhibition of NADPH oxidase could reduce myocardial oxidative stress (Qin et al. 2007).
Furthermore, NADPH oxidase depression decreases post-ischemia myocardial apoptosis, retarding heart failure development (Qin et al. 2007). Leeuwenburgh and colleagues designed an age-associated study with rats. The study demonstrated that aging decreased reduced GSH concentration and increased mitochondrial H2O2. Our study indicates that aging aggravates I/R injury. Additionally, aging increased myocardial apoptosis and depressed post-ischemic cardiac functional recovery (Jacob et al. 2010a; Shih et al. 2011). Meanwhile, aging also increased in vivo ROS. While our study presents data that are consistent with the existent literature, we further contribute evidence of the phenomenon occurring in humans.
It had been widely recognized that, except for ROS, RNS plays an important role in mediating myocardial apoptosis during I/R (Fan et al. 2011; Fan et al. 2005). NO may react with superoxide, whose production is increased during post-ischemic reperfusion, to form the more toxic nitrating and oxidant agent ONOO−, an RNS. Peroxynitrite is a substantial trigger of cardiomyocyte apoptosis. ONOO− has been shown to be highly reactive with a wide variety of molecules, including deoxyribose, cellular lipids, and protein sulfhydryl moieties, causing direct oxidative tissue damage. Several animal experiments tested the effects of aging upon in vivo RNS formation and demonstrated that aging resulted in increased nitration (Rebrin et al. 2007; Zhang et al. 2007). Currently, with the clinical trial, we did not find any significant difference in NOx and ONOO− formation between the adult and elderly populations. The activity of iNOS was assessed to determine the root cause of the above phenomenon. We tested iNOS activity, the major source of RNS, and surprisingly determined that aging did not increase post-ischemia iNOS activity. Conversely, iNOS activity in isolated WBCs in elderly patients is significantly lower than in the adult group.
To determine whether nitrate treatment affected iNOS activity, we carefully evaluated the medical treatment of the two study groups. No significant differences were found related to nitrate dose between two groups. Furthermore, we examined iNOS activity in rat cardiac tissue and obtained data consistent with the clinical trial. Advancing age may initiate decreased iNOS activity; iNOS may not be stimulated in the elderly fully post-I/R.
An additional interesting phenomenon was found in the animal experiments. As age increased, iNOS activity and RNS concentration in vivo increase from young to middle age. However, iNOS activity and RNS concentration in vivo decreased with the increasing age from middle-aged- to senescent animals. Although the exact mechanism responsible for this age-related bell-shaped alteration in RNS production remains unclear at the present time, these results from the animal experiment were, in part, consistent with our human study (from middle age to oldest), which might contribute to the decreasing of body’s reaction in senescent patients.
Taken together, results from the current study indicate that aging augmented in vivo ROS levels after I/R, but the effects of aging did not affect RNS. The novel insights gained from the current study should promote large scale and/or longer term clinical trials to confirm and enhance knowledge related to aging and to gain better mechanistic comprehension.
Limitations
There were significantly less culprit arteries found by PCI in the adult than elderly AMI group. Myocardial necrotic markers indicated more severe ischemia in the elderly. Due to natural limitations inherent in clinical trials, it is difficult to enroll identical patient cases (i.e., the same number of PCI-determined culprit arteries). In addition, diabetic status, blood pressure status, and pharmacological status were different between middle-aged- and senescent patients. All of the baseline differences might result in the change of ROS and RNS concentration in vivo. Therefore, we chose to perform a rat experiment, which could confirm that the baseline parameters were consistent in different aged groups.
Footnotes
Qian Fan and Mulei Chen contributed equally to the study.
Contributor Information
Qian Fan, Phone: +86-10-85231937, FAX: +86-10-65951064, Email: fanqian75@sina.com.
Xinchun Yang, Phone: +86-10-85231586, FAX: +86-10-65951064, Email: yangxc@medmail.com.cn.
References
- Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, et al. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol. 2003;285:H1015–1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- Fan Q, Gao F, Zhang L, Christopher TA, Lopez BL, Ma XL. Nitrate tolerance aggravates postischemic myocardial apoptosis and impairs cardiac functional recovery after ischemia. Apoptosis. 2005;10:1235–1242. doi: 10.1007/s10495-005-1455-5. [DOI] [PubMed] [Google Scholar]
- Fan Q, Yang XC, Liu Y, Wang LF, Liu SH, Ge YG, et al. Postconditioning attenuates myocardial injury by reducing nitro-oxidative stress in vivo in rats and in humans. Clin Sci (Lond) 2011;120:251–261. doi: 10.1042/CS20100369. [DOI] [PubMed] [Google Scholar]
- Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circulation research;107:1445-1453 [DOI] [PMC free article] [PubMed]
- Jacob MH, Da RJD, Jahn MP, Kucharski LC, Bello-Klein A, Ribeiro MF. Age-related effects of DHEA on peripheral markers of oxidative stress. Cell Biochem Funct. 2010;28:52–57. doi: 10.1002/cbf.1619. [DOI] [PubMed] [Google Scholar]
- Jacob MH, Janner Dda R, Araujo AS, Jahn MP, Kucharski LC, Moraes TB, et al. Redox imbalance influence in the myocardial Akt activation in aged rats treated with DHEA. Exp Gerontol. 2010;45:957–963. doi: 10.1016/j.exger.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- Liu M, Zhang P, Chen M, Zhang W, Yu L, Yang XC, et al. Aging might increase myocardial ischemia/reperfusion-induced apoptosis in humans and rats. Age (Dord) 2011;34(3):621–632. doi: 10.1007/s11357-011-9259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Simeone M, Patel R. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic Biol Med. 2007;43:271–281. doi: 10.1016/j.freeradbiomed.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Bregere C, Kamzalov S, Gallaher TK, Sohal RS. Nitration of tryptophan 372 in succinyl-CoA:3-ketoacid CoA transferase during aging in rat heart mitochondria. Biochemistry. 2007;46:10130–10144. doi: 10.1021/bi7001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol. 2011;57:9–17. doi: 10.1016/j.jacc.2010.08.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, et al. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia–reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106:I270–276. [PubMed] [Google Scholar]
- Zhang H, Tao L, Jiao X, Gao E, Lopez BL, Christopher TA, et al. Nitrative thioredoxin inactivation as a cause of enhanced myocardial ischemia/reperfusion injury in the aging heart. Free Radic Biol Med. 2007;43:39–47. doi: 10.1016/j.freeradbiomed.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]