Abstract
Falls represent a significant health risk in the elderly and often result in injuries that require medical attention. Reduced ability to control motion of the whole-body center of mass (COM) has been shown to identify elderly people at risk of falling. To explore effective preventive strategies and interventions, we studied adult age-related differences in multijoint coordination to control the COM during balance recovery. We used the uncontrolled manifold (UCM) analysis, which can decompose movement variability of joints into good movement variability (motor equivalent) and bad movement variability (nonmotor equivalent). The good variability does not affect the COM position, while the bad variability does. Twenty-nine subjects, including 16 healthy young (26.1 ± 4.5 year) and 13 older (74.6 ± 5.6 year) adults without systematic disease, neurological disease, or a severe degenerative condition stood on a flat platform, and received an unexpected backward translation. The older adults had similar amounts of joint movement as the young adults during balance recovery except for the thoracic–lumbar joint. However, the UCM analysis showed that the older adults changed their joint coordination pattern to control the COM and had a lower motor equivalent index with increased nonmotor equivalent variability (bad variability). We conclude that normal aging adults lose the compensatory strategy of flexibly controlling multiple joints when stabilizing the COM after receiving a balance perturbation.
Keywords: Posture, Joint coordination, Variability, Standing, Aging, Uncontrolled manifold
Introduction
Falls represent a significant health risk in the elderly and often result in injuries that require medical attention. These falls have serious physical, emotional, and financial impacts on the individual, as well as a serious impact on health care costs overall. Most falls occur after loss of stability during daily life activities, such as slipping while moving from sit-to-stand (Pavol et al. 2002), tripping while walking (Blake et al. 1988), or simply turning around (Nevitt et al. 1991). To provide effective preventive strategies and interventions, we must understand the mechanisms and factors that cause older adults to fall.
The primary goal during balance maintenance is to control the relative motion between the whole-body center of mass (COM) and its base of support (Shumway-Cook and Woollacott 2000; Lugade et al. 2011). Previous studies described different models and strategies to maintain optimal postural control in upright stance (Nashner and McCollum 1985; Winter 1990). Ankle and hip strategies restore the equilibrium by moving the body as a double-segment inverted pendulum, with counterphase motions at the ankles and hip. Previous studies have shown that the sensorimotor system uses the movement of all major body segments to stabilize the COM in healthy young adults (Scholz et al. 2007). Compensatory postural adjustments have also been described in terms of the multijoint coordination underlying the contribution of a variety of body segments to the recovery of stability. Substantial evidence supports the notion that movement variability is an essential feature of human motor behavior; this affords the sensorimotor system the necessary flexibility and adaptability to operate efficiently in a variety of performance contexts (Latash et al. 2002; Stergiou et al. 2006; Creath et al. 2008; Poston et al. 2008; Freitas et al. 2010). How normal aging affects the multijoint coordination pattern underlying the control of the COM during balance recovery is still unclear. This knowledge has the potential to contribute to the development of fall prevention strategies in older adults.
The sensorimotor system is known to have substantial redundancy at the joint, muscle, and neural levels. For example, when receiving a balance perturbation, one can use the hip joint to compensate for the ankle movement caused by the perturbation. One can also use the knee joint alone to compensate for the ankle movement. In this case, the hip and knee joints are the redundant joints that can help with balance recovery. Recent studies indicate that the neural controller takes advantage of motor redundancy, or more positively viewed, motor abundance (Latash 2000). Motor abundance has been shown to provide for flexible control of a variety of motor tasks and requires good multijoint coordination (Hsu and Scholz 2012).
The uncontrolled manifold (UCM) approach has been used in studying multijoint coordination in recent postural control research (Scholz and Schöner 1999; Latash et al. 2005; Hsu et al. 2007). The UCM approach relates individual joint contributions to the control of the COM. The UCM hypothesis assumes that the controller (sensorimotor system) acts in a state space of independent elemental variables. These are the smallest variables that describe the system at a selected level of analysis. Hypothetically, the controller can change these elemental variables independently of each other. In different studies, elemental variables have been associated with joint movements (Domkin et al. 2005; Freitas et al. 2010), moments of forces (Kang et al. 2004; Shim et al. 2008), muscle activations (Krishnamoorthy et al. 2003; Robert et al. 2008), and leg length (Auyang et al. 2009).
A previous study has shown that healthy young adults arranged their joint configuration in response to support surface perturbations by returning the COM to a similar state as preperturbation, which indicated that healthy young adults used a motor equivalent joint coordination pattern, a more flexible pattern, to stabilize their COM (Scholz et al. 2007). Many studies using the UCM approach to study joint coordination have found age-related differences in the structure of variability in pointing movements (Verrel et al. 2012) and multifinger force production (Shim et al. 2004; Olafsdottir et al. 2007). Healthy older adults used a less flexible joint coordination pattern compared with younger adults in those upper extremity movements. However, the whole-body joint coordination in upright balance control has not been investigated. The purpose of the present study was to determine to what extent healthy older adults use motor equivalent coordination patterns like young adults when recovering balance from a posture perturbation. We hypothesized that healthy older adults would use a less flexible coordination pattern to regain their balance after receiving a balance perturbation.
Methods
Subjects
According to a sample size analysis set with a power of 80 % and an alpha level of 5 %, 12 subjects were needed for each group. Sixteen young adults and 16 older adults were enrolled to compensate for an expected 20 % dropout rate. Eligible participants were given informed consent, and signatures were collected before enrollment. Older subjects lived independently and were free of systematic diseases, such as diabetus mellitus, neurological disease, or severe degenerative conditions. No subject had a history of lower extremity infirmity or pathology, or was suffering from any musculoskeletal disease at the time of testing that would affect the ability to perform the experiment. They had no history of falls 1 year prior to the testing. However, three older adults could not maintain their balance during the balance perturbation without taking a step, so their data were excluded in the analysis. All subjects reported that they were right-handed and right-footed. Height, weight, and lower extremity anthropometric measures were recorded for all subjects (Table 1). Prior to participation, each subject signed a consent form approved by the Human Subjects Compliance Committee.
Table 1.
Characteristics of the study participants
| Healthy young adult group (n = 16) | Healthy older adult group (n = 13) | |
|---|---|---|
| Age (years) | 26.1 ± 4.5 | 74.6 ± 5.6 |
| Gender | 8 females; 8 males | 5 females; 8 males |
| Weight (kg) | 67.8 ± 11.7 | 64.9 ± 7.6 |
| Height (cm) | 171.7 ± 0.1 | 163.4 ± 0.1 |
| Body mass index (kg/m2) | 22.8 ± 2.4 | 24.3 ± 2.7 |
| Exercise time per week (hours) | 3.25 ± 1.4 | 4.1 ± 2.2 |
| Mini-Mental State Examination (severe cognitive impairment, ≤9; moderate, 10–20; mild, 21–24) | 30 ± 0 | |
| Geriatric Depression Scale (severe depression, 20–30; mild, 10–19; normal, 0–9) | 0 ± 0 |
Setup and procedure
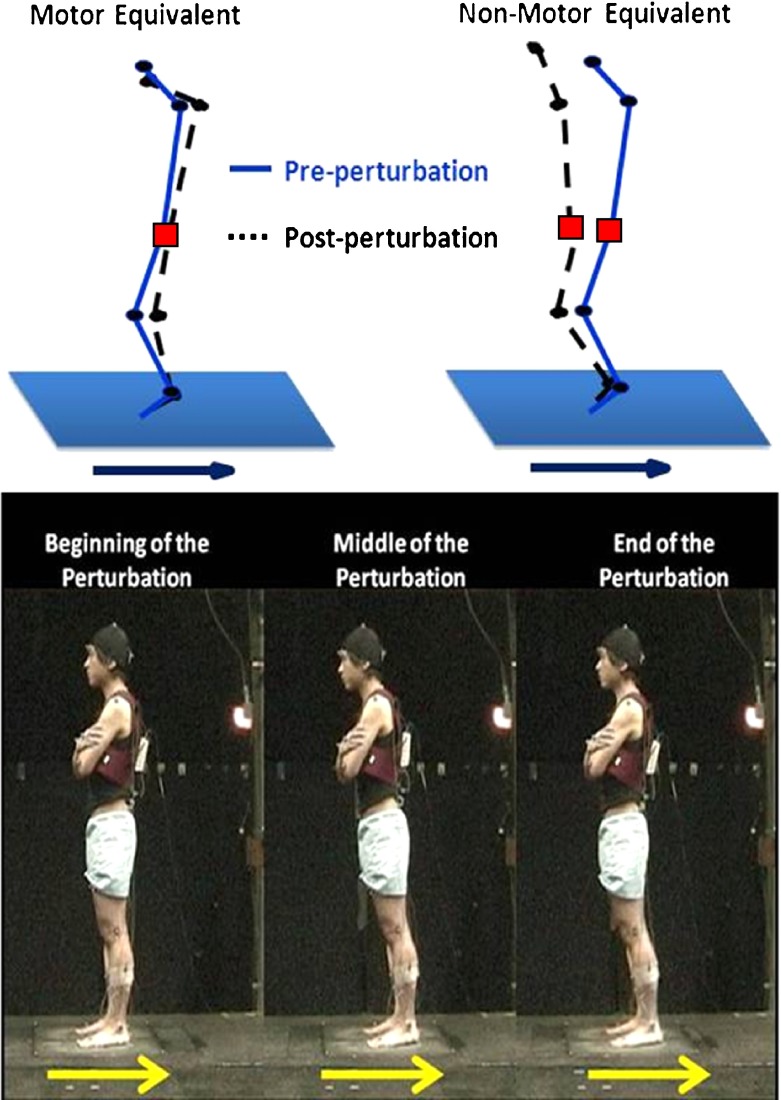
Subjects stood quietly on a movable platform in an upright position with their arms crossed over their chests (Fig. 1). Their feet were pointed in a natural stance and eyes were directed straight ahead. They were instructed to recover from a perturbation by returning to their initial upright position, without stepping or lifting their heels off the ground, if possible. The magnitude of the backward support surface translations was 15 cm with various velocities from 30 to 70 cm/s, which were used to elicit the postural recovery responses of the subject. Only results for 30 cm/s condition were presented here because 13 out of 16 older adults could maintain their balance without taking steps only in this condition. Trials that did not involve stepping were included in the analysis. Thus, data from the three healthy older adults who could not regain their balance without stepping were excluded in the UCM analysis. Each perturbation occurred in blocks of three repeated trials of the same magnitude, with the blocks given in a random order.
Fig. 1.
Illustration of motor equivalent (left) and nonmotor equivalent (right). The black circles are the joints, and the red square is the center of mass. The arrows show the direction of the perturbation
Experimental apparatus
Three-dimensional marker trajectories in space were collected by the ten-camera motion analysis system (Motion Analysis, Santa Rosa, CA, USA) with a sampling rate of 120 Hz. Twenty-nine retroreflective markers were placed bilaterally on bony landmarks of the subject similar to previously validated marker setups (Hahn and Chou 2004). The recorded coordinates of each reflective marker were smoothed using a MATLAB program (Mathworks, Version 7.0.1) with a 5-Hz low-pass, bi-directional second order Butterworth digital filter.
Dependent variables
Kinematic measures
The marker positions were used to calculate the sagittal plane joint angles at the ankle, knee, hip, between the 12th thoracic and 1st lumbar vertebrae junction (TLJ), and between the 7th cervical and 1st thoracic vertebrae junction (CTJ). Whole-body COM position in the sagittal plane was calculated as the weighted sum of the six-segment model, including the feet, shanks, thighs, pelvis, trunk, and head. The body was assumed to be symmetric. The segment lengths, widths, and circumferences of each subject were measured for calculation of each subject’s body COM. Locations of segmental centers of mass and mass proportions were estimated and used to compute the instantaneous position of the body’s COM (Winter 1990). The focus of this report is on control of the anterior–posterior (AP) position of the COM because the perturbation direction was posterior. The geometric model relating the COM position to the joint configuration, with origin at the ankle is:
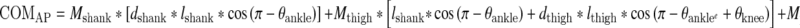 |
where θankle, …, θCTJ are the externally defined joint angles; lshank, …, lhead are the lengths of the respective segments calculated from the static calibration trial; dshank, …, dhead are the percentages of the segment lengths from the distal end where the mass of that segment lies; and Mshank, …, Mhead are the proportion of total body mass for each of these segments.
The COM excursion and the individual joint excursion were calculated from the maximum minus minimum data points during the active response phase. To examine the overall joint movements, the sum of the root mean square of the five joints’ excursions over the active response phase (defined below) was also computed. Because the balance perturbation was created using a moveable platform translation, this mechanical platform motion caused the movements of the subjects passively and created a passive response component in the subject’s body. Therefore, the active response component that was a corrective response to the platform translation came later (Runge et al. 1999). We defined an active response based on the COM velocity profile. The onset of the active response phase was defined as the time of first zero crossing in the positive direction; the active response was calculated during the 1-s time period after its onset (pink lines in Fig. 2).
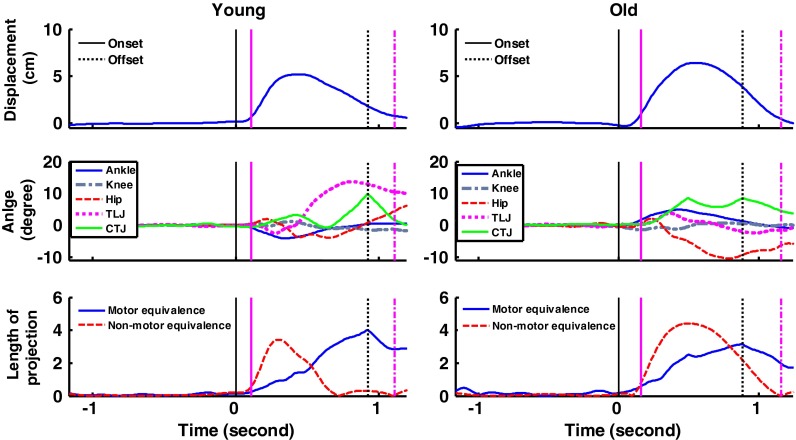
Fig. 2.
Perturbation responses of one young adult (left) and one older adult (right) resulting from a 15-cm support surface translation at 30 cm/s. Figures are excursion of COM position with the forward direction as positive (top), joint angle of five measured joints with flexion as positive (middle), and projections of the joint deviation vector ME vs. NME (bottom). The solid vertical lines indicate the onset of the perturbation (black) and onset of the active response phase (pink), while the vertical-dotted lines indicate the offset of the perturbation (black) and 1 s after the onset of active response phase (pink). Note that the hip and TLJ joint were moved in counterphase in the young subject while the hip joint went into extension without any compensatory movement in the older subject
Uncontrolled manifold analysis
In a uncontrolled manifold (UCM) model, the controller selects within the space of elemental variables a subspace (a manifold, UCM) corresponding to a value of a performance variable that needs to be stabilized. In the present study, we were interested in how movements of the individual joints (elemental variables) affect the COM position (the performance variable) during balance recovery. The controller arranges covariations among the joints such that their variance has relatively little effect on the COM position. The movement variability of joints can be viewed as consisting of two components, goal-equivalent variability and nongoal-equivalent variability (Verrel et al. 2012). In the present study, we quantified how much of the joint variability assists the body to return the COM position to its preperturbation state and how much of it changes the COM position.
The joint coordination pattern to stabilize the COM was examined using the UCM analysis for which the methodology has been previously validated (Scholz et al. 2007). The UCM approach can partition postural variability into motor equivalent (ME) and nonmotor equivalent (NME) components. In the present study, the ME can be seen as “good variability” that helps the body to restore the COM position to its preperturbation state and affords flexibility in dealing with an unexpected balance perturbation. On the contrary, the NME can be seen as “bad variability” that takes the COM position away from its preperturbation state (Fig. 1). UCM analysis was performed on each trial to obtain the index of motor equivalence with the following steps:
Computing the reference joint configuration
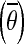 , which is the mean joint configuration of the ankle, knee, hip, TLJ, and CTJ joint angle (θankle,θknee,θhip,θTLJ, and θCTJ) during the baseline period over the 1 s before the perturbation onset.
, which is the mean joint configuration of the ankle, knee, hip, TLJ, and CTJ joint angle (θankle,θknee,θhip,θTLJ, and θCTJ) during the baseline period over the 1 s before the perturbation onset.Computing the joint deviation vector
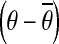 , which is the difference between the current joint configuration at each point in the trial and the reference joint configuration.
, which is the difference between the current joint configuration at each point in the trial and the reference joint configuration.- Calculating the Jacobian matrix (J), which relates changes in COM position to changes in joint postures (the geometrical model)
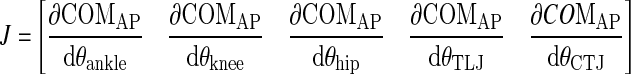
- Computing the null-space of the Jacobian matrix (ɛ) based on the reference joint configuration
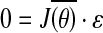
- Projecting joint deviation vectors
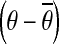 into the nullspace and into its orthogonal space (range space)
into the nullspace and into its orthogonal space (range space)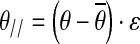
where θ// is the vector of joint configuration projected to the nullspace of Jacobian, and θ⊥ is the vector of joint configuration projected orthogonal to the nullspace of Jacobian.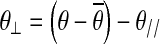
- Computing resultant length and normalizing each projection to the appropriate degrees of freedom
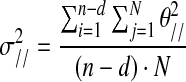
where σ2//is variance within the UCM, and σ2⊥ is variance in the joint space orthogonal to the UCM. N is the number of time sample, n is the total number of joints (i.e., five joints), and d is the number of special dimensions of COM (i.e., one dimension in the sagittal plane).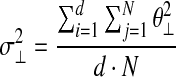
Resulting measures were the lengths of projection of the joint deviation vector and were categorized as two components: motor equivalent (ME), which is the deviation vector projection into the nullspace (σ2//), indicating the extent to which the difference between the current joint configuration and the reference joint configuration, tended to take the COM back to a preperturbation position corresponding to the reference joint configuration. We refer to ME as “good variability”. Nonmotor equivalent (NME) is the joint deviation vector projection into the orthogonal space (σ2⊥), indicating the extent to which the difference between the current joint configuration and the reference joint configuration, tended to lead to a different COM position. We refer to NME as “bad variability.”
-
Defining the motor equivalent index (ME index) as the synergy index: ME index for the entire period of the active response was defined as the ratio of the two projection components
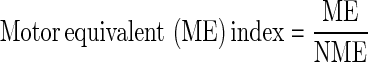
ME-index >1 suggests that the subject used a motor equivalent joint coordination, that is, a more flexible pattern, to regain balance stability. On the contrary, ME index <1 suggests that the subject used a nonmotor equivalent joint coordination, that is, a less flexible pattern, to regain balance stability.
Clinical evaluations
The balance ability for the older adults was determined using the Timed Up and Go Test (Shumway-Cook et al. 2000), the Berg Balance Scale Test (Berg et al. 1992), and the Dynamic Gait Index (Shumway-Cook and Woollacott 2000). The young adult group also performed the Timed Up and Go Test for later group comparison. The young adult group did not perform the Berg Balance Scale Test and Dynamic Gait Index because of ceiling effects in this group.
Statistics
A mixed-design multivariate analysis of variance tests was conducted to analyze differences between the two groups in COM excursion pre-/postperturbation, COM excursions during active response phase, five joint excursions, sum of the five joint excursions, and ME index. An internal Bonferroni correction was used to avoid the accumulation of type I error.
The measure of motor equivalence was obtained with the mean of each subject’s ME and NME values for each trial that were computed across the duration of the active response phase. A two-way mixed-design ANOVA was performed to examine the between-subjects factor (young vs. old) and variance component (ME vs. NME). A Pearson correlation was used to determine the correlations between clinical evaluation and center of mass control (ME index) using the data from older adults. The alpha level was set to 0.05. All statistical analyses were conducted using SPSS, release 18.0.1 (SPSS Inc., Chicago, IL, USA).
Results
Excursion of the center of mass and individual joints
The COM motion of a representative older adult and young adult are shown in Fig. 2 (upper panel). The COM excursion was not significantly different between groups for the active response phase (mean ± SD: young, 4.95 ± 1.04 cm; old, 5.33 ± 1.33 cm; F(1,27) = 0.75, p = 0.39) nor for the difference between baseline period and the first data point after the end of the active response period, that is, the pink dashed line minus the mean of the 1-s period in Fig. 2 (young, 1.83 ± 2.01 cm; old, 2.59 ± 2.17 cm; F(1,27) = 0.97, p = 0.33).
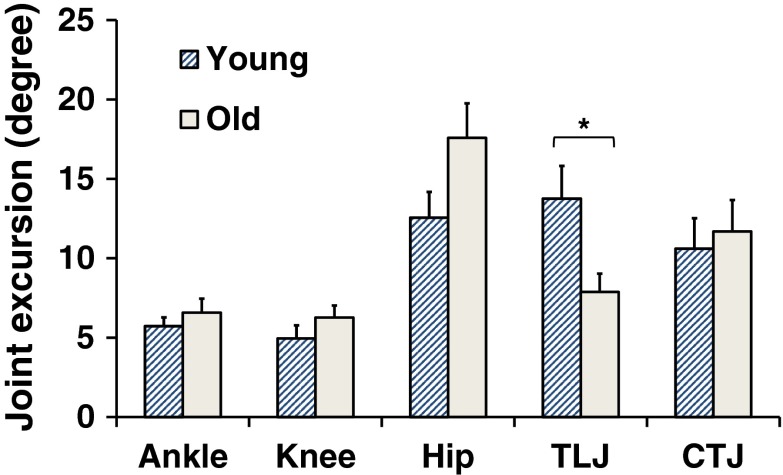
The motion of the five examined individual joints in the same representative individuals are shown in Fig. 2 (middle panel). Most individual joint excursions during the active response phase were not significantly different between groups, except for the thoracic–lumbar junction (TLJ) [F(1,27) = 5.41, p = 0.03] (Fig. 3). Although the hip joint excursion seems very different between groups, this difference did not reach significance [F(1,27) = 3.58, p = 0.07]. The sum of the five joint excursions was also not significantly different between the two groups [mean ± SD: young, 47.56 ± 22.34°; old, 49.85 ± 19.48°; F(1,27) = 0.09, p = 0.76]. These results suggest that these five individual joints might self-organize to stabilize the COM position in a form of multi-joint coordination. However, how well the subjects compensated for their joint movements with this multi-joint coordination was further examined using the UCM analysis.
Fig. 3.
Joint excursions (maximum minus minimum data points) during the active response phase for each joint angle and the two age groups. Error bars represent standard error of the mean (SEM). *p < 0.05
Uncontrolled manifold analysis
The joint coordination pattern that subjects used to stabilize the COM position was examined by measuring the ME index using the UCM analysis. The ME index is the ratio of motor equivalent variability to nonequivalent variability. The ME index in the young adults was >1 (1.63 ± 1.32), while the ME index was <1 in the older adults (0.82 ± 0.37). The difference between groups was significant [F(1,27) = 4.58, p = 0.04].
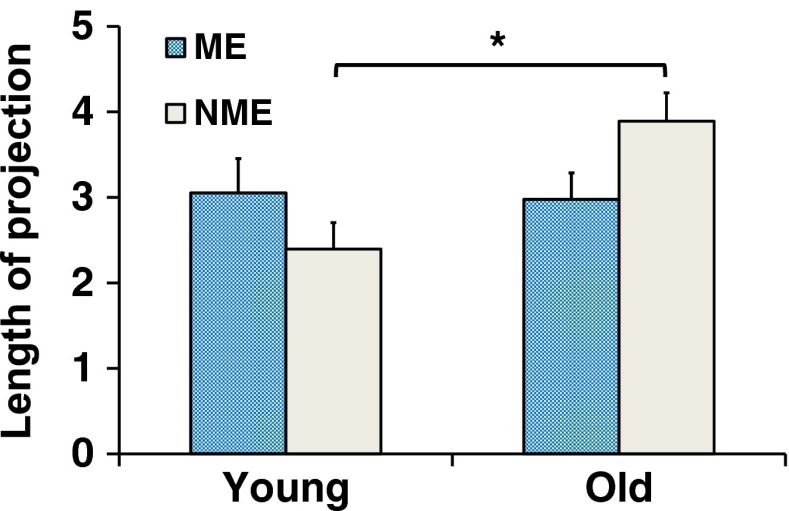
Figure 2 (bottom panel) shows examples for perturbation responses of the same representative individuals. The young adult had higher motor equivalent projections of the joint deviation vector (ME, good variability) than nonmotor equivalent projections (NME, bad variability) for half of the active response phase whereas the older adult had a higher NME than ME for most of the active response phase. Figure 4 presents the group averages of ME and NME. A two-way analysis of variance revealed a significant main effect of the variance projection by group [F(1,27) = 9.17, p = 0.005]. NME was significantly higher in older adults than in young adults (p = 0.0001), whereas ME was not significantly different between groups (p = 0.69).
Fig. 4.
Mean measures of motor equivalent (ME) and nonmotor equivalent (NME) variance projection during the active response phase for young adults and older adults. Mean value was computed over the active response phase. Error bars represent standard error of the mean (SEM). *p < 0.05
Clinical test correlates
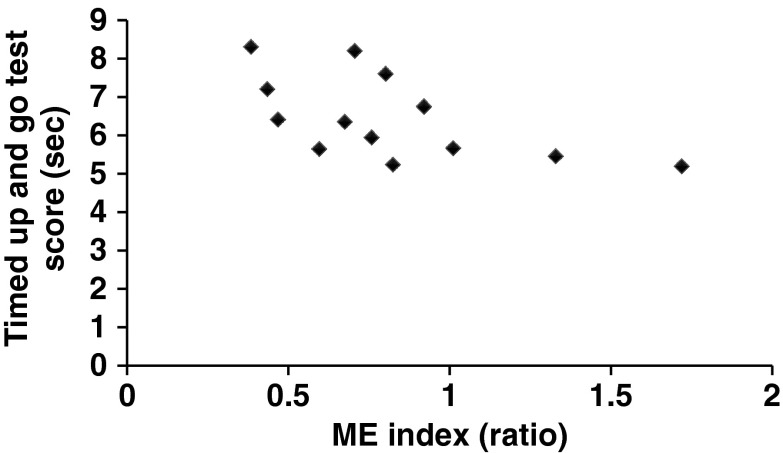
Older adults had longer Timed Up and Go Test times (6.46 ± 0.30 s) when compared to the young adults (5.41 ± 0.17 s; t = −3.21, p = 0.003). Moreover, the scores of the Timed Up and Go Test in older adults were moderate correlated with the ME index (Table 2 and Fig. 5). The scores of the Berg Balance Scale Test (53.92 ± 2.29; full score = 56) and Dynamic Gait Index (23.31 ± 1.03; full score = 24) in the older adults were both close to the full score. No clinical tests correlated with sum of five joint excursions, motor equivalent projection, or nonmotor equivalent projection itself (Table 2).
Table 2.
Correlation coefficient of the clinical evaluation in the older adult group (n = 13)
| Sum of joint excursions | Motor equivalent variance projection (ME) | Nonmotor equivalent variance projection (NME) | Motor equivalent index (ME index) | |||||
|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | |
| Timed Up and Go Test | −0.40 | 0.09 | −0.42 | 0.08 | −0.03 | 0.47 | −0.59 | 0.04* |
| Berg Balance Scale Test | 0.46 | 0.09 | 0.39 | 0.10 | 0.35 | 0.12 | 0.16 | 0.30 |
| Dynamic Gait Index | 0.31 | 0.15 | 0.33 | 0.14 | −0.01 | 0.48 | 0.35 | 0.12 |
*p < 0.05
Fig. 5.
Correlation between the Timed Up and Go Test and the ME index for the older adult group
Discussion
The purpose of this study was to determine to what extent healthy older adults preserved a motor equivalent coordination pattern for postural control when recovering from a postural perturbation. Our hypothesis was confirmed that older adults used a less flexible pattern with lower motor equivalent indexes (defined as the ratio between motor equivalent variance projection ME and nonmotor equivalent variance projection NME) when compared to young adults.
Movement variability may reflect flexible control of a motor task (Hsu et al. 2007). The capability of humans to produce varied solutions to a movement or task offers the flexibility to handle unexpected or challenging environments and is a major source of variability in movement patterns. However, an excessive variability may also reflect the presence of injuries or disease, altering motor control patterns (Hamill et al. 1999). Thus, an appropriate amount of movement variability is necessary for adapting to a new movement pattern (Harbourne and Stergiou 2009). This process requires good coordination from the neural controller by organizing available degrees of freedom, that is, the individual joints (Latash et al. 2010).
Studies have shown that the normal aging process increases (Barak et al. 2006; Christou and Enoka 2011; Paterson et al. 2011) or decreases variability (Gariépy et al. 2008) at different levels. Older adults who had a history of falls had increased variability in gait parameters (Paterson et al. 2011) and the kinematic measures in ankle plantar flexion, hip extension, and hip flexion at higher walking speeds (Barak et al. 2006). In bisegmental coordination studies, postural sway was measured at the head–hip or shoulder–hip (Accornero et al. 1997; Gariépy et al. 2008). Older adults exhibited a more rigid stance and higher cross-correlations (decreased variability) between two segments than the younger adults. Our findings are in line with these studies; however, we have increased evidence, as we measured five joints along the longitudinal axis of the body instead of only two joints.
Individual element variability (joint angle in the present study) and performance variability (COM position in the present study) are different aspects of balance control. Most studies only focus on one or the other (Barak et al. 2006; Pajala et al. 2007; Paterson et al. 2011). Even in the studies that have examined both types of variability, the direct linkage between the two types of variability is unclear (Schieppati et al. 2002; Gariépy et al. 2008; Van Ooteghem et al. 2010). Uncontrolled manifold (UCM) analysis provides a straightforward linkage between the individual element and the performance variability using a geometrical model (“Kinematic measures”). UCM analysis quantifies the influence of the individual element variability on the performance variability, that is, the structure of the joint variability. UCM analysis measures how small changes in the individual joints affect the COM position. Therefore, the structure of the joint variability can be identified as good variability, which does not affect the COM position, or bad variability, which does.
The UCM hypothesis assumes that the neural controller organizes the elemental variables to achieve desired values of important performance variables. Multijoint postural control is achieved not only by corrections at one joint but also by multiple corrections at different joints coordinated by the sensorimotor system (Schieppati et al. 2002; Hsu et al. 2007; Kiemel et al. 2008). The controller aims to limit bad variability (NME), while it allows relatively large good variance (ME). The normal aging process alters the motor control mechanism in many ways. In our study, the COM and individual joint excursions were not different between groups, but the structure of joint variability changed. We found increased bad variability, which would change the COM position in the older adults, while the good variability was not different between groups. The ME index was >1 in the young adults, but was <1 in the older adults, which suggests that the older adults used a less flexible joint coordination pattern.
We also observed decreased joint movement at the TLJ in the older adults when compared to the young adults. The older adults had reduced ability to use TLJ to compensate for the movement of other joints. These results imply that movements among joints during balance recovery were not well organized in stabilizing the COM position in normal aging adults. Like the example subjects shown in Fig. 2, the hip and TLJ joint moved in counterphase in the young subject during postural recovery, while the hip joint went into extension without any compensatory movement in the TLJ joint or any other joints in the older subject. Traditionally, hip joint is the major joint that is used for balance recovery during large balance perturbations (Runge et al. 1999). The finding of the current study shows that the trunk also contributes to balance recovery and supports our hypothesis that balance recovery is a multijoint coordination process. Therefore, the trunk control needs to be emphasized when designing exercise training program for fall prevention. Perhaps core stability exercise could be used for improving balance control ability.
Other studies using UCM analysis to study age-related changes in coordination also reported decreased good variability or increased bad variability in aging adults. Bad variability increased in finger force coordination tasks when older adults were asked to perform with accurate force and moment production (Park et al. 2011); older adults also showed a lower synergy index (Shinohara et al. 2004; Kapur et al. 2010). Good variability decreased in older adults compared to young adults in manual pointing movements with respect to basic kinematic variables (Verrel et al. 2012). The present study was the first study to investigate age-related changes in joint coordination using UCM analysis for upright stance. The findings of this study might provide information for the development of fall prevention programs. For example, clinicians could use the ME index as a grading system for balance ability when designing rehabilitation protocols. Therapy for a patient with a low ME index would emphasize joint coordination training for balance recovery.
In the present study, we found no strong correlation between clinical measurements and ME nor NME. This could be due to a ceiling effect of scores on the Berg Balance Scale and Dynamic Gait Index. Most of the older adults recruited in this study exercised regularly and had an average of 4 h exercise time per week. This could contribute to the high clinical measurements for balance and walking ability. Only the Timed Up and Go Test showed a moderate correlation with the ME index, possibly because the Timed Up and Go Test is a timed measurement and thus has no ceiling effect (Shumway-Cook et al. 2000). Based on this finding, the UCM analysis could be used to be an indicator of risk of falling. Clinicians could thus use the results of UCM analysis to indicate a person’s balance and walking ability and predict his/her risk of falling.
The present study has limitations. The UCM model in the current study only focused on motion in the sagittal plane. Whether any coordination changes occurred in the frontal plane could not be examined in the current UCM model. A three-dimensional (3D) UCM model that can account for 3D rotation is needed. A previous study found that older people usually lost balance taking sideways steps to recover balance, and mediolateral sway is most highly correlated with falls (Maki and McIlroy 2006). In the current study, we did not give mediolateral perturbations or include stepping reactions that could lead to falls; only backward translations were given. Lastly, the older adults recruited in the present study were relatively active in terms of exercise habits. Whether older adults with a sedentary life would show increased changes in joint coordination patterns during postural recovery could be a topic for the future development of this line of research.
Conclusion
In this study, we found that the use of motor abundance during balance recovery was reduced in healthy older adults relative to young adults. The results of the UCM analysis can be used to detect poor balance coordination in the elderly. Our findings suggest that training in multijoint coordination needs to be addressed in fall prevention program. The training task should involve all relevant joints as a group, instead of training each joint individually. Future studies should consider correlating the fall history of older adults with the ME index, using the ME index as an indicator of high falls risk, and explore the structure of movement variability related to postural control in patients with balance disorders.
Acknowledgments
We thank Masahiro Fujimoto and Shiu-Ling Chiu for helping with the data collection. This study was supported by NIH grant no. AG05317 awarded to Dr. Marjorie Woollacott in the USA and NSC grant no. 99-2320-B-002-003-MY2 awarded to Dr. Wei-Li Hsu in Taiwan.
References
- Accornero N, Capozza M, Rinalduzzi S, Manfredi GW. Clinical multisegmental posturography: age-related changes in stance control. Electroencephalogr Clin Neurophysiol. 1997;105:213–219. doi: 10.1016/S0924-980X(97)96567-X. [DOI] [PubMed] [Google Scholar]
- Auyang AG, Yen JT, Chang YH. Neuromechanical stabilization of leg length and orientation through interjoint compensation during human hopping. Exp Brain Res. 2009;192:253–264. doi: 10.1007/s00221-008-1582-7. [DOI] [PubMed] [Google Scholar]
- Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: a dynamic approach. Phys Ther. 2006;86:1501–1510. doi: 10.2522/ptj.20050387. [DOI] [PubMed] [Google Scholar]
- Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992;73:1073–1080. [PubMed] [Google Scholar]
- Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, Fentem PH, Bassey EJ. Falls by elderly people at home: prevalence and associated factors. Age Ageing. 1988;17:365–372. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- Christou E, Enoka R. Aging and movement errors when lifting and lowering light loads. Age. 2011;33:393–407. doi: 10.1007/s11357-010-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, Jeka JJ. The role of vestibular and somatosensory systems in intersegmental control of upright stance. J Vestib Res. 2008;18:39–49. [PMC free article] [PubMed] [Google Scholar]
- Domkin D, Laczko J, Djupsjobacka M, Jaric S, Latash ML. Joint angle variability in 3D bimanual pointing: uncontrolled manifold analysis. Exp Brain Res. 2005;163:44–57. doi: 10.1007/s00221-004-2137-1. [DOI] [PubMed] [Google Scholar]
- Freitas SM, Scholz JP, Latash ML. Analyses of joint variance related to voluntary whole-body movements performed in standing. J Neurosci Meth. 2010;188:89–96. doi: 10.1016/j.jneumeth.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy C, Hasson CJ, Van Emmerik REA, Caldwell GE. Age-related decrease in degrees of freedom in postural control during quiet stance. J Biomech. 2008;41:S24. doi: 10.1016/S0021-9290(08)70024-1. [DOI] [Google Scholar]
- Hamill J, van Emmerik RE, Heiderscheit BC, Li L. A dynamical systems approach to lower extremity running injuries. Clin Biomech. 1999;14:297–308. doi: 10.1016/S0268-0033(98)90092-4. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Chou LS (2004) Age-related reduction in sagittal plane center of mass motion during obstacle crossing. J Biomech 37:837–844 [DOI] [PubMed]
- Harbourne RT, Stergiou N. Movement variability and the use of nonlinear tools: principles to guide physical therapist practice. Phys Ther. 2009;89:267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WL, Scholz JP (2012) Motor abundance supports multitasking while standing. Hum Mov Sci. doi:10.1016/j.humov.2011.07.017 [DOI] [PMC free article] [PubMed]
- Hsu WL, Scholz JP, Schoner G, Jeka JJ, Kiemel T. Control and estimation of posture during quiet stance depends on multijoint coordination. J Neurophysiol. 2007;97:3024–3035. doi: 10.1152/jn.01142.2006. [DOI] [PubMed] [Google Scholar]
- Kang N, Shinohara M, Zatsiorsky VM, Latash ML. Learning multi-finger synergies: an uncontrolled manifold analysis. Exp Brain Res. 2004;157:336–350. doi: 10.1007/s00221-004-1850-0. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zatsiorsky VM, Latash ML. Age-related changes in the control of finger force vectors. J Appl Physiol. 2010;109:1827–1841. doi: 10.1152/japplphysiol.00430.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiemel T, Elahi AJ, Jeka JJ. Identification of the plant for upright stance in humans: multiple movement patterns from a single neural strategy. J Neurophysiol. 2008;100:3394–3406. doi: 10.1152/jn.01272.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle synergies during shifts of the center of pressure by standing persons. Exp Brain Res. 2003;152:281–292. doi: 10.1007/s00221-003-1574-6. [DOI] [PubMed] [Google Scholar]
- Latash M. There is no motor redundancy in human movements. There is motor abundance. Mot Contr. 2000;4:259–261. doi: 10.1123/mcj.4.3.259. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schoner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev. 2002;30:26–31. doi: 10.1097/00003677-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Latash ML, Krishnamoorthy V, Scholz JP, Zatsiorsky VM. Postural synergies and their development. Neural Plast. 2005;12:119–130. doi: 10.1155/NP.2005.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Levin MF, Scholz JP, Schoner G. Motor control theories and their applications. Medicina (Kaunas) 2010;46:382–392. [PMC free article] [PubMed] [Google Scholar]
- Lugade V, Lin V, Chou LS. Center of mass and base of support interaction during gait. Gait Posture. 2011;33:406–411. doi: 10.1016/j.gaitpost.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention. Age Ageing. 2006;35:ii12–ii18. doi: 10.1093/ageing/afl078. [DOI] [PubMed] [Google Scholar]
- Nashner LM, McCollum G. The organization of human postural movements: a formal basis and experimental synthesis. Behav Brain Sci. 1985;8:135–172. doi: 10.1017/S0140525X00020008. [DOI] [Google Scholar]
- Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46:M164–M170. doi: 10.1093/geronj/46.5.M164. [DOI] [PubMed] [Google Scholar]
- Olafsdottir H, Zhang W, Zatsiorsky VM, Latash ML. Age-related changes in multifinger synergies in accurate moment of force production tasks. J Appl Physiol. 2007;102:1490–1501. doi: 10.1152/japplphysiol.00966.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajala S, Era P, Koskenvuo M, Kaprio J, Tolvanen A, Rantanen T. Genetic and environmental contribution to postural balance of older women in single and dual task situations. Neurobiol Aging. 2007;28:947–954. doi: 10.1016/j.neurobiolaging.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Park J, Sun Y, Zatsiorsky VM, Latash ML. Age-related changes in optimality and motor variability: an example of multifinger redundant tasks. Exp Brain Res. 2011;212:1–18. doi: 10.1007/s00221-011-2692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson K, Hill K, Lythgo N. Stride dynamics, gait variability and prospective falls risk in active community dwelling older women. Gait Posture. 2011;33:251–255. doi: 10.1016/j.gaitpost.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Runtz EF, Edwards BJ, Pai YC. Age influences the outcome of a slipping perturbation during initial but not repeated exposures. J Gerontol A Biol Sci Med Sci. 2002;57:M496. doi: 10.1093/gerona/57.8.M496. [DOI] [PubMed] [Google Scholar]
- Poston B, Enoka JA, Enoka RM. Endpoint accuracy for a small and a large hand muscle in young and old adults during rapid, goal-directed isometric contractions. Exp Brain Res. 2008;187:373–385. doi: 10.1007/s00221-008-1309-9. [DOI] [PubMed] [Google Scholar]
- Robert T, Zatsiorsky VM, Latash ML. Multi-muscle synergies in an unusual postural task: quick shear force production. Exp Brain Res. 2008;187:237–253. doi: 10.1007/s00221-008-1299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge CF, Shupert CL, Horak FB, Zajac FE. Ankle and hip postural strategies defined by joint torques. Gait Posture. 1999;10:161–170. doi: 10.1016/S0966-6362(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Giordano A, Nardone A. Variability in a dynamic postural task attests ample flexibility in balance control mechanisms. Exp Brain Res. 2002;144:200–210. doi: 10.1007/s00221-002-1028-6. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schöner G, Hsu WL, Jeka J, Horak F, Martin V. Motor equivalent control of the center of mass in response to support surface perturbations. Exp Brain Res. 2007;180:163–179. doi: 10.1007/s00221-006-0848-1. [DOI] [PubMed] [Google Scholar]
- Shim JK, Lay BS, Zatsiorsky VM, Latash ML. Age-related changes in finger coordination in static prehension tasks. J Appl Physiol. 2004;97:213–224. doi: 10.1152/japplphysiol.00045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Hsu J, Karol S, Hurley BF. Strength training increases training-specific multifinger coordination in humans. Mot Contr. 2008;12:311–329. doi: 10.1123/mcj.12.4.311. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Scholz JP, Zatsiorsky VM, Latash ML. Finger interaction during accurate multi-finger force production tasks in young and elderly persons. Exp Brain Res. 2004;156:282–292. doi: 10.1007/s00221-003-1786-9. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. Motor control: theory and practical applications. Baltimore: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–129. doi: 10.1097/01.NPT.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- Van Ooteghem K, Frank JS, Allard F, Horak FB. Aging does not affect generalized postural motor learning in response to variable amplitude oscillations of the support surface. Exp Brain Res. 2010;204:505–514. doi: 10.1007/s00221-010-2316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrel J, Lovden M, Lindenberger U (2012) Normal aging reduces motor synergies in manual pointing. Neurobiol Aging 33:200.e1–200.e10 [DOI] [PubMed]
- Winter DA. Anatomy biomechanics and control of balance during standing and walking. Waterloo: Biomechanics; 1990. [Google Scholar]