Abstract
Saccadic impairment in Alzheimer’s disease (AD) was found in horizontal saccades. The present study extends investigation to vertical saccades in a large number of subjects, including AD and amnestic mild cognitive impairment (aMCI). We examined both horizontal and vertical saccades in 30 healthy elderly, 18 aMCI, and 25 AD. Two tasks were used: gap (fixation target extinguishes prior to target onset) and overlap (fixation stays on after target onset). Eye movements were recorded with the Eyeseecam system. (1) Robust gap effect (shorter latencies in gap than in overlap) exists for AD and aMCI patients as for healthy elderly; (2) abnormal long latency of saccades in gap and overlap tasks for AD relative to healthy elderly and aMCI patients; (3) longer latency for aMCI patients than for healthy elderly for the overlap task; (4) significant correlation between scores of Mini-Mental State Examination (MMSE) and latencies of saccades considering the AD group only; (5) higher coefficient of variation in latency for AD patients than for healthy elderly and for aMCI patients; (6) variability of accuracy and speed is abnormally higher in AD patients than in aMCI and healthy elderly. Abnormalities of latency and latency–accuracy–speed variability reflect deficits of cerebral areas involved in the triggering and execution of saccades; latency of saccades can be used as follow-up test for aMCI and AD patients with its significant correlation with the changes of MMSE scores.
Keywords: Alzheimer’s disease, aMCI, Saccades, Gap, Overlap, Variability
Introduction
Study of saccades is an excellent tool in investigating cognitive function during normal aging and neurodegenerative diseases. Particularly, a variety of existing ocular motor tests (e.g., gap versus overlap) allows testing automatic versus controlled initiated saccades hypothetically involving different cortical–subcortical ocular motor networks. Next, we will review brief studies of saccades in aged individuals, healthy or patients.
In the gap paradigm, the fixation point disappears some time prior to target onset while, in the overlap paradigm, the fixation point remains illuminated during the target presentation. In addition, latencies of saccades in the gap paradigm are generally reduced as compared with the overlap paradigm. This is called the gap effect (Saslow 1967) and could be due to attentional disengagement (Fischer and Breitmeyer 1987; Fischer and Weber 1993; Hoffman and Subramaniam 1995; Shepherd et al. 1986), to advance motor preparation (Dorris et al. 1997; Rolfs and Vitu 2007) or to disinhibition in the superior colliculus because of the offset of the fixation point (Reuter-Lorenz et al. 1991; Findlay and Walker 1999). Different cortical–subcortical areas, such as, superior colliculus (Isa and Kobayashi 2004), posterior parietal cortex (Kapoula et al. 2004; Kapoula et al. 2001), or frontal eye field (Kurkin et al. 2003) could be involved in the generation saccades. However, Abel et al. (2002) demonstrated that AD patients had a gap effect of similar magnitude to normal subjects.
As mentioned above, study of saccades can give information about brain function. Thus, seeking the correlation between saccade properties namely latency and measures of cognitive function (e.g., Mini-Mental State Examination (MMSE)) is an excellent tool. The MMSE test (Folstein et al. 1975) assesses five cognitive abilities: orientation to time and space; registration and ability to repeat words; attention span and arithmetic; memory and recall named objects; language. A saccade task (gap or overlap) as rudimentary as it can appear, still can share, many of the complex cognitive operations that are required by MMSE, namely orientation to time and location of the saccade target, attention, and ability to register ongoing and past target locations over repetitive trials. Thus, one could expect a correlation between saccade latency and MMSE scores. However, some studies (Abel et al. 2002; Hershey et al. 1983) did not find significant correlation between severity of dementia in AD and the latency of prosaccades while other studies reported a significant correlation between the measures of cognitive impairment and reaction time of prosaccades (Bylsma et al. 1995; Pirozzolo and Hansch 1981). Besides prosaccades, there are some studies examining correlation between MMSE scores and properties of other types of eye movements. For instance, a significant correlation between the MMSE scores and error rates of antisaccades (subjects have to suppress saccade to target and saccade voluntarily opposite) was found in some studies (Boxer et al. 2006a; Crawford et al. 2005), while Kaufman et al.(2012) did not find such correlation. Finally, performance on visual tracking tasks was measured in groups of Alzheimer-type dementia, pseudodementia of depression, and elderly normal controls. Smooth pursuit tracking errors were identified by counting the number of catch-up saccades required to compensate for failure of the smooth pursuit system. The group with Alzheimer-type dementia had significantly worse (p < 0.0001) smooth pursuit tracking than either pseudodementia patients or elderly normal controls (Hutton et al. 1984).
Another important issue is the existence of eye movement abnormalities in patients with amnestic mild cognitive impairment (aMCI). A preliminary study from our group (Yang et al. 2011) of horizontal saccades only revealed significantly longer latency and higher variability of accuracy–speed in patients with AD than in healthy elderly or patients with aMCI; no abnormality was found for aMCI. There was no significant difference of the coefficient of variation in latency among these groups of subjects, but the number of subjects was small. In addition, because of small number of group subjects, we did not report either the express type of latency of saccades or the correlation between MMSE scores and latencies of saccades.
The present study aims to compare healthy aged subjects, AD at mild to moderate stage and aMCI patients in terms of their performance in two tasks, gap and overlap, including all four directions (left, right, up, and down). The study focus on gap overlap tasks as such tasks are simple and can be readily done even when the disease is advanced. Moreover, the study aims to examine if and how performances on such simple saccade tasks correlate with MMSE and other clinical measures. Another novelty is the analysis of all parameters of saccades (accuracy, speed, latency) both in terms of means and of the coefficient of variation. Such comprehensive saccade examination allows revealing important specific results for the different groups studied.
Methods
Participants
We studied normal aged control subjects, patients with aMCI, and patients with AD of mild to moderate severity, without ophthalmological or other neuropsychiatric disorders. All subjects had normal, or corrected to normal, visual acuity without group difference by age or gender. Most of the patients were not on anti-dementia medications. A few cases were on clinical trial of blind, placebo-controlled AD symptomatic medications.
All patients underwent a screening process that included a review of their medical history, physical and neurological examinations, laboratory tests, and magnetic resonance imaging (MRI) analysis. The clinical assessment of mild cognitive impairment or dementia included neuropsychological tests, as well as behavioral and psychiatric interviews conducted by the attending psychiatrist.
Amnestic MCI were diagnosed based on the following criteria (Petersen et al. 2001): (1) memory complaint, preferably corroborated by a spouse or relative, (2) objective memory impairment, (3) normal general cognitive function, (4) intact activities of daily living, and (5) absence of dementia. We have amended the amnestic MCI diagnostic criteria of the Petersen MMSE cut-off score in order to be consistent with the low level of education in elderly Chinese people. The original MMSE was developed by Folstein et al. (1975). The culturally adapted Chinese version of the Mini-Mental State Examination was established in 1988 by R. Katzman et al. (1988). These authors found that, with the Chinese version of MMSE, AD patients who had not been educated (NO ED) exhibited MMSE scores of <18; those with elementary school education exhibited MMSE scores of <21; and those with higher than middle school education exhibited MMSE scores of <25. In the present study, the aMCI analysis was carried out on NO ED patients with MMSE cut-off scores of ≥18, elementary school educated patients with MMSE cut-off scores of ≥21, and higher than middle school educated patients with MMSE cut-off scores of ≥25.
The neuropsychological battery which was used only to determine MCI subtype in the present study included: Wechsler Memory Scale (WMS) Verbal Associates immediate and 30-min delayed test, Rey Auditory Verbal Learning and 30-min Delayed Test, WMS-Digit Span, Category Naming Test-Animals, Clock Drawing Test. We rated the MCI patients’ cognitive impairment in seven domains: memory, attention, language, visual–spatial, orientation, calculation, and executive function according to the neuropsychological battery and MMSE. Based on the assessment, we retained aMCI patients and excluded impairment in a single non-memory domain (single, non-memory domain MCI subtype) and impairment in two or more domains (multiple domains, slightly impaired MCI subtype). AD patients recorded scores of <4 on the Hachinski Ischemia Scale and showed no history of significant systemic or psychiatric conditions, or traumatic brain injuries that could compromise brain function. All AD patients were required to have fewer than two lacouna ischemia (of diameter <1 cm), as revealed by MRI fluid-attenuated inversion recovery sequence scanning.
The cognitively normal elderly formed the normal control (NC) group was of independently functioning community dwellers. They had no history of cognitive decline, neurological or psychiatric disorders, or uncontrolled systemic medical disorders.
Following such procedures, the recruited participants consisted of 25 individuals (seven men) with AD (ranging from 60 to 83 years; mean, 73.5 ± 8.2 years), MMSE ranging from 8 to 26 (mean, 15.7 ± 4.4); 18 patients (seven men) with MCI, age ranging from 59 to 91 years (mean, 77.6 ± 10.7 years), MMSE from 20 to 28 (mean, 25.2 ± 2.4). Thirty healthy subjects (15 men, age- and education-matched to aMCI or AD patients), age ranging from 60 to 82 years (mean, 73.8 ± 9.4 years), MMSE from 28 to 30 (mean, 29.3 ± 0.8). All clinical characteristics of subjects are summarized in Table 1. Estimated duration of disease and the degree of autonomy measured by the activity of daily living scale are also shown in Table 1. Informed consent was obtained from all participants, and the study was approved by the institutional review board of Shanghai Mental Health Center.
Table 1.
Subject demographics
| Control | MCI | AD | |
|---|---|---|---|
| n | 30 | 18 | 25 |
| Age (years) | 73.8 ± 9.4 | 77.6 ± 10.7 | 73.5 ± 8.2 |
| Gender (m/f) | 15/15 | 7/11 | 7/18 |
| Education (years) | 11.4 ±3.4 | 12.0 ± 3.7 | 9.4 ± 4.1 |
| MMSE | 29.4 ± 0.8 | 25.2 ± 2.4 | 15.7 ± 4.4 |
| ADL (max. 56) | 14.6 ± 3.2 | 18.3 ± 5.4 | 30.7 ± 10.5 |
| Estimated duration of disease (years) | NA | 4.2 ± 2.6 | 4.1 ± 2.4 |
MMSE Mini Mental State Examination, ADL activities of daily living
Visual display
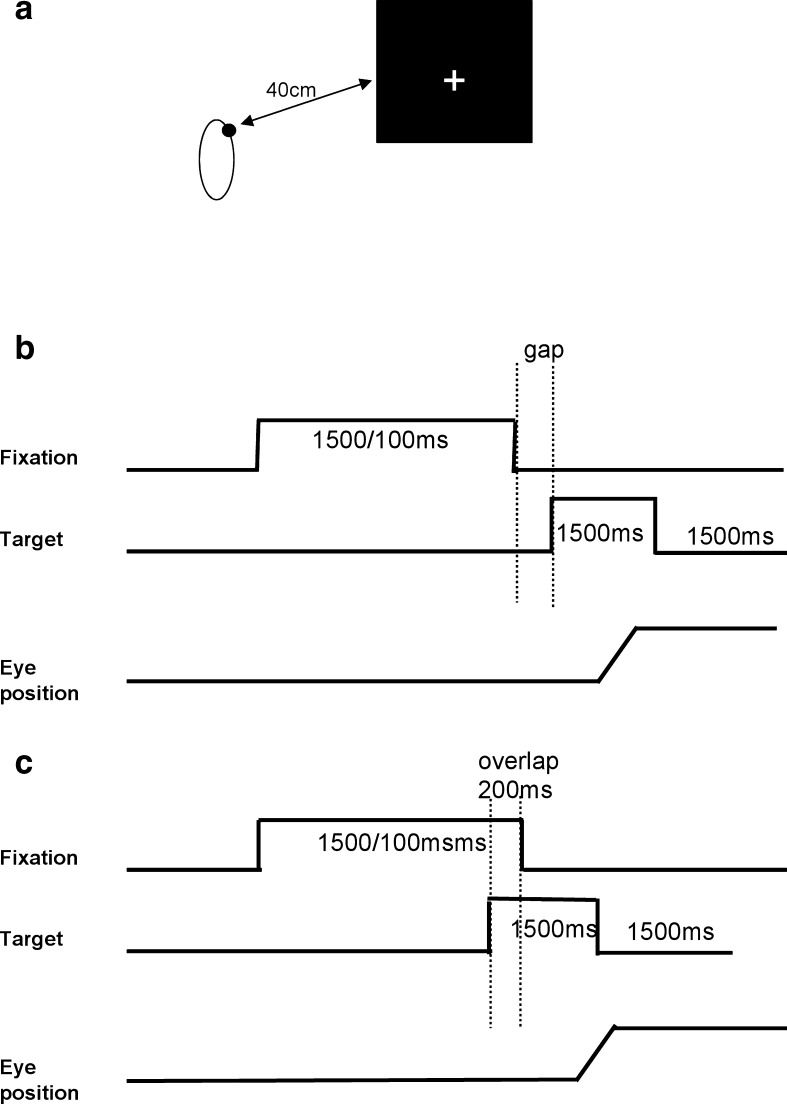
The visual display, shown in Fig. 1a, white luminous signal ‘+’ was used on a black computer screen. The signal ‘+’ was presented at the center of the screen for fixation, and when presented at an eccentricity of ±10° horizontally and vertically, it was as for the stimulation target. The subject was comfortably seated in an adapted chair, his head resting on a chin and frontal support. The subject viewed binocularly; all targets were highly visible. The test was conducted in one dim room.
Fig. 1.
Experimental design. a Spatial arrangement. Five white luminous ‘plus signs’ were used on a black computer screen at 57 cm from the subject, one at the center of the screen; the others at an eccentricity of ±10° horizontally or vertically. b, c Temporal arrangement. Each trial started by lighting one central ‘plus’ during approximately 1,500–2,000 ms as fixation. For the gap task, between the fixation offset and the target onset, there was a gap of 200 ms (b); for the overlap task, the fixation stays on 200 ms after the target onset (c). The target, one of the eccentric ‘+’, appears for 1,500 ms
Fixation and oculomotor tasks: gap and overlap tasks
Each trial started by lighting a fixation signal ‘+’ at the center that stayed on for a random period between 1.5 and 2 s. In the gap task, there was a time interval of 200 ms between the offset of the fixation signals and the onset of the saccade target. The target signal was kept on for 1.5 s (Fig. 1b). In the overlap task, the fixation signal remained illuminated for 200 ms after the target signal appeared. The target stayed on also for another 1.5 s (Fig. 1c). Subjects were required to make a saccade to the target point as rapidly and accurately as possible. A period of complete darkness of 500 ms was for break. Subjects were instructed to use this period for blinks. The total mean length of each trial was about 4 s. In each block, only gap or overlap task was used randomly for two horizontal directions, targets at 10o, left or right (12 trials for each direction, total 48 trials). One block for each task lasted 4 min.
A calibration sequence was performed at the beginning; the target made the following predictive sequence: center, 10o to left; center, 10o to right; center, 10o to up; center, 10o to down; four times; the target stayed at each location for 1 s. From these recordings, we extracted calibration factors which should be verified to be satisfied by the Eyeseecam system.
Eye movement recording
Horizontal and vertical eye movements were recorded binocularly with a video-oculography, Eyeseecam system (University of Munich Hospital, Clinical Neuroscience, Munich, Germany, see http://eyeseecam.com/). The sampling rate of the Eyeseecam system was 222 Hz. The optimal spatial resolution was approximately 0.010.
Data analysis
From the two individual calibrated eye position signals, we derived the conjugate signal (left eye + right eye)/2. The onset and the offset of saccades were defined as the time when conjugate eye velocity exceeded or dropped below 10 % of the peak velocity. The process was performed automatically by the computer, and the verification was made by visual inspection of the individual eye position and velocity traces. For both gap and overlap tasks, latency was measured as the time between target onset and saccade onset. To estimate the accuracy, we used the amplitude of primary main saccade relative to the target eccentricity (gain = saccade amplitude/target eccentricity). Mean velocity was calculated as amplitude/duration. Peak velocity was calculated as the maximum value of velocity during saccade. To evaluate the variability of parameters, we calculated the coefficient of variation (CV), and the relative standard deviation expressed as a unitless proportion of each subject mean (van Beers 2007; Peltsch et al. 2009).
Eye movements in the wrong direction, with latency shorter than 80 ms (anticipation) or longer than 1500 ms, or contaminated by blinks were rejected. For healthy elderly, 5 % of trials (ranged from 5 % to 10 %); for aMCI patients, 7 % of trials (5 % to 12 %), and for patients with AD, 16 % of trials (from 10 % to 25 %) had to be rejected, the most frequent reason being the blinks.
Statistical
A three-way analysis of variance (ANOVA) was performed on individual mean values of each parameter with the between-subjects factor, group (healthy, aMCI, and AD), and the within-subjects factors, the oculomotor task (gap, overlap) and the direction (left, right, up, and down). Post hoc comparisons were done with the least significant differences test. For the express latency and the coefficient of variation of each parameter, the non-parametric Kruskal-Wallis and Mann–Whitney U tests were used for comparisons between groups. The Bravais–Pearson correlation coefficient was used for evaluation of the correlation between MMSE scores and latencies of saccades. All statistical analyses were run by use of the software “Statistic.”
Results
Latency
Mean latency
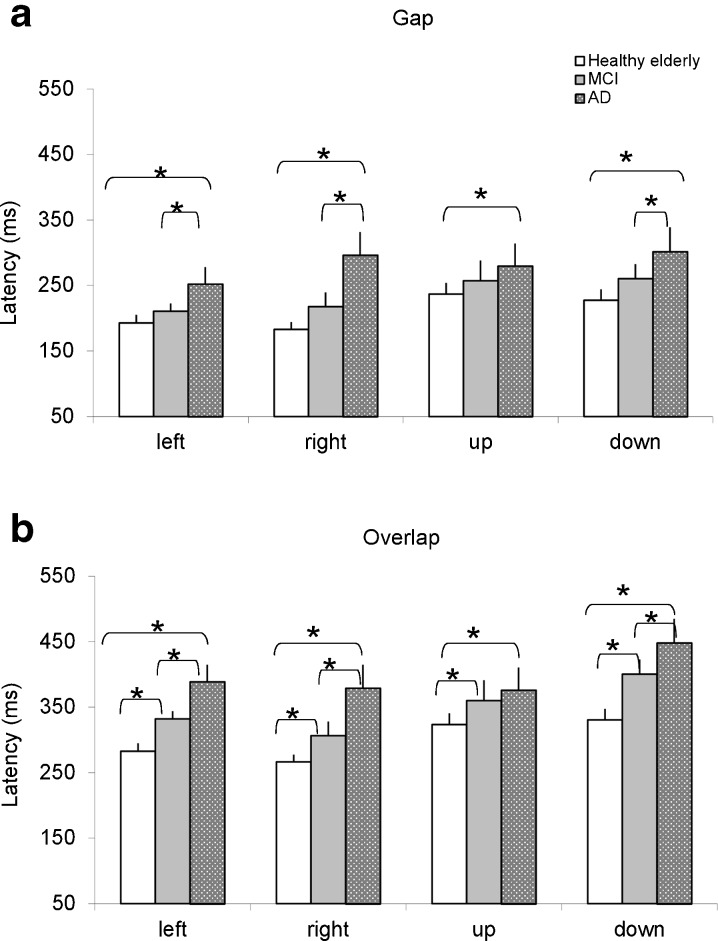
Figure 2 shows group mean latency with standard error for different directions (left, right, up, and down) in healthy elderly, aMCI, and AD patients for tasks gap (a) and overlap (b), respectively. The three-way ANOVA showed a gap effect, i.e., significantly shorter latencies for gap task than for overlap task (F1,70 = 107.7, p < 0.001) and a group effect, i.e., significantly different latencies among the three groups (F2,70 = 6.4, p < 0.001), but no direction effect (F3,210 = 0.8, p = 0.4). Further post hoc comparisons showed that the gap effect was significant for all three groups of subjects and for all directions (all p < 0.001). The mean values of gap effect were 88, 113, and 115 ms for healthy elderly, aMCI, and AD patients, respectively. For the gap task, AD patients had significantly longer latency than healthy elderly; relative to aMCI latencies in AD patients were longer, for all directions (all p < 0.05), except for upward saccades). For the overlap task, AD patients also showed significantly longer latency than healthy elderly for all directions and relative to aMCI patients for all directions (all p < 0.05, except for upward saccades); moreover, aMCI patients had significantly longer latency than healthy elderly for all directions (all p < 0.05).
Fig. 2.
Group mean latency of saccades with standard error for leftward, rightward, upward, and downward saccades in healthy elderly, MCI, and AD in conditions gap (a) and overlap (b, c); asterisks indicate significant difference (p < 0.05)
Correlation between mean latency and MMSE
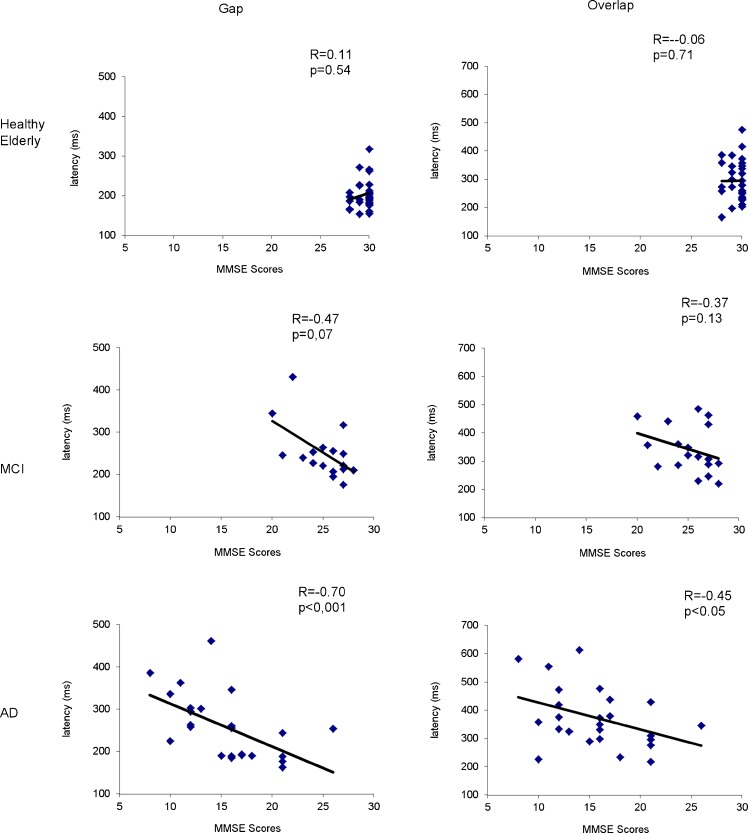
Figure 3 presents the correlation between MMSE scores and individual mean latency in the task gap (a) or overlap (b) for three groups of subjects, healthy elderly, aMCI, and AD. The Bravais–Pearson correlation coefficient was highly significant (p < 0.05 for both gap and overlap tasks), i.e., the lower the MMSE, the longer the latency of saccades is, for AD patients. However, such correlation does not exist for groups of healthy elderly or aMCI patients (all p > 0.05).
Fig. 3.
Correlation between MMSE scores and latencies of saccades in conditions gap and overlap for three groups of subjects, healthy elderly, aMCI, and AD; p < 0.05 indicates significant correlation, i.e., the lower the MMSE scores, the longer latencies of saccades are
Coefficient of variation in saccade latency
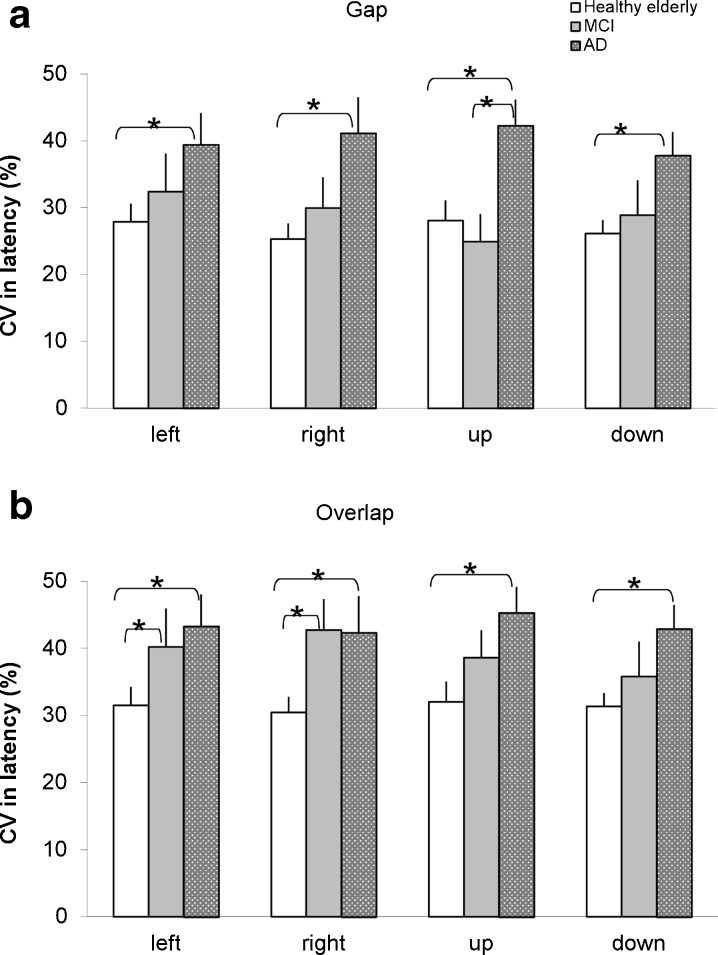
Figure 4 shows group mean CV in latency for the four directions under the gap (a) and the overlap (b) tasks for healthy elderly, for aMCI and for AD in individuals. The percentage of variability was relatively high (>25 %) for all groups. The Kruskal–Wallis test showed significant group effect on the CV in latency for both tasks (all p < 0.05). Further Mann–Whitney U test showed higher CV in latency for AD patients than for healthy elderly for all directions and both tasks (all p < 0.05) and also higher CV in latency for AD patients than for aMCI patients for some conditions (for upward saccades in the gap task, for leftward and rightward saccades in the overlap task, p < 0.05).
Fig. 4.
Group mean coefficient of variation (CV) in saccade latency for both horizontal and vertical saccades in healthy elderly, MCI, and AD patients under conditions gap (a) and overlap (b). Asterisks indicate significant difference (p < 0.05)
Accuracy and velocity
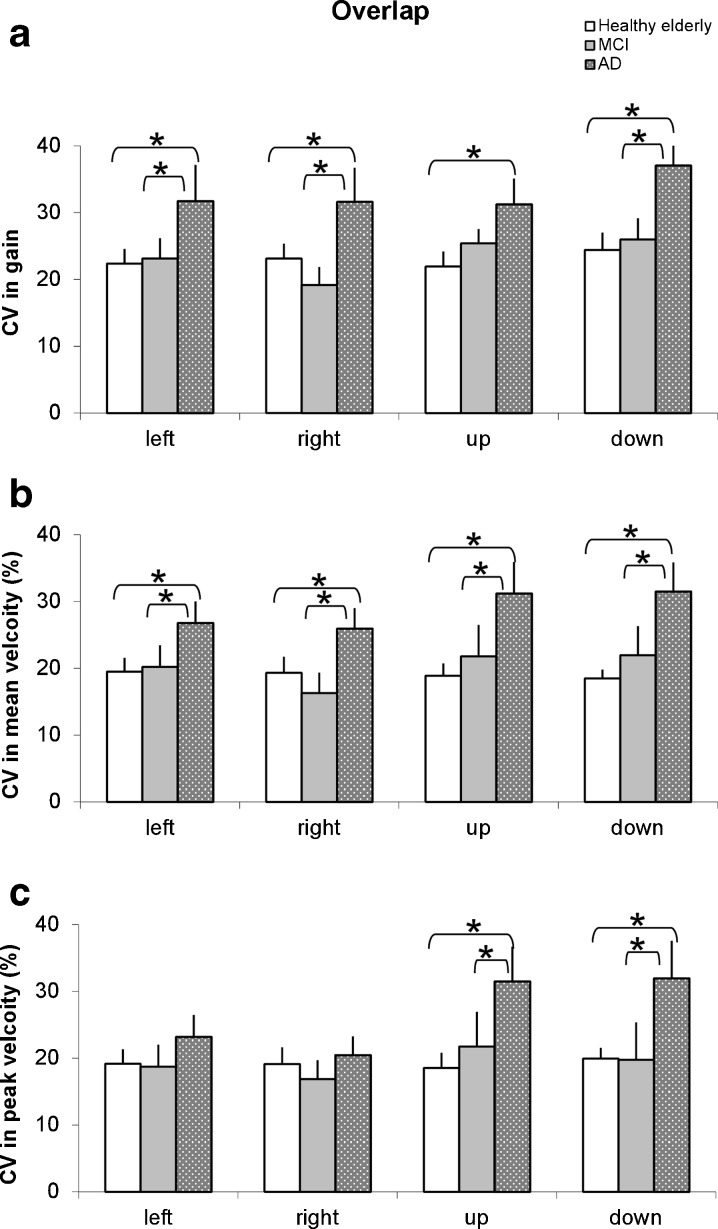
Accuracy and velocity were analyzed for both tasks and for the three groups of subjects. There was no significantly difference for the accuracy (gain) and the velocity (both mean and peak velocity) among three groups of subjects. However, the CV of these parameters was significantly different for different groups of subjects but in the overlap task only. Figure 5 shows the CV in gain (a), the CV in mean velocity (b), and CV in peak velocity (c) for all directions in healthy elderly, aMCI, and AD subjects. For the CV in gain, AD patients showed significantly higher values than healthy elderly (all p < 0.05 for all directions); they also showed significantly higher values than aMCI patients (for all directions all p < 0.05 except for upward saccades). For the CV in mean velocity, AD patients showed significantly higher values than both healthy elderly and aMCI patients for all directions (all p < 0.05). For the CV in peak velocity, AD patients showed significantly higher values than both healthy elderly and aMCI patients for vertical saccades only (all p < 0.05).
Fig. 5.
Group mean coefficient of variation (CV) in saccade gain (a), mean velocity (b), and peak velocity (c) for both horizontal and vertical saccades in healthy elderly, MCI, and AD patients under the overlap condition. Asterisks indicate significant difference (p < 0.05)
Discussion
The main results are: (1) Robust gap effect (shorter latencies in gap than in overlap) exists for AD and MCI patients as for healthy elderly; (2) abnormal long latency of saccades in gap and overlap tasks for AD relative to healthy elderly and MCI patients; (3) longer latency for MCI patients than for healthy elderly for the overlap task; (4) significant correlation between MMSE scores and latencies of saccades considering AD patients only; (5) higher CV in latency for AD patients than for healthy elderly and for MCI patients; (6) variability of accuracy and of speed is abnormally higher in AD patients than in MCI and healthy elderly. These results will be discussed below.
Gap effect for all groups
The gap effect was strong for all groups, even stronger for patients. Our observation of a gap effect (longer latency in the overlap task than in the gap task) in AD patients is compatible with the study of Abel et al. (2002). They reported that AD patients had a gap effect of similar magnitude to normal subjects while, in our study, such gap effect is much longer for AD patients (115 ms) and for aMCI patients (113 ms) than for healthy elderly (88 ms). Perhaps this is related to the increase of latency in the overlap task relative to controls; the increase of saccadic latency in AD patients and in aMCI patients is about 70 ms and 26 ms in the gap task while it goes up to 100 ms and 52 ms in the overlap task, respectively. Moreover, our study shows such strong gap effect for both horizontal and vertical saccades.
To summarize, our study shows for the first time a gap effect in AD patient for all four directions and of higher magnitude than for controls. This is attributed to more severe deficit of the saccade triggering mechanism in the overlap task, presumably activating an extended cortical circuit including FEF (Kapoula et al. 2010; Vernet et al. 2009). It is interesting that aMCI patients show the same behavior, e.g., stronger gap effect than controls, similarly to AD patients. aMCI patients seem to have more problems with the overlap task. This is clearly seen in the results discussed below, showing a significant increase of latency relative to controls only for the overlap task only. Such differential deficit can increase the difference between gap and overlap latencies (the gap effect).
Global but not uniform latency abnormality in AD patients
The present results concerning saccades are compatible with some previous studies (Garbutt et al. 2008; Moser et al. 1995; Shafiq-Antonacci et al. 2003) that reported longer latencies of saccades in AD patients than healthy elderly. Many studies demonstrated that elderly subjects commonly showed an increase in saccade reaction time (Munoz et al. 1998; Sharpe and Zackon 1987; Warabi et al. 1984; Yang et al. 2006). According to Pitt and Rawles (1988), saccadic latencies were estimated to increase with age by 0.76 % per year in normal controls. Neural events that determine saccadic latency include visual processing, decision making, and sensory-to-oculomotor transformation of signals. Normal visual acuity (Schlotterer et al. 1984), normal spatial frequency contrast sensitivity (Schlotterer et al. 1984), and normal early components of the pattern reversal visual evoked response (Wright et al. 1984) in AD patients, suggesting functional integrity of the primary visual patterns. However, dysfunction of the frontal lobe (Boxer et al. 2006b) of temporoparietal cortex (Whitwell et al. 2010) had been noted in AD patients. Moreover, parietal lobe lesions cause latency prolongation of saccades (Pierrot-Deseilligny et al. 1991). Area 7 of the parietal lobe is especially vulnerable to degeneration in AD patients (Brun and Englund 1981). Therefore, degeneration of the posterior parietal cortex and/or frontal lobe may explain the prolongation of saccadic latency in AD patients.
It is important to note that increase of latency occurred for all four directions and for both tasks. This is compatible with known physiology for a common cortical circuit controlling saccades triggering in all directions. Yet, it is also important to note that the increase relative to controls was not uniform for the two tasks. It was higher for the overlap task. As mentioned above, this can be attributed to higher cortical complexity of saccades in such task involving both parietal and frontal oculomotor areas (Kapoula et al. 2010; Vernet et al. 2009).
Specific latency deficits in aMCI
Note that aMCI patients showed longer latency for both horizontal and vertical saccades than for healthy elderly, but in the overlap condition only. This is different from our previous preliminary study (Yang et al. 2011) that was limited to few subjects and to horizontal saccades only. Here, with more complex tasks inducing horizontal and vertical saccades, we show latency abnormality but for the overlap task only. Presumably, in such task, aMCI patients had more difficulty in shifting their attention and making the decision.
To our knowledge, such a specific saccade latency effect is reported in aMCI at the first time. This observation has clinical relevance for early diagnosis and follow-up of such patients. The overlap task is more demanding in terms of cortical resources and thus more sensitive even to moderate cognitive impairment.
Correlation between latencies of saccades and MMSE scores
Our results showing the significant correlation between MMSE scores and latency of saccades in both gap and overlap tasks for the group of patients with AD are compatible with a few other studies (Bylsma et al. 1995; Pirozzolo and Hansch 1981). Bylsma et al. (1995) found such latency correlations with changes in MMSE existing over test sessions (including follow-up investigation). Our data offer strong evidence here for a direct relationship between cognitive measures and simple reaction time of saccades involving a higher cortical regulatory role in sensory–motor integration. The sensitivity of simple saccade latency to presumed neuro-patho-physiological status supports the hypothesis that saccade latency performance accesses a cardinal function dimension of the central nervous system. For example, Garbutt et al. (2008) also found that the Alzheimer’s disease patients had smaller parietal and occipital lobe volumes than the controls, and correlations were identified with the latency of visually guided saccades but not with the latency of anti-saccades. Visually guided saccades are believed to be controlled predominately by the parietal cortex (Kapoula et al. 2004; Pierrot-Deseilligny et al. 1995; Yang and Kapoula 2004). Garbutt et al. (2008) suggested that saccade latency may be correlated with atrophy of visual cortical regions. In some other studies, no such correlation was found between MMSE scores and latency of saccades (Abel et al. 2002; Hershey et al. 1983). Perhaps this is due to their small group (11 patients with dementia) while Bylsma et al. (1995) examined 31 AD patients, and our study, 25 AD patients. Moreover, in our study, the mean MMSE score was lower (15.7) than in the study of Hershey or Bylsma cited above (19.2 and 20.55, respectively). In their review on antisaccades, Kaufman et al. (2010) pointed that most studies have focused on patients at the moderate to severe stages of AD, which may exaggerate differences between AD and controls and may strengthen the correlation between MMSE scores and error rates. Thus, further investigation with even more patients and with a more spread range of MMSE scores is required to confirm that a specific causal link exists between the cortical degenerative changes in Alzheimer’s disease and the disturbances of cognitive and psychomotor function.
Abnormal variability in the accuracy and the speed for AD or aMCI patients in overlap only
Recall that accuracy was evaluated by the mean gain (saccade amplitude/target eccentricity). Similarly to our previous study (Yang et al. 2011), AD patients showed normal accuracy and speed as healthy elderly and aMCI patients. This result suggests that the pontine burst units in charge of the speed of saccades are intact in both AD and aMCI. Even though the accuracy of saccades has its complex control involving cortical–subcortical and cerebellar areas (Leigh and Zee 2006), here, the mean group gain showed no significant difference between AD patients and aMCI or healthy subjects. Recall that AD patients were at mild or moderate stage of the disease. Perhaps, when only pathology of cortical areas is involved in AD patients, it does not drive to changes of their mean accuracy. However, in the present study, the variability of accuracy and speed was found to be higher in AD patients than in healthy elderly or in aMCI patients. It is important that such effects were present in the overlap task only. Normally, elderly subjects make many saccades to within 10 % of the amplitude of target execution (Van Gisbergen et al. 1981; Yang and Kapoula 2008). In this study, healthy elderly made saccades within about 15 %, a little higher than in previous study. Yet, the higher variability (30 %) in the overlap condition for AD patients suggests that their initial saccades were well out of the range of target execution. Such variability reflects the deficient motor error signal driving the saccade and could be due to the cerebral cortical degeneration of Alzheimer’s disease. For example, neurons in the frontal (Bizzi 1968) and parietal (Andersen and Mountcastle 1983) cortex encode eye position; and some neurons in the frontal eye fields probably encode motor errors (Goldberg and Bruce 1981). In addition, AD patients commonly have bilateral temporoparietal degeneration which can affect subject’s attention (Kim et al. 2007). As mentioned above, the neural circuit for controlling saccades in the overlap task seems to be more complex than that in the gap condition, involving more cortical areas.
In conclusion, the study highlights the importance of saccade tests in normal and pathological ageing, particularly the importance of gap–overlap tasks showing differential and specific deficits in AD versus aMCI patients. The study establishes a link between MMSE and saccade latency which has direct clinical significance. The use of saccades tasks in aMCI patients is a novel approach, and we hope it will stimulate further intensive research.
Acknowledgments
Experimental design and methods were conceived and developed by the IRIS group (Z Kapoula); support by the PICS CNRS (no: 4197). Case recruitment was supported by grant from China Ministry of Science and Technology (no: 2009BAI77B03).
Contributor Information
Qing Yang, Phone: +33-1-56095066, FAX: +33-1-560950 66, Email: yangqing165@hotmail.fr, Email: qing.yang@parisdescartes.fr.
Shifu Xiao, Email: xiaoshifu@msn.com.
Zoi Kapoula, Email: zoi.kapoula@egp.aphp.fr.
References
- Abel LA, Unverzagt F, Yee RD. Effects of stimulus predictability and interstimulus gap on saccades in Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;13(4):235–243. doi: 10.1159/000057702. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Mountcastle VB. The influence of the angle of gaze upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci. 1983;3(3):532–548. doi: 10.1523/JNEUROSCI.03-03-00532.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E. Discharge of frontal eye field neurons during saccadic and following eye movements in unanesthetized monkeys. Exp Brain Res. 1968;6(1):69–80. doi: 10.1007/BF00235447. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, Lisberger SG. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26(23):6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, Weiner MW, Rosen HJ. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology. 1981;5(5):549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Bylsma FW, Rasmusson DX, Rebok GW, Keyl PM, Tune L, Brandt J. Changes in visual fixation and saccadic eye movements in Alzheimer's disease. Int J Psychophysiol. 1995;19(1):33–40. doi: 10.1016/0167-8760(94)00060-R. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Higham S, Renvoize T, Patel J, Dale M, Suriya A, Tetley S. Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer's disease. Biol Psychiatry. 2005;57(9):1052–1060. doi: 10.1016/j.biopsych.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Pare M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17(21):8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behav Brain Sci. 1999;22(4):661–674. doi: 10.1017/s0140525x99002150. [DOI] [PubMed] [Google Scholar]
- Fischer B, Breitmeyer B. Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologia. 1987;25(1A):73–83. doi: 10.1016/0028-3932(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav Brain Sci. 1993;16:553–610. doi: 10.1017/S0140525X00031575. [DOI] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, Dean D, Kramer J, Neuhaus J, Miller BL, Lisberger SG, Boxer AL. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. 2008;131(Pt 5):1268–1281. doi: 10.1093/brain/awn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Frontal eye fields in the monkey: eye movements remap the effective coordinates of visual stimuli. Society for Neuroscience. 1981;7:131. [Google Scholar]
- Hershey LA, Whicker L, Jr, Abel LA, Dell'Osso LF, Traccis S, Grossniklaus D. Saccadic latency measurements in dementia. Arch Neurol. 1983;40(9):592–593. doi: 10.1001/archneur.1983.04050080092023. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57(6):787–795. doi: 10.3758/BF03206794. [DOI] [PubMed] [Google Scholar]
- Hutton JT, Nagel JA, Loewenson RB. Eye tracking dysfunction in Alzheimer-type dementia. Neurology. 1984;34(1):99–102. doi: 10.1212/WNL.34.1.99. [DOI] [PubMed] [Google Scholar]
- Isa T, Kobayashi Y. Switching between cortical and subcortical sensorimotor pathways. Prog Brain Res. 2004;143:299–305. doi: 10.1016/S0079-6123(03)43029-X. [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Isotalo E, Muri RM, Bucci MP, Rivaud-Pechoux S. Effects of transcranial magnetic stimulation of the posterior parietal cortex on saccades and vergence. Neuroreport. 2001;12(18):4041–4046. doi: 10.1097/00001756-200112210-00037. [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Yang Q, Coubard O, Daunys G, Orssaud C. Transcranial magnetic stimulation of the posterior parietal cortex delays the latency of both isolated and combined vergence-saccade movements in humans. Neurosci Lett. 2004;360(1–2):95–99. doi: 10.1016/j.neulet.2004.01.077. [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Yang Q, Vernet M, Dieudonne B, Greffard S, Verny M. Spread deficits in initiation, speed and accuracy of horizontal and vertical automatic saccades in dementia with Lewy bodies. Front Neurol. 2010;1:138. doi: 10.3389/fneur.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Zhang MY, Ouang Ya Q, Wang ZY, Liu WT, Yu E, Wong SC, Salmon DP, Grant I. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai Dementia Survey. J Clin Epidemiol. 1988;41(10):971–978. doi: 10.1016/0895-4356(88)90034-0. [DOI] [PubMed] [Google Scholar]
- Kaufman LD, Pratt J, Levine B, Black SE. Antisaccades: a probe into the dorsolateral prefrontal cortex in Alzheimer's disease. A critical review. J Alzheimers Dis. 2010;19(3):781–793. doi: 10.3233/JAD-2010-1275. [DOI] [PubMed] [Google Scholar]
- Kaufman LD, Pratt J, Levine B, Black SE. Executive deficits detected in mild Alzheimer's disease using the antisaccade task. Brain and Behavior. 2012;2(1):15–21. doi: 10.1002/brb3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Lee BH, Seo SW, Moon SY, Jung DS, Park KH, Heilman KM, Na DL. Attentional distractibility by optokinetic stimulation in Alzheimer disease. Neurology. 2007;69(11):1105–1112. doi: 10.1212/01.wnl.0000276956.65528.d4. [DOI] [PubMed] [Google Scholar]
- Kurkin S, Takeichi N, Akao T, Sato F, Fukushima J, Kaneko CR, Fukushima K. Neurons in the caudal frontal eye fields of monkeys signal three-dimensional tracking. Ann N Y Acad Sci. 2003;1004:262–270. doi: 10.1196/annals.1303.023. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movement. 4. New York: Oxford University Press; 2006. [Google Scholar]
- Moser A, Kompf D, Olschinka J. Eye movement dysfunction in dementia of the Alzheimer type. Dementia. 1995;6(5):264–268. doi: 10.1159/000106957. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121(4):391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Peltsch A, Hemraj A, Garcia A, Munoz DP (2009) Age-related trends in saccade characteristics among the elderly. Neurobiol Aging 32:669-79 [DOI] [PubMed]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114(Pt 3):1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI. Cortical control of saccades. Ann Neurol. 1995;37(5):557–567. doi: 10.1002/ana.410370504. [DOI] [PubMed] [Google Scholar]
- Pirozzolo FJ, Hansch EC. Oculomotor reaction time in dementia reflects degree of cerebral dysfunction. Science. 1981;214(4518):349–351. doi: 10.1126/science.7280699. [DOI] [PubMed] [Google Scholar]
- Pitt MC, Rawles JM. The effect of ageing on saccadic latency and velocity. Neuro-ophthalmology. 1988;8:123–129. doi: 10.3109/01658108808996031. [DOI] [Google Scholar]
- Reuter-Lorenz PA, Hughes HC, Fendrich R. The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Percept Psychophys. 1991;49(2):167–175. doi: 10.3758/BF03205036. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Vitu F. On the limited role of target onset in the gap task: support for the motor-preparation hypothesis. J Vis. 2007;7(10):1–20. doi: 10.1167/7.10.7. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am. 1967;57(8):1024–1029. doi: 10.1364/JOSA.57.001024. [DOI] [PubMed] [Google Scholar]
- Schlotterer G, Moscovitch M, Crapper-McLachlan D. Visual processing deficits as assessed by spatial frequency contrast sensitivity and backward masking in normal ageing and Alzheimer's disease. Brain. 1984;107(Pt 1):309–325. doi: 10.1093/brain/107.1.309. [DOI] [PubMed] [Google Scholar]
- Shafiq-Antonacci R, Maruff P, Masters C, Currie J. Spectrum of saccade system function in Alzheimer disease. Arch Neurol. 2003;60(9):1272–1278. doi: 10.1001/archneur.60.9.1272. [DOI] [PubMed] [Google Scholar]
- Sharpe JA, Zackon DH. Senescent saccades. Effects of aging on their accuracy, latency and velocity. Acta Otolaryngol. 1987;104(5–6):422–428. doi: 10.3109/00016488709128270. [DOI] [PubMed] [Google Scholar]
- Shepherd M, Findlay JM, Hockey RJ. The relationship between eye movements and spatial attention. Q J Exp Psychol A. 1986;38(3):475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- van Beers RJ. The sources of variability in saccadic eye movements. J Neurosci. 2007;27(33):8757–8770. doi: 10.1523/JNEUROSCI.2311-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gisbergen JA, Robinson DA, Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol. 1981;45(3):417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]
- Vernet M, Yang Q, Gruselle M, Trams M, Kapoula Z. Switching between gap and overlap pro-saccades: cost or benefit? Exp Brain Res. 2009;197(1):49–58. doi: 10.1007/s00221-009-1887-1. [DOI] [PubMed] [Google Scholar]
- Warabi T, Kase M, Kato T. Effect of aging on the accuracy of visually guided saccadic eye movement. Ann Neurol. 1984;16(4):449–454. doi: 10.1002/ana.410160405. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, Senjem ML, Knopman DS, Petersen RC, Dickson DW, Josephs KA. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010;75(21):879–887. doi: 10.1212/WNL.0b013e3181feb2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Harding GF, Orwin A. Presenile dementia—the use of the flash and pattern VEP in diagnosis. Electroencephalogr Clin Neurophysiol. 1984;57(5):405–415. doi: 10.1016/0013-4694(84)90069-5. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kapoula Z. TMS over the left posterior parietal cortex prolongs latency of contralateral saccades and convergence. Invest Ophthalmol Vis Sci. 2004;45(7):2231–2239. doi: 10.1167/iovs.03-1291. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kapoula Z. Aging does not affect the accuracy of vertical saccades nor the quality of their binocular coordination: a study of a special elderly group. Neurobiol Aging. 2008;29(4):622–638. doi: 10.1016/j.neurobiolaging.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kapoula Z, Debay E, Coubard O, Orssaud C, Samson M. Prolongation of latency of horizontal saccades in elderly is distance and task specific. Vision Res. 2006;46(5):751–759. doi: 10.1016/j.visres.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Yang Q, Wang T, Su N, Liu YY, Xiao SF, Kapoula Z. Long latency and high variability in accuracy-speed of prosaccades in Alzheimer's disease at mild to moderate stage. Dement Geriatr Cogn Disord Ext. 2011;1:318–329. doi: 10.1159/000333080. [DOI] [PMC free article] [PubMed] [Google Scholar]