Abstract
We assessed lifelong environmental enrichment effects on possible age-related modifications in emotional behaviors, spatial memory acquisition, retrieval of recent and remote spatial memory, and cholinergic forebrain systems. At the age of 1 month, Long–Evans female rats were placed in standard or enriched rearing conditions and tested after 3 (young), 12 (middle-aged), or 24 (aged) months. Environmental enrichment decreased the reactivity to stressful situations regardless of age. In the water maze test, it delayed the onset of learning deficits and prevented age-dependent spatial learning and recent memory retrieval alterations. Remote memory retrieval, which was altered independently of age under standard rearing conditions, was rescued by enrichment in young and middle-aged, but unfortunately not aged rats. A protected basal forebrain cholinergic system, which could well be one out of several neuronal manifestations of lifelong environmental enrichment, might have contributed to the behavioral benefits of this enrichment.
Keywords: Acetylcholine, Aging, Anxiety, Basal forebrain, Enriched environment, Recent and remote memory, Striatum
Introduction
The identification of factors enhancing cognitive and emotional health in older people becomes of increasing importance with the general population aging (see Depp et al. 2010 for a recent review). In rodents, age-related learning and memory impairments can be attenuated by a relatively short (a few weeks) exposure to environmental conditions providing sensory, motor, and cognitive stimulations, as well as sustained social interactions (e.g., Fernandez et al. 2004; Frick and Fernandez 2003; Harburger et al. 2007). Such environmental enrichment has also been shown to attenuate defensive behaviors elicited by potential threats in adult rodents. For instance, it decreases freezing responses to a fear-conditioned context (Barbelivien et al. 2006; Benaroya-Milshtein et al. 2004) and reduces anxiety-like behavior (e.g., Galani et al. 2007), an observation recently confirmed in aged rats (Leal-Galicia et al. 2008). The impact of environmental enrichment on aging-related impairments might depend on the exposure duration, longer enrichment leading to attenuation or even complete prevention of the age-related cognitive decline (Bennett et al. 2006; Kobayashi et al. 2002) but also on the age of enrichment onset (Bouet et al. 2011; Freret et al. 2011). We recently found that environmental enrichment during the whole post-weaning life limited the age-related alterations of Morris water maze learning capabilities in rats; it also ameliorated memory persistence for at least 24 h after the end of training (Harati et al. 2011). This finding suggests that enrichment could have an impact on consolidation processes. Whether it might allow the maintenance of spatial memory at longer delays, however, has never been investigated.
The neurobiological mechanisms involved in the protective effects of environmental enrichment on aging processes remain poorly understood. Even if age-related cognitive deficits could be the consequence of multiple alterations rather than that of dysfunctions in (or of) a particular anatomically and/or neurochemically defined network or system, it is noteworthy that aged rats show a consistent reduction of cholinergic markers, especially in the basal forebrain. Interestingly, the extend of these cholinergic alterations correlate with the severity of some cognitive deficits (Aubert et al. 1995; Baskerville et al. 2006; Herzog et al. 2003; Stemmelin et al. 2000; Sugaya et al. 1998). In a recent study, we found that aged rats exposed to lifelong enrichment had more cholinergic neurons in the basal forebrain and striatum as compared with their standard counterparts. This observation suggests that part of the enrichment-induced preservation of spatial learning and memory capabilities might rely on better neuronal survival in the cholinergic system (Harati et al. 2011). However, the question of when, over life, this positive effect of enrichment becomes detectable remains open.
In the current study, we therefore assessed the effects of lifelong enrichment on (1) emotional behaviors, (2) both recent and remote spatial memory, and (3) the status of the cholinergic forebrain system at three ages (young adult, middle-aged, and aged).
Materials and methods
Subjects and housing conditions
Experimental protocols and animal care were in compliance with the national (council directive 87–848, 19 October 1987, Ministère de l’Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale) and international (directive 86–609, 24 November 1986, European Community) laws and policies (personal authorization N° 67–167 for A.B., N° 67–289 for M.M., and N° 67–215 for J-C.C.; H.H. under the formers’ responsibility).
Female Long–Evans rats (Centre d’Elevage René Janvier, Le Genest-St-Isles, France) were delivered to the laboratory at the age of 4 weeks. They were randomly assigned to one of two different housing conditions. Non-enriched (standard) rats were housed in groups of two in transparent Makrolon cages (46 × 26 × 15 cm). Enriched rats were housed in groups of 10–12 in two contiguous wire-mesh cages (112 × 40 × 40 cm) connected by two openings: Various objects (tunnels, toys, chains, etc.) were placed in both cages and changed five times a week. Food and water were provided ad libitum at different locations. The cages were placed in a temperature- (22 ± 1°C) and humidity (55 ± 5%)-controlled room under a 12:12 h light–dark cycle (lights on at 8:00 am). In the standard condition, direct handling of the rats was limited to manipulations accompanying cage cleaning; water and food renewal did not require physical contact with the rats. In the enriched condition, rats were handled once every 3 weeks when the cages were changed. As these cages had a floor made of metallic grids, the sawdust underneath each cage could be changed without having to handle the rats. After a period of differential housing, which lasted 3, 12, or 24 months, rats were isolated in transparent Makrolon cages (42 × 26 × 15 cm) for 1 week. Then, they were weighed and gently handled for 1 min/day over two consecutive days before the onset of behavioral testing.
Behavioral testing was started when the young adult (Y) rats were aged 4 months and had spent 3 months in the enriched/standard housing conditions (11 enriched rats/11 standard rats), when the middle-aged (MA) rats were 13 months old and had spent 12 months in the enriched/standard housing conditions (10 enriched rats/10 standard rats), and when aged (AG) rats were 25 months old and had spent 24 months in the enriched/standard housing conditions (8 enriched rats/8 standard rats).
Rats were first tested in the plus-maze, in order to avoid any effect of other testing-related manipulations on anxiety-related behaviors (e.g., Rodgers and Dalvi 1997). Basal corticosterone level was then evaluated, and contextual fear conditioning and Morris water maze testing were performed subsequently. No individual determination of the estrous cycle was carried out in our young adult and middle-aged rats; aged female rats are in a permanent diestrous stage after 17 months (LeFevre and McClintock 1988). Under our housing conditions (absence of male rats), the estrous cycle does not synchronize, as females are kept in facilities isolated from the ones where males are kept (Schank 2001). In addition, based on the literature, the impact of the hormonal status on cognitive performance in rats is relatively negligible, and some results even show no fluctuations of performance during the cycle in the Long–Evans strain (e.g., Berry et al. 1997; Stackman et al. 1997). Therefore, considering the number of animals in each experimental group, estrous cycle influence on behavioral and biochemical outputs might have at best contributed to some between-subject variability in each group, but most probably not to an overall group effect. Thus, although a contribution of the hormonal status as a potential bias of our observations cannot be discarded, this bias was most probably minimal. It seems nevertheless prudent to consider that the results reported hereafter do not generalize to male rats (for more details on this issue, see Harati et al. 2011).
Behavioral tests
Elevated plus-maze
The apparatus was made of black Plexiglas and consisted of two opposing open arms (50 cm long × 10 cm wide × 1.5 cm high borders) and two opposing enclosed arms (50 cm long × 10 cm wide × 40 cm high borders) fixed to a central platform (10 × 10 cm2) in a cross shape. The maze was elevated to a height of 73 cm above the floor and illuminated with four halogen lamps placed in the four corners of the test room, providing an identical illumination to each open arm (10 lux) and a weaker one to each of the closed ones (2.5 lux). A camera fixed 200 cm above the maze was connected to a video monitor placed in an adjacent room, from where the experimenter could follow the displacements of the rats. Between two rats, the apparatus was wiped with a solution of 70% alcohol. Testing was performed between 9:00 am and 12:00 pm. Rats were brought to the testing room in a transport cage and left there for 5 min before the test was started. The 5-min test began immediately after placing rats on the central platform of the maze, head facing a closed arm (the same for all rats). The entries in each arm (whenever the four paws were in an arm) and in the central platform were recorded by the experimenter using a computer driven by a home-made software. The total number of arms entries was computed to assess motor activity in the maze. The time spent in the open arms and the number of entries in these arms were expressed as the percent of the total time spent in the arms and the percent of the total number of arms entries, respectively, and computed as index of anxiety. The experimenter was blind to the rats’ rearing conditions.
Contextual fear conditioning
Conditioned fear was assessed 1 week after the elevated plus-maze test. Eight identical conditioning chambers (25 × 27 × 18 cm3) were used. Each chamber was made of transparent plastic with a transparent ceiling and placed in ventilated (background noise between 65.7 and 70.2 dB) light- and sound-attenuated boxes (57 × 38 × 38 cm3, Campden Instruments LTD). An illumination of 6 lux was maintained by a bulb through a frosted plastic plate. Each chamber was equipped on one wall with a 1-cm-diameter hole, light indicators (always off), and a loudspeaker. A camera (MCT-210 MS, OptoVision, Toulouse, France) was fitted inside each box, above the center of the chamber, and monitored the entire chamber from the top through a 2.45 mm wide angle lens. The grid floor of each chamber consisted of parallel 0.3 cm diameter stainless-steel bars spaced 0.8 cm apart. A sawdust tray was placed under the grid floor. Shock delivery was controlled by a computerized interface (Med-PC, Med Associates, Inc., St Albans, VT, USA). Automatic measurements of freezing were performed as described by Marchand et al. (2003). Briefly, video signals of two sets of four cameras were sent to a PC-type microcomputer (Pentium type 660 MHz, 512 Mo RAM) equipped with a Scion LG3 video capture card (Scion Corporation, Frederick, Maryland, USA) via two Quad-type multiplexers (Computar QSMX-II). Data acquisition was carried out by a script written under the “Scion Image” software. It allowed the monitoring of all eight chambers at a sampling rate of 1 Hz. Data analysis was conducted with a set of procedures written under Excel® Visual Basic®, which allowed the computation of percentage of time spent freezing over blocks of selected duration.
Conditioning and testing took place between 8:30 and 11:30 am. For conditioning, one footshock (0.6 mA before scrambling, 0.8 s) was delivered 2 min after the placement of the rat in the chamber. The rat was removed from the chamber after one additional minute. On the following day, conditioned contextual fear was assessed by placing rats in the conditioning chamber for a 2-min session.
Morris water-maze
Four weeks after fear conditioning, place acquisition was tested in the Morris water-maze. The maze consisted of a circular pool (diameter 160 cm, height 60 cm) filled with water to half its height (i.e., 30 cm) and virtually divided into four equal quadrants. The water (20°C) was made opaque with powdered milk. A circular platform, 11 cm in diameter, was placed in the pool, 1 cm underneath the water surface. The pool was located in an experimental room with many extra-maze cues (e.g., chair, computer, desk, cages, lights, pictures hanging on the wall, fan, etc.). A video-tracking system (Noldus, Wageningen, The Netherlands) was used to collect various aspects of the rat’s behavior. For each trial, the rat was placed in the pool, facing the wall, at one of eight fixed starting points in a random order. It was given a maximum of 60 s to reach the submerged platform. When the rat climbed onto the platform, it was left on it for 10 s, before being removed, and given the next trial. When the rat failed to find the platform within 60 s, it was gently guided to it by the experimenter and was left there for 10 s. Each day, the rats were given four consecutive trials for which they were released from a different starting point in a randomized order. During five consecutive days (first training period), the platform was at the same location. Twenty-four hours after the last training day, the platform was removed, and all rats were given a single 60-s trial (1 day probe trial). Twenty-four hours later, the rats were given further training. The platform was replaced at the same location as for the initial training, and all rats were given five additional training days (second training period). Finally, after a 25-day rest delay, all rats were subjected to another probe trial (25 days probe trial). Training days are generally considered to provide a measure of spatial learning. Probe trials are considered to measure the capability to retrieve the spatial memory, as well as its strength and precision. After the 25-day post-acquisition probe trial, AG rats were tested over two consecutive days with a visible platform in order to verify that they had the motivational, motor, and visual abilities to perform the task. On the second day, the platform (Plexiglas colored black to enhance its visibility; 11 cm in diameter) protruding 1 cm above the water surface was placed at another location. Four successive trials were conducted over two consecutive days as previously described (Harati et al. 2011).This precaution was not taken in Y and MA rats as we previously demonstrated that enrichment did not affect performance at these ages in the visible platform version of the task (Harati et al. 2011).
For the acquisition of the platform location, the distances swum to reach the platform were corrected according to the method described by Lindner (1997). To facilitate data analysis, scores were averaged for the first and the second periods of training (mean of the 5 days in each case). The precise time course of learning performance has been reported previously in the same type of rat populations (Harati et al. 2011). For the probe trials, we analyzed the time spent in the target quadrant (i.e., where the platform was located during training), the latency to the first swim over the former platform location, and the number of annulus crossings (the annulus zone covering the area of the platform enlarged by a 10-cm annulus). For the visible platform task, distances swum to reach the platform were analyzed for each trial.
Blood sampling and corticosterone measurement
Two days after the plus-maze test, blood sampling was performed between 8.00 and 9.00 am to minimize circadian variability in corticosterone levels. To reduce disturbance in the housing room, home cages were transported by hand in an adjacent experimental room where blood sampling was performed according to the method of Fluttert et al. (2000). The animal was gently held in place in a folded towel, and its tail was lightly incised with a razor blade, about 2 cm from the tip. By gently stroking the tail from the base to the tip, about 300 μl of blood were collected in an EDTA-treated capillary tube. Collection time was less than 1 min. After 5 min of centrifugation (4°C; 12,000 rpm), approximately 100 μl of plasma was collected and kept at −80°C until determination. Plasmatic corticosterone concentrations were measured using a standard radio-immunoassay kit and procedure (MP Biomedicals, Dia Sorin, Anthony). Samples containing the corticosterone were diluted in steroid diluent (1:200). Then, radioactive tracer (cortico-I125) and anti-corticosterone antibody were added in hemolysis tubes to the samples containing the corticosterone or calibrator’s samples. They were incubated for 2 h, before 0.5 mL of the precipitation solution was added in every tube at room temperature (i.e., about 22°C). The tubes were then centrifuged at 2,000×g during 15 min, at 4°C. The supernatants were eliminated, and the radioactivity present in the pellets was counted in a scintillation counter connected to a computer, which established the “calibrator curve” and determined the quantity of corticosterone present in the samples.
ChAT immunohistochemistry
Perfusion and preparation of tissue sections
Ten days after the last probe trial of the water maze test, each rat was injected with an overdose of sodium pentobarbital (180 mg/kg) and transcardially perfused with 60 ml of a phosphate-buffered 4% paraformaldehyde solution (pH 7.4; 4°C). The brain was extracted, post-fixed for 4 h in the same fixative, and then transferred into a 0.1 M phosphate-buffered 30% sucrose solution for about 36–40 h (4°C). Brains were frozen using isopentane (−40°C) and subsequently kept at −80°C until sectioning. Coronal sections, 40 μm thick, were cut on a freezing microtome (−20°C) and collected according to the following schedule: Four successive sections were discarded before one was collected approximately from Bregma +1.7 mm to Bregma +1 mm for the striatum. For the other structures, they were collected serially from Bregma +1 mm to Bregma −1.6 mm. All sections were kept in a cryopreservative medium at −20°C until staining.
Immunohistochemistry
The sections were rinsed three times for 10 min in PBS (0.1 M, pH 7.4) containing 0.02% merthiolate (PBS + M) before being soaked for 1 h in 5% normal donkey serum (BioWest, Nuaillé, France) in PBS + M containing 0.5% Triton X-100. The sections were then transferred without rinsing into the primary antibody solution, which contained a goat polyclonal antibody directed against choline-acetyltransferase (ChAT) (1:500; Chemicon International, AB 144 P, Temecula, CA). The incubation with the primary antibody lasted one night and was followed by three PBS + M rinses. Then, the sections were soaked for 1 h in a buffer solution containing donkey anti-goat biotinylated antibody (1:500; Vector Laboratories International, AP 180 B, Burlingame, CA). For the primary and the secondary antibodies, the sections were incubated at room temperature (22°C). After three PBS + M washes, the sections were exposed for 1 h in a standard avidin–biotin–peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). The sections were then rinsed twice in PBS + M and once in 0.6% Tris-buffer (pH = 7.6) and subsequently reacted with the Vector Peroxidase substrate kit DAB (SK-4100, Vector Laboratories). Finally, after three PBS + M rinses, the sections were mounted onto gelatine-coated slides, dried at room temperature, dehydrated, and then cover-slipped.
Quantification
The counting of ChAT-positive neurons was performed as previously described (Harati et al. 2008). Briefly, anatomical landmarks were used to select the location of counting frames in the whole striatum, the medial septum/vertical diagonal band of Broca (MS/vDBB), and the nucleus basalis magnocellularis (NBM). Positive neurons were counted in one section in the left and right hemispheres under a microscope (Olympus: Vanox–AHBT3), using a calibrated eyepiece grid to facilitate counting (Olympus: WH 10X2-H). Sections were selected from each region at about the same level of anteriority in each rat. Only cells that were clearly distinguishable from the background (i.e., clear-cut contrast and well-delineated borders) were counted. Then, the mean number of ChAT-positive neurons per section was calculated for each rat.
Statistical analysis
Most data were subjected to analysis of variance (ANOVA) with “Age” and “Housing condition” as between-subject factors and, when required, "Training Period" as a within-subject factor (i.e., for water-maze acquisition performance). The ANOVAs were completed by post hoc comparisons using the Newman–Keuls (NK) multiple range test. In some cases, which are indicated in the “Results” section and were motivated by the general layout of the data indicating obvious differences, multiple comparisons were performed in the absence of significant interactions between main factors, as advocated by Howell (1998). For analysis of probe-trial performance, the time spent in the target quadrant was compared with chance level (15 s) using a Student’s t test. Regression analyses were performed between some behavioral and immuhistochemical variables using the Spearman correlation test. The threshold for rejecting the null hypothesis was 0.05 throughout.
Results
Body weight
The body weight increased with age, but this effect was more pronounced in the standard vs. enriched groups (in grams—Y 233 ± 4, MA 329 ± 13, AG 415 ± 13 for standard groups and Y 233 ± 4, MA 301 ± 6, AG 359 ± 17 for enriched groups). The ANOVA showed significant effects of “Age” (F2/52 = 122.10, p < 0.0001) and “Housing condition” (F1/52 = 12.32, p < 0.01), and there was a significant interaction between both factors (F2/52 = 3.97, p < 0.05). Post hoc comparisons indicated that the body weight of MA and AG enriched rats was significantly lower than that of their standard counterparts (p < 0.05 and p < 0.001, respectively); in the aged rats, the difference amounted to about 55 g in average.
Behavioral data
Elevated plus-maze
The total number of arms visited during the test appeared very similar in all groups (Y 15.63 ± 1.41, MA 16 ± 1.27, AG 18.5 ± 1.63 for standard groups and Y 17.63 ± 0.6, MA 15.8 ± 0.82, AG 13.62 ± 1.05 for enriched groups). The ANOVA showed no significant effect of “Age” or “Housing condition”, but there was a significant interaction between the two factors (F 2/52 = 4.25, p < 0.05), the difference between standard and enriched AG rats achieving significance (p = 0.05, NK). Figure 1 shows that both the percent time in the open arms (Fig. 1a) and the percent entries in the open arms (Fig. 1b) increased with age, especially in the rats reared under standard conditions. These two variables were also higher in Y- and MA-enriched rats than in their standard counterparts. The ANOVAs partly confirmed these observations, as they indicated a significant “Age” effect (F 2/52 = 4.12, p < 0.05 and F 2/52 = 3.28, p < 0.05, respectively) but no significant effect of the “Housing condition.” Nevertheless, a trend towards a significant interaction between the two factors was found for the percent time in the open arms (F 2/52 = 2.62, p = 0.08), which was higher in AG standard rats than in their MA and Y counterparts (NK, p < 0.05 and p < 0.01, respectively). Such a difference was not observed among enriched rats. The means percent time and entries in the open arms were close to chance in AG rats (i.e., 50% time and entries for standard rats, 50% entries for enriched rats) such a ceiling effect might obscure an effect of enrichment in the other groups of age. Therefore, two additional analyses were made. For the first one, the same variables were analyzed, but data from AG groups were excluded. There was a significant effect of “Housing condition” (F 1/38 = 6.08, p < 0.05) for the percent time in the open arms, but only a trend toward a significant effect for the percent entries (F 1/38 = 3.15, p = 0.08). We found no effect of “Age” and no interaction. For the second analysis, the mean duration of open arm visits (time in open arms/number of open arm entries) was analyzed for all groups of rats. As illustrated in Fig. 1c, the mean duration of open arm visits seems higher in aged rats as compared with their younger counterparts but also higher in enriched rats as compared with their standard counterparts. The ANOVA showed significant effects of “Age” (F 2/52 = 5.25, p < 0.05) and “Housing condition” (F 1/52 = 3.58, p < 0.05), but no interaction between these two factors. Post hoc comparisons indicated that AG rats significantly differed from both Y and MA rats (p < 0.05 in each case), which did not differ from each other.
Fig. 1.
Mean (+SEM) percent time spent in the open arms (a), percent entries in open arms (b), and duration of open arm visits (c) in the elevated plus-maze. Statistics: currency sign significantly different from 50% (dashed line), p < 0.05 (Student t tests); section sign, significantly different from young rats in the same housing condition; dollar sign, significantly different from middle-aged rats in the same housing condition; p < 0.05 (NK) in each case. Group abbreviations are Y for young, MA for middle-aged, and AG for aged rats
Contextual fear conditioning
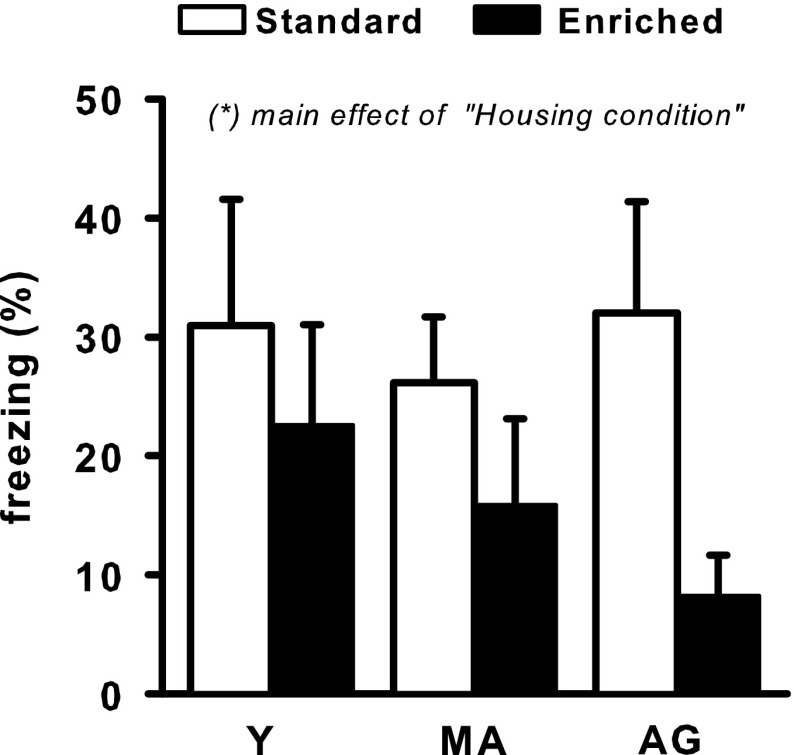
During the conditioning session, all rats were active before footshock delivery (data not shown), and the statistical analysis revealed no significant effects of “Age” and “Housing condition,” or an effect of their interaction. During the minute that followed footshock delivery, freezing scores were moderately increased in each group of rats, and all the groups exhibited comparable freezing levels (Y 36 ± 6.8, MA 29 ± 7, AG 37 ± 9 for standard group and Y 30.5 ± 5.7, MA 27 ± 8.5, AG 16 ± 7.1 for enriched ones; there were no effects of “Age,” “Housing condition,” and of the interaction between these factors). As illustrated in Fig. 2, moderate freezing scores were also obtained during context re-exposure 24 h later. This figure shows that freezing scores of standard rats were similar in all age groups but were lower in enriched rats, especially in the AG group. The ANOVA, however, showed no significant effect of “Age.” There was a significant effect of “Housing condition” (F 1/52 = 4.46, p < 0.05), but, despite the marked difference among both AG groups, there was no significant interaction between “Age” and “Housing condition.
Fig. 2.
Freezing scores presented as a mean (+SEM) percent time spent at freezing during re-exposure to the conditioning context. Statistical analyses showed a significant main effect of “Housing condition”. Group abbreviations are as in Fig. 1
Water maze
Acquisition
Figure 3 indicates that the average distance swum to reach the platform decreased from the first to the second training period in all groups and that the age-dependent degradation of performance appeared more limited in enriched than in standard rats, especially during the second training period. The ANOVA revealed significant effects of “Training Period" (F 1/52 = 154.68, p < 0.0001), “Age” (F 2/52 = 13.55, p < 0.0001), and “Housing condition” (F 1/52 = 32.79 p < 0.0001), and all interactions between these factors were significant (Age × Housing condition, F 2/52 = 3.3, p = 0.05; Age × Training Period, F 2/52 = 4.14 p < 0.05; Age × Housing condition × Training Period, F 2/52 = 4.05, p < 0.05). Post hoc comparisons indicated that, in each group, the distance to reach the platform decreased from the first to the second training period (p < 0.001), indicating an overall improvement of performance with further training. The comparisons also revealed that, during the first period of training, the distance was significantly longer in MA and AG rats housed in standard condition as compared with their Y counterparts (p < 0.01, NK). In enriched rats, however, only the performance of AG rats was significantly impaired (p < 0.05). During the second period of training, AG standard rats remained poor performers compared with their Y and MA counterparts (p < 0.01, NK). The beneficial effect of the enrichment was significant in MA rats during the first period of training (p < 0.001, NK) and in AG rats during both periods of training (p < 0.01, NK).
Fig. 3.
Distances to reach the platform (means ± SEM) during the two 5-day-long training periods in the water maze acquisition (20 trials in each period, 4 trials/day). Statistics: ampersand symbol, significantly different from the first period of training within the same group; asterisk, significantly different from standard rats at the same age and period; section sign, significantly different from young rats in the same housing condition at the same period; dollar sign, significantly different from middle-aged rats in the same housing condition at the same period; p < 0.05 (NK) in each case. Group abbreviations are as in Fig. 1
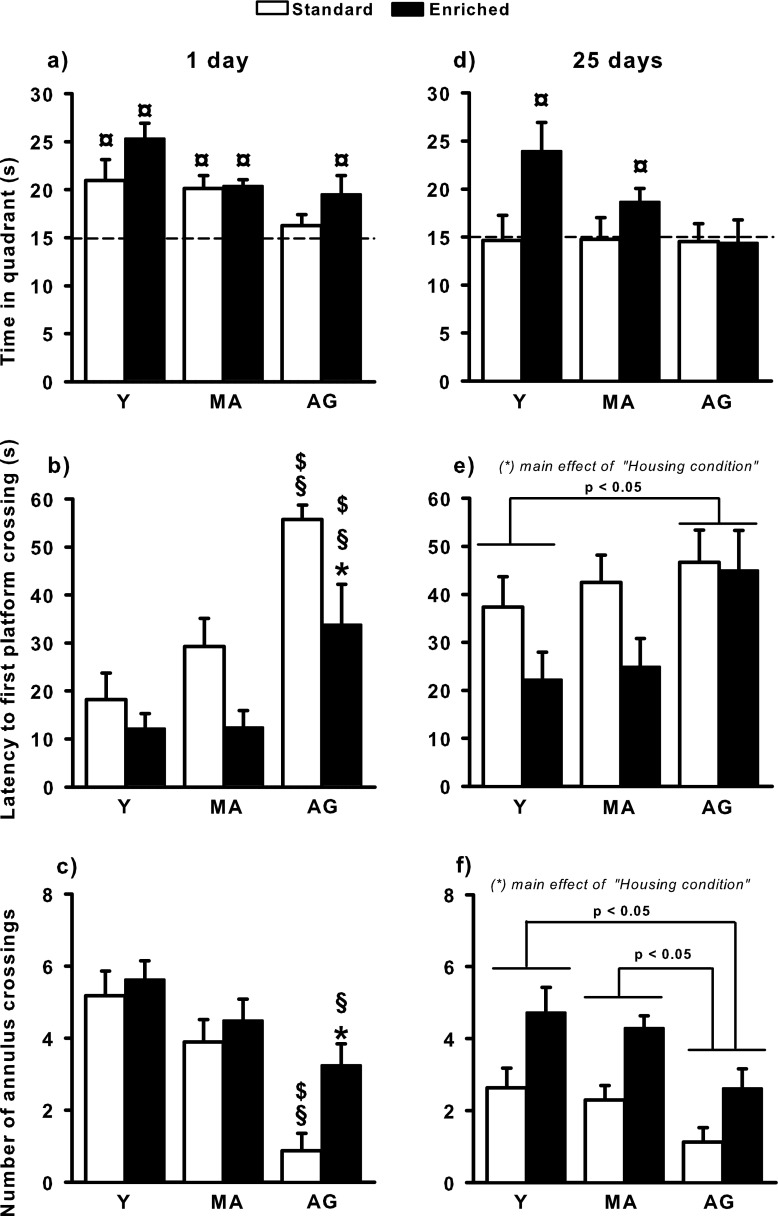
Probe trial after a 1-day rest (recent memory)
Regardless of their housing conditions, Y and MA rats spent significantly more time than chance (i.e., 15 s) in the target quadrant (p < 0.05; Fig. 4a). In contrast, in AG rats, only those housed in the enriched environment performed above chance (p = 0.05). The latency to the first swim over the former platform location (Fig. 4b) and the number of annulus crossings (Fig. 4c) were also affected by age, especially in the standard conditions. The ANOVA showed significant effects of “Age” (F 2/52 = 17.45, p < 0.0001 for latency and F 2/52 = 15.23, p < 0.0001 for annulus crossings) and “Housing condition” (F 1/52 = 12.69, p < 0.001 for latency and F 1/52 = 5.44, p < 0.05 for annulus crossings), but no significant interaction between the two factors. For each variable, post hoc comparisons which, given the layout of the figures, were performed despite the absence of significant interactions, indicated that AG standard rats were impaired in comparison with their enriched counterparts, but also with the Y and MA standard ones (p < 0.01). It is noteworthy that AG-enriched rats were nevertheless impaired as compared with the Y-enriched ones (p = 0.05) and to the MA-enriched ones, but only for the latency to the first swim over the former platform location (p < 0.05).
Fig. 4.
Probe trial performance (mean + SEM) assessed 1 day (a, b, c) and 25 days (d, e, f) after the end of training. Panels a and d show the time spent in the target quadrant (the dashed line indicates chance level). Panels b and e show the latency to first platform crossing. Panels c and f show the number of annulus crossings. Statistics: currency sign significantly different from chance level, p < 0.05 (Student’s t test); section sign, significantly different from young rats in the same housing condition; dollar sign, significantly different from middle-aged rats in the same housing condition; asterisk, significantly different from standard rats at the same age; p < 0.05 (NK) in each case. Group abbreviations are as in Fig. 1
Probe trial after a 25-day rest (remote memory)
Only Y- and MA-enriched rats spent an average time in the target quadrant that significantly exceeded chance (p < 0.05; Fig. 4d). The latency to the first swim over the former platform location (Fig. 4e) and the number of annulus crossings (Fig. 4f) were affected by age, although performance was better in the enriched groups except for latencies in aged rats. The ANOVA indicated significant effects of “Age” (F 2/52 = 3.18, p < 0.05 for latency and F 2/52 = 6.15, p < 0.01 for annulus crossings) and “Housing condition” (F 1/52 = 4374, p < 0.05 for latency and F 1/52 = 18.96, p < 0.0001 for annulus crossings), but no significant interaction between the two factors. Post hoc comparisons revealed that the latency to reach the former platform location for the first time was higher in AG rats as compared with Y rats (p < 0.05), and the difference between AG and MA rats was close to significance (p = 0.06). They also indicated that AG rats crossed the annulus less frequently than MA and Y rats (p < 0.01).
Visible platform
Even if AG-enriched rats seem to swim a shorter distance than AG standard ones to reach the visible platform on the first trial (in centimeters, 329 ± 91 vs. 592 ± 135), their performances were similar on the last one (in centimeters, 155 ± 29 vs. 194 ± 40). The ANOVA indicated significant effects of “Day” (F 1/14 = 13.74, p < 0.01) and “Trial” (F 3/42 = 2.99, p < 0.05), but no effect of “Housing condition” or an interaction between any of these factors.
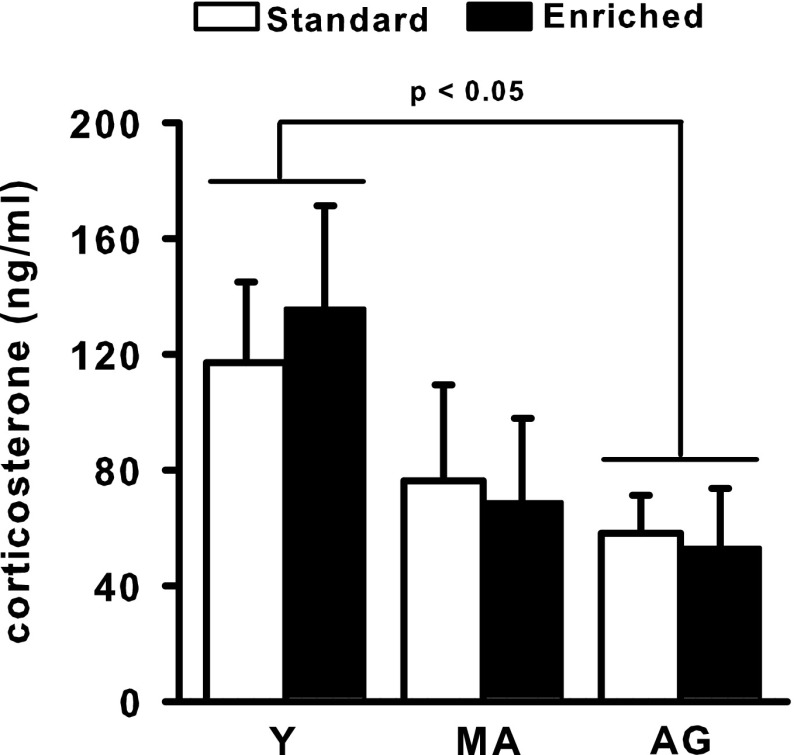
Basal corticosterone level
Three of the blood samples could not be assayed due to insufficient plasma yield. The corresponding rats were excluded from the analysis so that the final counts used for corticosterone level analyses were as follows—11 enriched and 11 standard Y rats, nine enriched and eight standard MA rats, and eight enriched and eight standard AG rats. Basal corticosterone levels decreased over age but did not seem to be influenced by the housing condition (Fig. 5). The ANOVA indicated a significant effect of “Age” (F 2/49 = 3.43, p < 0.05), due to corticosterone levels that were lower in AG rats than in Y rats (p = 0.05). There were no significant effects of "Housing condition" and no "Age × Housing condition" interaction.
Fig. 5.
Basal level of corticosterone (mean + SEM). All samples were collected during the second hour of the lights-on phase. Group abbreviations are as in Fig. 1
Immunohistochemical data
Anti-ChAT immunostaining
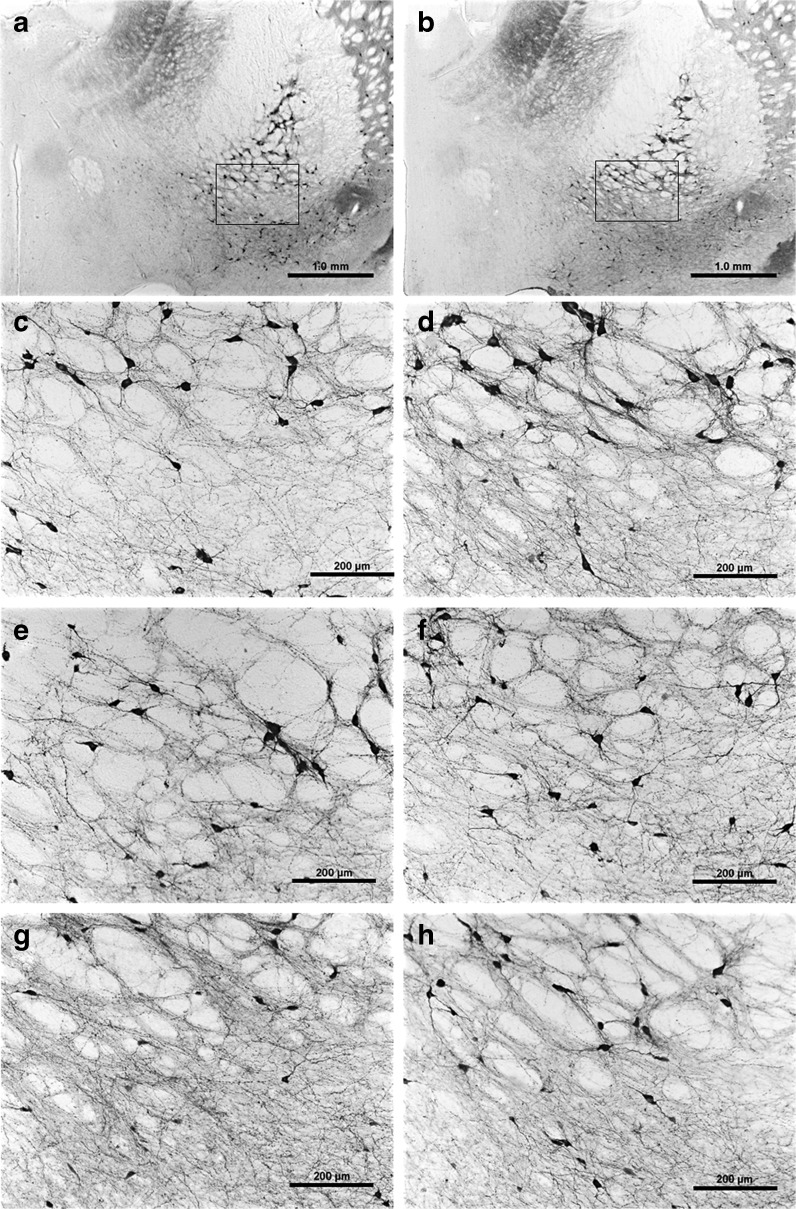
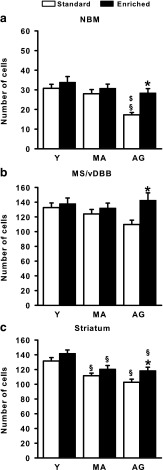
Typical examples of ChAT-positive immunostaining in the NBM are shown in Fig. 6 and quantitative evaluations in the NBM, the MS/vDBB, and the striatum are illustrated in Fig. 7. This figure shows that, in the NBM (Fig. 7a) and the MS/vDBB (Fig. 7b), the number of ChAT-positive neurons decreased over age in standard rats and remained at the level found in Y rats in the AG-enriched ones.
Fig. 6.
Representative photomicrographs of coronal sections through the NBM showing ChAT-immunoreactive neurons in young standard (a, c), young enriched (b, d), middle-aged standard (e), middle-aged enriched (f), aged standard (g), and aged enriched (h) rats. The rectangles shown in photographs a and b indicate the location of the images taken at a higher magnification (c to h) and where counting was done. Note the lower number of ChAT-positive neurons in g as compared with h. Scale bar is 1,000 μm in a and b, 200 μm in c to h
Fig. 7.
Number of ChAT-positive neurons (means + SEM/section) in the NBM (a), the MS/vDBB (b), and the striatum (c). Statistics: asterisk significantly different from standard rats at the same age; section sign, significantly different from young rats in the same housing condition; dollar sign, significantly different from middle-aged rats in the same housing condition; p < 0.05 (NK) in each case. Group abbreviations are as in Fig. 1
In the NBM, ANOVA showed significant effects of “Age” (F 2/52 = 8.31, p < 0.001) and “Housing condition” (F 1/52 = 9.09, p < 0.01) but no significant interaction between both factors. Post hoc comparisons indicated that the AG standard rats had significantly less ChAT-positive neurons than their Y and MA counterparts, but also than the AG-enriched rats (NK, p < 0.01), which did not differ from their environment-matched MA and Y counterparts. In the MS/DBB, ANOVA revealed no significant effects of “Age” or Age × Housing condition interaction, but there was a significant “Housing condition” effect (F 1/52 = 6.70, p < 0.05), which was due to a higher number of ChAT-positive neurons in AG-enriched vs. AG standard rats (p < 0.05, NK).
In the striatum (Fig. 7c), the number of ChAT-positive neurons decreased over age, whatever the housing condition, although remaining higher in enriched vs. standard rats. The ANOVA showed significant effects of “Age” (F 2/52 = 18.71, p < 0.001) and “Housing condition” (F 1/52 = 9.66, p < 0.005), but the interaction between both factors was not significant. Post hoc comparisons indicated that the number of ChAT-positive neurons was lower in MA and AG rats than in Y rats, whatever the housing conditions (p < 0.05). The number of ChAT-positive neurons was also higher in AG-enriched vs. AG standard rats (p < 0.05, NK).
Regression analyses
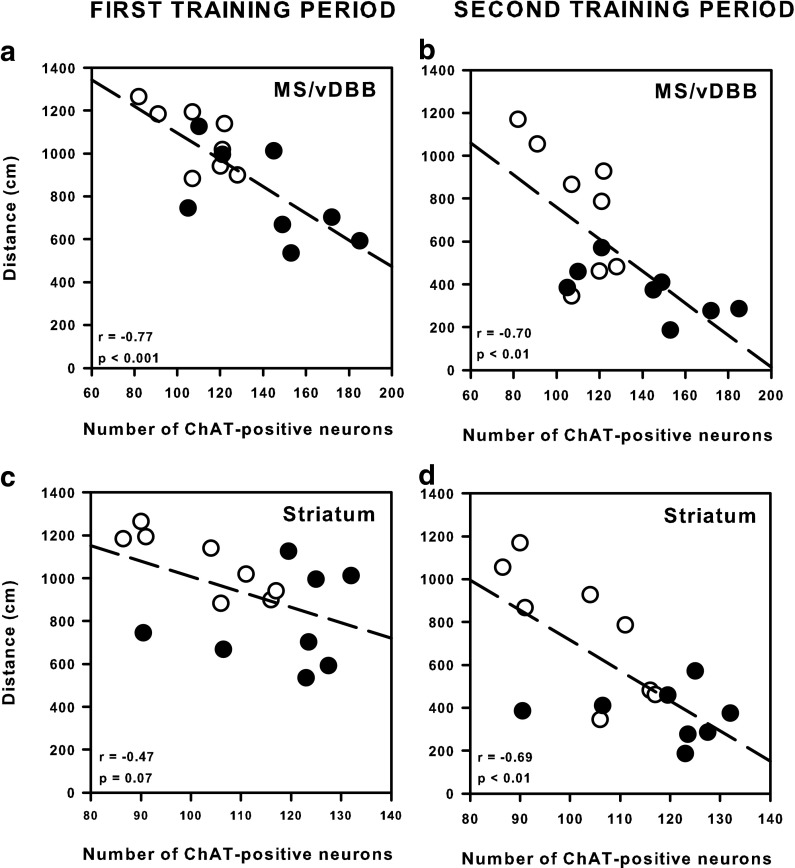
In order to examine whether the neurobiological markers modified by enriched housing conditions (i.e., ChAT-positive neurons in all structures investigated) have a functional significance with regard to the age-related behavioral deficits, we performed regression analyses between these markers and behavioral performance within populations of Y, MA, and AG rats (Baxter and Gallagher 1996). Concerning ChAT-positive neurons in the striatum and the MS/vDBB, we found no significant correlation in Y and MA rats. However, a negative correlation was found in the Y rats between the distance to the platform during the second training period and the number of ChAT-positive neurons in the NBM (r = −0.56, p < 0.01): The higher the former, the lower the latter. By contrast, in AG rats, there was no significant correlation between the number of ChAT-positive neurons in the NBM and performances in the water maze, but a negative correlation was found between the number of ChAT-positive neurons in the MS/vDBB as well as in the striatum and the distance to the platform during the first (r = −0.77, p < 0.001 and r = −0.47, p = 0.07, respectively) and the second (r = −0.70, p < 0.01 and r = −0.69, p < 0.01, respectively) training periods (Fig. 8).
Fig. 8.
Correlations between water maze performance recorded during the first (a and c) and the second (b and d) training periods and immunohistochemical data. Only aged rats (AG) were considered. Regression lines (dashed) and correlation plots of the number of ChAT-positive neurons in the MS/VDBB (a and b) or the striatum (c and d) vs. average distance to reach the platform in standard (open circle) and enriched (filled circle) rats. The correlation coefficients and p values are given in the bottom leftcorner of each panel
Discussion
Basal corticosterone levels
Basal corticosterone levels were lower in aged rats than in young adult rats. This observation is consistent with previous studies (e.g., Cano et al. 2008; Cizza et al. 1994), although inconsistent with others (e.g., Bizon et al. 2001; Montaron et al. 2006; Workel et al. 2001). Among factors that could account for the discrepant findings, the time of the light–dark cycle at which the samples were collected may be of major relevance (Cano et al. 2008). Confirming previous reports in adult rats (e.g., Bakos et al. 2009; Leal-Galicia et al. 2007; Schrijver et al. 2002; but see Moncek et al. 2004), we observed that enrichment did not influence the basal corticosterone level, whatever the age.
Emotional behaviors
In the plus-maze, our data suggest a decrease in anxiety-related behavior over aging, in agreement with previous studies (Pisarska et al. 2000; Torras-Garcia et al. 2005, but Boguszewski and Zagrodzka 2002; Bessa et al. 2005). As this decrease was not observed in middle-aged rats, it is probably not a phenomenon protracting over lifespan. In fact, in aged rats, the number of entries in the open vs. closed arms was balanced. The same applies to the time spent in the open vs. closed arms, but only in rats reared in standard condition. It is possible that, contrary to aged enriched rats, aged standard rats did not discriminate open from closed arms. Our observations in young and middle-aged enriched rats are in line with previous studies showing that environmental enrichment reduced anxiety-like behavior (Galani et al. 2007; Leal-Galicia et al. 2007, 2008; Pena et al. 2006).
Our fear-conditioning data confirmed that the freezing response towards a context previously associated with a footshock was not altered by age and that contextual fear memory lasted for at least 24 h in aged rats (Houston et al. 1999; Oler and Markus 1998; but see Ward et al. 1999). In all age groups, enrichment attenuated contextual freezing. Even if this result is inconsistent with previous studies showing that enrichment tended to increase contextual fear (Briand et al. 2005; Duffy et al. 2001; Tang et al. 2001), we previously showed (Barbelivien et al. 2006) that if, in adult rats, enrichment increased fear to a background context (i.e., when a tone perfectly predicts footshock occurrence), it concomitantly decreased fear to a foreground context (i.e., no predictive tone), as found in the current study at all ages. Therefore, the lower freezing scores that we observed in enriched rats may reflect a genuine decrease in fear rather than a deficit in contextual conditioning.
Altogether, the plus-maze and the fear conditioning data indicate that environmental enrichment increases the rat’s ability to cope with stressful situations. It did not modify the plasma corticosterone basal level but might have decreased the HPA axis sensitivity to stress. In line with this hypothesis, enriched mice re-exposed to a passive avoidance apparatus were shown to display less freezing responses and weaker corticosterone release than standard mice (Benaroya-Milshtein et al. 2004).
Spatial learning and memory
Our water maze data confirmed that lifelong environmental enrichment has a beneficial impact on age-related spatial memory deficits (Harati et al. 2011). Several studies have shown that spatial memory deficits can be observed at an age of 12 months (Bizon and Gallagher 2003; Bizon et al. 2009; Markowska 1999; Wyss et al. 2000). In the current study, training started when middle-age rats were 15 months old. These rats displayed a weak acquisition but no recent memory recall deficit. The weakness of this deficit contrasts with the severity of the deficit observed in aged standard rats over the first training period and its corresponding probe trial (Bizon et al. 2009; Harati et al. 2011; Muir et al. 1999). This impact of age was less pronounced in enriched rats, middle-aged ones performing at the level of their young adult counterparts and better than the standard ones during acquisition. Aged enriched rats, despite weaker learning performance, performed better than their standard counterparts, and memory was maintained for 1 day. Overall, these results suggest that environmental enrichment not only delayed the onset of spatial learning/retention deficits but also reduced their severity.
With prolonged training (second period), aged enriched rats performed not differently from young adult and middle-aged rats, thereby confirming the preservation of a genuine spatial learning capacity. By contrast, despite their slight improvement, the low performance observed in aged standard rats further emphasizes the severity of their deficit. However, they remained able to learn the cued version of the task (visible platform) as previously shown (Harati et al. 2011). Only young adult and middle-aged enriched rats exhibited evidence for remote memory. To our knowledge, the effect of enrichment on remote spatial memory has not been investigated so far, but a recent study suggests that its beneficial effect on remote memory might be limited to some kinds of learning experience. Using a passive avoidance task in mice, Leget et al. (2012) reported that enrichment did not improve remote memory (8 weeks). The prevention of spontaneous forgetting in young and middle-aged rats may be related to their ability to form a memory trace that was less sensitive to degradation and to better deal with spatial cue saliency, which contributes to persistence of memory traces (Lopez et al. 2008). It is also noteworthy that, in both young (Bruel-Jungerman et al. 2005, 24 h and 48 h) and aged rats (Leal-Galicia et al. 2008, 24 h), enrichment increases the vividness of memory for object recognition. Even if this form of memory is more labile than the one explored in our study, these results show that enrichment enhances memory persistence. The enrichment-induced neurobiological modifications that might explain effects on memory persistence are numerous. Indeed, environmental enrichment increases hippocampal neurogenesis and survival of newly formed neurons (e.g., Bruel-Jungerman et al. 2005), CREB activation (e.g., Williams et al. 2001), long-term potentiation (Duffy et al. 2001), and brain-derived neurotrophic factor levels (Bakos et al. 2009). All these factors contribute to consolidation and thus memory persistence (Bekinschtein et al. 2007; Bruel-Jungerman et al. 2005; Sekeres et al. 2010; Suzuki et al 2011). Interestingly, the capability of enriched environment to enhance long-term memory for object recognition was lost when neurogenesis was blocked (Bruel-Jungerman et al. 2005).
Spatial cognition and forebrain cholinergic neurons
Our data confirmed an age-related decrease of the number of ChAT-positive neurons in the basal forebrain under standard rearing conditions (Baskerville et al. 2006; De Lacalle et al. 1996; Stemmelin et al. 2000). Alteration of the cholinergic system originating in the MS/vDBB has been implicated in the age-related spatial memory impairments (Aubert et al. 1995; Baskerville et al. 2006; Sugaya et al. 1998). This view was challenged as selective cholinergic lesions of the septal region did not affect water maze performance or produced only modest alterations (e.g., Baxter et al. 1995). The persistence of a spatial memory, however, may be compromised when such lesions also affect the NBM (Parent and Baxter 2004; Traissard et al. 2007). Thus, because the loss of basal forebrain cholinergic neurons occurred in both the septo-hippocampal and the basalo-cortical systems, its contribution to the age-related spatial memory impairments is well possible. As enrichment prevented this loss, it is tempting to suggest that such prevention could be part of the mechanism underlying improved acquisition/memory performance in aged rats, a view also supported by the negative correlation between the number of ChAT-positive neurons in the MS/vDBB and water maze acquisition performances in aged rats only. Interestingly, in a mouse model of Alzheimer’s disease (Berardi et al. 2007), enriched housing prevented both the cognitive decline and the age-dependent loss of basal forebrain cholinergic neurons.
Our results confirm that the number of cholinergic interneurons in the striatum decrease over aging (Colombo and Gallagher 1998; Stemmelin et al. 2000). Enriched rats appeared less affected than standard rats. In aged rats, we also found a negative correlation between the number of striatal ChAT-positive interneurons and water maze performance during the two training periods, as reported earlier (Colombo and Gallagher 1998; Stemmelin et al. 2000). Even though the MWM task is generally considered to depend on hippocampal functions, other structures, among which the striatum, are known to contribute (e.g., Devan et al. 1996; Shirakawa and Ichitani 2004; Miyoshi et al. 2012). Thus, it can be suggested that the positive effect of enriched environment on water maze learning may also be related to the environmental impact on the survival of striatal cholinergic neurons. Along this line, it is noteworthy that aging-dependent alterations of cholinergic pharmacodynamics in the striatum were correlated with levels of water maze performance (Cassel et al. 2007) and that the M2/M4 muscarinic receptor coupling in the striatum of cognitively impaired aged rats is reduced (Nieves-Martinez et al. 2012).
Conclusion
We have shown that lifelong environmental enrichment preserved the abilities to acquire and retrieve recent—unfortunately not remote—spatial memory from aging-related degradation in rats. It also attenuated emotional reactions in stressful situations. Furthermore, as the positive effects of enrichment on basal forebrain and striatal cholinergic neurons were only evident in aged rats, our results suggest that enrichment protected these neuronal population against their age-related alterations. Even if the protective effects of enrichment on basal forebrain cholinergic neurons might be related to increased nerve growth factor production (O’Callaghan et al. 2009; Pham et al. 2002), as these neurons are known to require it for their survival, the mechanisms involved in its protective effects on striatal cholinergic interneurons remain to be determined.
The functional impact of lifelong environmental enrichment most probably occurs conjointly at different neurobiological levels, and the conjunction of these multilevel, environment-triggered changes might be determinant in the attenuation of (or protection against) the cognitive alterations accompanying aging under standard rearing conditions. In such a view, the herein described effects of the enriched environment on cholinergic markers is possibly only one of the environmental impacts accounting for better cognition in aged rats.
Acknowledgments
The authors would like to express their gratefulness to O. Bildstein, G. Edomwony, and O. Egesi for their valuable and constant investment in the care provided to the rats and the outstanding management of the enriched environment over a 2-year period. The authors are especially grateful to Christine Demangeat and Rémy Sapin, who performed the plasmatic corticosterone level measurements. This study was supported by the Université de Strasbourg and the CNRS.
Footnotes
Hayat Harati and Alexandra Barbelivien are equivalent co-first authors.
Jean-Christophe Cassel and Monique Majchrzak are equivalent co-last authors.
References
- Aubert I, Rowe W, Meaney MJ, Gauthier S, Quirion R. Cholinergic markers in aged cognitively impaired Long-Evans rats. Neurosci. 1995;67:277–292. doi: 10.1016/0306-4522(95)00056-O. [DOI] [PubMed] [Google Scholar]
- Bakos J, Hlavacova N, Rajman M, Ondicova K, Koros C, Kitraki E, Steinbusch HWM, Jezova D. Enriched environment influences hormonal status and hippocampal brain derives neurotrophic factor in a sex dependent manner. Neurosci. 2009;164:788–797. doi: 10.1016/j.neuroscience.2009.08.054. [DOI] [PubMed] [Google Scholar]
- Barbelivien A, Herbeaux K, Oberling P, Kelche C, Galani R, Majchrzak M. Environmental enrichment increases responding to contextual cues but decreases overall conditioned fear in the rat. Behav Brain Res. 2006;169:231–238. doi: 10.1016/j.bbr.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Baskerville KA, Kent C, Nicolle MM, Gallagher M, McKinney M. Aging causes partial loss of basal forebrain but no loss of pontine reticular cholinergic neurons. NeuroReport. 2006;17:1819–1823. doi: 10.1097/WNR.0b013e32800fef5a. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Neurobiological substrates of behavioural decline: models and data analytic strategies for individual differences in aging. Neurobiol Aging. 1996;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995;109:714–722. doi: 10.1037/0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Berardi N, Braschi C, Capsoni S, Cattaneo A, Maffei L. Environmental enrichment delays the onset of memory deficits and reduces neuropathological hallmarks in a mouse model of Alzheimer-like neurodegeneration. J Alzheimers Dis. 2007;11:359–370. doi: 10.3233/jad-2007-11312. [DOI] [PubMed] [Google Scholar]
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav Neurosci. 1997;111:267–274. doi: 10.1037/0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Oliveira M, Cerqueira JJ, Almeida OF, Sousa N. Age-related qualitative shift in emotional behavior: paradoxical findings after re-exposure of rats in the elevated-plus maze. Behav Brain Res. 2005;162:135–142. doi: 10.1016/j.bbr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Helm KA, Han JS, Chun HJ, Pucilowska J, Lund PK, Gallagher M. Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behaviorally characterized young and aged Long-Evans rats. Eur J Neurosci. 2001;14:1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fisher 344 rats. Neurobiol Aging. 2009;30:645–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguszewski P, Zagrodzka J. Emotional changes related to age in rats—a behavioral analysis. Behav Brain Res. 2002;133:323–332. doi: 10.1016/S0166-4328(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Bouet V, Freret T, Dutar P, Billard JM, Boulouard M. Continuous enriched environment improves learning and memory in adult NMRI mice through theta burst-related-LTP independent mechanisms but is not efficient in advanced aged animals. Mech Ageing Dev. 2011;132:240–248. doi: 10.1016/j.mad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Briand LA, Robinson TE, Maren S. Enhancement of auditory fear conditioning after housing in a complex environment is attenuated by prior treatment with amphetamine. Learn Mem. 2005;12:553–556. doi: 10.1101/lm.95905. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Cano P, Cardinali DP, Spinedi E, Esquifino AI. Effect of aging on 24-hour pattern of stress hormones and leptin in rats. Life Sci. 2008;83:142–148. doi: 10.1016/j.lfs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Cassel J-C, Lazaris A, Birthelmer A, Jackisch R. Spatial reference- (not working- or procedural-) memory performance of aged rats in the water maze predicts the magnitude of sulpiride-induced facilitation of acetylcholine release by striatal slices. Neurobiol Aging. 2007;28:1270–1285. doi: 10.1016/j.neurobiolaging.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Cizza G, Calogero AE, Brady LS, Bagdy G, Bergamini E, Blackman MR, Chrousos GP, Gold PW. Male Fischer 344/N rats show a progressive central impairment of the hypothalamic-pituitary-adrenal axis with advancing age. Endocrinol. 1994;134:1611–1620. doi: 10.1210/en.134.4.1611. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Gallagher M. Individual differences in spatial memory and striatal ChAT activity among young and aged rats. Neurobiol Learn Mem. 1998;70:314–327. doi: 10.1006/nlme.1998.3857. [DOI] [PubMed] [Google Scholar]
- De Lacalle S, Cooper JD, Svendsen CN, Dunnett SB, Sofroniew MV. Reduced retrograde labelling with fluorescent tracer accompanies neuronal atrophy of basal forebrain cholinergic neurons in aged rats. Neurosci. 1996;75:19–27. doi: 10.1016/0306-4522(96)00239-4. [DOI] [PubMed] [Google Scholar]
- Depp C, Vahia IV, Jeste D. Successful aging: focus on cognitive and emotional health. Annu Rev Clin Psycho. 2010;6:527–550. doi: 10.1146/annurev.clinpsy.121208.131449. [DOI] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobio Learn Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CI, Collazo J, Bauza Y, Castellanos MR, Lopez O. Environmental enrichment–behavior–oxidative stress interactions in the aged rat: issues for a therapeutic approach in human aging. Ann NY Acad Sc. 2004;1019:53–57. doi: 10.1196/annals.1297.012. [DOI] [PubMed] [Google Scholar]
- Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000;34:372–378. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- Freret T, Billard JM, Schumann-Bard P, Dutar P, Dauphin F, Boulouard M, Bouet V (2011) Rescue of cognitive aging by long-lasting environmental enrichment exposure initiated before median lifespan. Neurobio Aging doi.org/10.1016/j.neurobiolaging.2011.09.028 [DOI] [PubMed]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/S0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Galani R, Berthel MC, Lazarus C, Majchrzak M, Barbelivien A, Kelche C, Cassel J-C. The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol Learn Mem. 2007;88:1–10. doi: 10.1016/j.nlm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Harati H, Barbelivien A, Cosquer B, Majchrzak M, Cassel J-C. Selective cholinergic lesions in the rat nucleus basalis magnocellularis with limited damage in the medial septum specifically alter attention performance in the five-choice serial reaction time task. Neurosci. 2008;153:72–83. doi: 10.1016/j.neuroscience.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Harati H, Majchrzak M, Cosquer B, Galani R, Kelche C, Cassel J-C, Barbelivien A. Attention and memory in aged rats: impact of lifelong environmental enrichment. Neurobiol Aging. 2011;32:718–736. doi: 10.1016/j.neurobiolaging.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Lambert TJ, Frick KM. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behav Brain Res. 2007;185:43–48. doi: 10.1016/j.bbr.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CD, Nowak KA, Sater M, Bruno JP. Microdialysis without acetylcholinesterase inhibition reveals an age-related attenuation in stimulated cortical acetylcholine release. Neurobiol Aging. 2003;24:861–863. doi: 10.1016/S0197-4580(02)00226-9. [DOI] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Méthodes statistiques en sciences humaines. Paris: De Boeck Université; 1998. [Google Scholar]
- Kobayashi S, Ohashi Y, Ando S. Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. J Neurosci Res. 2002;70:340–346. doi: 10.1002/jnr.10442. [DOI] [PubMed] [Google Scholar]
- Leal-Galicia P, Saldivar-Gonzalez A, Morimoto S, Arias C. Exposure to environmental enrichment elicits differential hippocampal cell proliferation: role of individual responsiveness to anxiety. Dev Neurobiol. 2007;67:395–405. doi: 10.1002/dneu.20322. [DOI] [PubMed] [Google Scholar]
- Leal-Galicia P, Castaneda-Bueno M, Quiroz-Baez R, Arias C. Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem. 2008;90:511–518. doi: 10.1016/j.nlm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behaviour. Biol Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Leger M, Bouet V, Freret T, Darmaillacq AS, Dacher M, Dauphin F, Boulouard M, Schumann-Bard Environmental enrichment improves recent but not remote memory in association with a modified brain metabolic activation profile in adult mice. Behav Brain Res. 2012;228:22–29. doi: 10.1016/j.bbr.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem. 1997;68:203–220. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- Lopez J, de Vasconcelos AP, Cassel J-C. Environmental cue saliency influences the vividness of a remote spatial memory in rats. Neurobiol Learn Mem. 2008;90:285–289. doi: 10.1016/j.nlm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Marchand AR, Luck D, DiScala G. Evaluation of an improved automated analysis of freezing behaviour in rats and its use in trace fear conditioning. J Neurosci Methods. 2003;126:145–153. doi: 10.1016/S0165-0270(03)00076-1. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi E, Wietzikovski EC, Bortolanza M, Boschen SL, Canteras NS, Izquierdo I, Da Cunha C. Behav Brain Res. 2012;226:171–178. doi: 10.1016/j.bbr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Muir JL, Fischer W, Bjorklund A. Decline in visual attention and spatial memory in aged rats. Neurobiol Aging. 1999;20:605–615. doi: 10.1016/S0197-4580(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Nieves-Martinez E, Haynes K, Childers SR, Sonntag WE, Nicolle MM. Muscarinic receptor/G-protein coupling is reduced in the dorsomedial striatum of cognitively impaired aged rats. Behav Brain Res. 2012;227:258–264. doi: 10.1016/j.bbr.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits on the radial maze and in fear conditioning: hippocampal processing and consolidation. Hippocampus. 1998;8:402–415. doi: 10.1002/(SICI)1098-1063(1998)8:4<402::AID-HIPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena Y, Prunell M, Dimitsantos V, Nadal R, Escorihuela RM. Environmental enrichment effects in social investigation in rats are gender dependent. Behav Brain Res. 2006;174:181–187. doi: 10.1016/j.bbr.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Pham TM, Winblad B, Granholm AC, Mohammed AH. Environmental influences on brain neurotrophins in rats. Pharmacol Biochem Behav. 2002;73:167–175. doi: 10.1016/S0091-3057(02)00783-9. [DOI] [PubMed] [Google Scholar]
- Pisarska M, Mulchahey JJ, Welge JA, Geracioti TD, Jr, Kasckow JW. Age-related alterations in emotional behaviors and amygdalar corticotropin-releasing factor (CRF) and CRF-binding protein expression in aged Fischer 344 rats. Brain Res. 2000;877:184–190. doi: 10.1016/S0006-8993(00)02606-8. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/S0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Behav. 2002;71:129–139. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- Sekeres MJ, Neve RL, Frankland PW, Josselyn SA. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn Mem. 2010;17:280–283. doi: 10.1101/lm.1785510. [DOI] [PubMed] [Google Scholar]
- Shank JC. Do Norway rats (Rattus norvegicus) synchronize their estrous cycle? Physiol Biochem Behav. 2001;73:209–224. doi: 10.1016/s0031-9384(00)00395-4. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Ichitani Y. Prolonged initiation latency in Morris water maze learning in rats with ibotenic acid lesions to medial striatum: effects of systemic and intranigral muscimol administration. Brain Res. 2004;1030:193–200. doi: 10.1016/j.brainres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Stemmelin J, Lazarus C, Cassel S, Kelche C, Cassel J-C. Immunohistochemical and neurochemical correlates of learning deficits in aged rats. Neurosci. 2000;96:275–289. doi: 10.1016/S0306-4522(99)00561-8. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol Aging. 1998;19:351–361. doi: 10.1016/S0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu L-C, Zhao M-G, Xu H, Shang Y, Endoh K, Iwamoto T, Mamiya N, Okano E, Hasegawa S, Mercaldo V, Zhang Y, Maeda R, Ohta M, Josselyn SA, Zhuo M, Kida S. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011;31:8786–8802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacol. 2001;41:779–790. doi: 10.1016/S0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Torras-Garcia M, Costa-Miserachs D, Coll-Andreu M, Portell-Cortes I. Decreased anxiety levels related to aging. Exp Brain Res. 2005;164:177–184. doi: 10.1007/s00221-005-2240-y. [DOI] [PubMed] [Google Scholar]
- Traissard N, Herbeaux K, Cosquer B, Jeltsch H, Ferry B, Galani R, Pernon A, Majchrzak M, Cassel JC. Combined damage to entorhinal cortex and cholinergic basal forebrain neurons, two early neurodegenerative features accompanying Alzheimer’s disease: effects on locomotor activity and memory functions in rats. Neuropsychopharmacol. 2007;32:851–871. doi: 10.1038/sj.npp.1301116. [DOI] [PubMed] [Google Scholar]
- Ward MT, Oler JA, Markus EJ. Hippocampal dysfunction during aging I: deficits in memory consolidation. Neurobiol Aging. 1999;20:363–372. doi: 10.1016/S0197-4580(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, McCoy JG. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol Behav. 2001;73:649–6. doi: 10.1016/S0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- Workel JO, Oitzl MS, Fluttert M, Lesscher H, Karssen A, de Kloet ER. Differential and age-dependent effects of maternal deprivation on the hypothalamic–pituitary–adrenal axis of brown Norway rats from youth to senescence. J Neuroendocrinol. 2001;13:569–580. doi: 10.1046/j.1365-2826.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Chambless BD, Kadish I, van Groen T. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol Aging. 2000;21:671–681. doi: 10.1016/S0197-4580(00)00132-9. [DOI] [PubMed] [Google Scholar]