Abstract
Melatonin and serotonin rhythms, which exhibit a close association with the endogenous circadian component of sleep, are attenuated with increasing age. This decrease seems to be linked to sleep alterations in the elderly. Chrononutrition is a field of chronobiology that establishes the principle of consuming foodstuffs at times of the day when they are more useful for health, improving, therefore, biorhythms and physical performance. Our aim was to analyze whether the consumption of cereals enriched with tryptophan, the precursor of both serotonin and melatonin, may help in the reconsolidation of the sleep/wake cycle and counteract depression and anxiety in 35 middle-aged/elderly (aged 55–75 year) volunteers in a simple blind assay. Data were collected for 3 weeks according to the following schedule: The control week participants consumed standard cereals (22.5 mg tryptophan in 30 g cereals per dose) at breakfast and dinner; for the treatment week, cereals enriched with a higher dose of tryptophan (60 mg tryptophan in 30 g cereals per dose) were eaten at both breakfast and dinner; the posttreatment week volunteers consumed their usual diet. Each participant wore a wrist actimeter that logged activity during the whole experiment. Urine was collected to analyze melatonin and serotonin urinary metabolites and to measure total antioxidant capacity. The consumption of cereals containing the higher dose in tryptophan increased sleep efficiency, actual sleep time, immobile time, and decreased total nocturnal activity, sleep fragmentation index, and sleep latency. Urinary 6-sulfatoxymelatonin, 5-hydroxyindoleacetic acid levels, and urinary total antioxidant capacity also increased respectively after tryptophan-enriched cereal ingestion as well as improving anxiety and depression symptoms. Cereals enriched with tryptophan may be useful as a chrononutrition tool for alterations in the sleep/wake cycle due to age.
Keywords: Chrononutrition, Actigraphy, Elderly, Tryptophan, Serotonin, Melatonin
Introduction
Aging is a very complex process that is related to circadian rhythm disruption and produces sleep disturbances (Huang et al. 2002) and other physiological or psychological dysfunctions (Barnard and Nolan 2008) including impaired nutrient absorption (Meier and Sturm 2009; Woudstra and Thomson 2002), immunosenescence (Canizzo et al. 2011; Hutt et al. 2010; Ortega et al. 2000), decrease in hormone levels (Ferrari and Magri 2008), or neuronal death (Shankar 2010; Qureshi and Parvez 2007). Adequate daily sleep is an important part of a healthy and productive lifestyle. In fact, it is well-known that sleep characteristics are involved in maintaining a good health and quality of life (Ancoli-Israel et al. 1997; Tsou 2011). Sleep disorders can result in diverse consequences including deleterious effects on immune system, psychiatric diseases and symptoms, or even increased risk of coronary diseases and diabetes mellitus type 2 among others. In this way, sleep disruptions leads to deterioration in the quality of life (Faraut et al. 2011; Tsou 2011; Asplund 2000). On the other hand, the consequences of numerous disorders are directly linked to sleep. These include obstructive sleep apnea (Russell and Duntley 2011), untreated diabetes (Kachi et al. 2012), obesity (Yiengprugsawan et al. 2012), dementia, and Alzheimer or Parkinson's diseases, among others (Mondragón-Rezola et al. 2010; Witting et al. 1990).
About 30 % of people over 50 years suffer from sleep problems (Zhdanova et al. 2001). This is partially attributed to age-related shorter amplitude and advance in phase of circadian rhythms (Van Someren 2000; Dijk et al. 2000). In many cases, circadian rhythm disruption is associated with medication due to its stimulating or sedating effects (Velayos 2009; Ironside et al. 2010; Ancoli-Israel et al. 1997).
Oldest groups are especially prone to impaired sleep (Huang et al. 2002). Nocturnal sleep is an active physiologic process that seems to contribute to the neutralization of free radicals through immune system (Paredes et al. 2009a), consolidates memory (Maquet et al. 2010), and reconstitutes body tissues (Reiter et al. 2007). Many hormones and neurotransmitters are involved in sleep regulation (Sánchez et al. 2010; Kotronoulas et al. 2009) being of importance the indole melatonin and the neurotransmitter serotonin (Hajak et al. 1991; Silber and Schmitt 2009). Since both serotonin and melatonin circulating levels decrease with increasing age, the occurrence of sleep problems in the elderly may be a consequence of the age-related impairment in the synthesis and secretion of both the neurotransmitter and the indole (Zhdanova et al. 2001).
Tryptophan is an essential amino acid that humans have to obtain from the diet (Yao et al. 2011; Sarris and Byrne 2011). This amino acid is the precursor of serotonin and melatonin and participates in the regulation of circadian rhythms (Hajak et al. 1991; Garau et al. 2006; Paredes et al. 2009b; Franco et al. 2012). It has been reported that age wanes the transport of tryptophan across the blood–brain barrier (Tang and Melethil 1995; Porter et al. 2005). Also, tryptophan hydroxylase, which catalyzes the rate-limiting step in the biogenesis of both serotonin and melatonin in cells, declines with aging due to oxidation by reactive oxygen species and alterations in the phosphorylation cascade that modulates enzyme activity (Hussain and Mitra 2004).
The serotonergic system is implicated in sleep, mood, and cognition. It has been shown that age also provokes changes in the synthesis and secretion of serotonin. Dysfunctions in the pathways where this neurotransmitter is involved can produce depression, anxiety disorders, cognitive dysfunctions, bipolar disorders, and obsessive–compulsive disorders (Cubero et al. 2011; Mendelsohn et al. 2009; Markus et al. 2005).
The indole melatonin is mainly produced during dark hours by the pineal gland from serotonin and entrains circadian rhythms (Bubenik and Konturek 2011). Specialized photoreceptive cells in the retina detect light (Jung-Hynes et al. 2010) and this information goes directly to the suprachiasmatic nuclei through the retinohypothalamic tract and indirectly through the geniculohypothalamic tract (Sánchez et al. 2008c). N-acetyltransferase, which constitutes the limiting step in melatonin synthesis in the pineal gland, produces the indole only during darkness (Sánchez et al. 2008b). Studies have reported that sleep/wake circadian rhythm disruption due to aging is related to a decline in melatonin levels, causing negative effects on health (Garrido et al. 2010) and mood (Lam 2008).
Depression and anxiety disorders are pointed as consequences, among many others, of circadian rhythm disturbances in the elderly (Most et al. 2010). Sleep restriction and sleep fragmentation can be produced by work, lifestyle, or medical conditions which have been reported to produce attention problems, fatigue, and/or mood disorders (Reynolds and Banks 2010).
During aging, cellular functions decline due to free radical action on macromolecules. In this way, free radicals and oxidative stress have been recognized as important causes involved in aging and several physiological dysfunctions related to age (Meydani 2001; Mammucari and Rizzuto 2010). It is well-known that diet with high levels of antioxidants may enhance life quality (Reiter et al. 2005; Rizwan et al. 2011)
In previous reports, we showed that tryptophan administration was able to reverse the age-related changes in the circulating levels of melatonin and serotonin in both mammals and ringdoves (Aparicio et al. 2007; Paredes et al. 2007; Sánchez et al. 2008a, b). Also, we reported that diets enriched with tryptophan itself or with foodstuffs containing high levels of this amino acid were able to both consolidate sleep in newborns (Cubero et al. 2005, 2007) and improve sleep and antioxidant capacity levels in young, middle-aged, and elderly individuals (Garrido et al. 2009, 2010). Thus, the aim of the present work was to evaluate the effect of tryptophan-enriched cereals on sleep, serotonin, melatonin, total antioxidant capacity levels, and mood in elderly humans.
Materials and methods
Participants and experimental design
The study was carried out in 35 elderly volunteers aged 55–75 years (26 females and 9 males) with normal weight who suffered from sleep difficulties, mainly sleep onset and sleep fragmentation problems; all participants were Caucasian. Before entering the assessment, all subjects were physically and psychologically screened by the doctor of the assay. The following criteria were used for participant selection: they were healthy, had right clinical analysis (only some of them suffered from hypertension), nonsmokers, not alcohol or drug abusers, their sleep onset took more than 1 h, or they experienced more than three nocturnal awakenings. None of them reported sleep pathologies or depression during the assay. All subjects gave written informed consent to participate in the study (Table 1).
Table 1.
Characteristics of participants
| Age | 62 ± 0.82 years |
| Education | 13.96 ± 0.71 years |
| BMI | 25.45 ± 0.97 |
| High blood pressure | 17 % |
Age and education are expressed in years (mean ± SEM). High blood pressure was the only comorbid disease present in this clinical assay
BMI body mass index
The participants stayed in their principal homes during the assay. They were given instructions to have the most similar environments and the best possible sleep hygiene conditions including temperature (17–20°C), humidity (50–70 %), lightning, and routine before going to sleep.
This study was approved by the Ethical Committee of the University of Extremadura (Badajoz, Spain) in accordance with the Declaration of Helsinki, the Council of Europe, and the Universal Declaration of UNESCO on Human Rights Biomedicine and Human Genome.
Diets
For the first week, every participant consumed control cereals (22.5 mg tryptophan in 30 g cereals per dose) at breakfast and dinner. The treatment week (second week) volunteers consumed 30 g of tryptophan-enriched cereals (containing 60 mg tryptophan) at breakfast and dinner. Finally, there was a posttreatment week (third week) with no cereals, in which volunteers consumed their habitual diet.
Control cereals were the commercial formula Blevit Plus 8 cereales© with a content of 75 mg tryptophan/100 g cereals. Tryptophan-enriched cereals were Blevit Plus 8 cereales© modified with a content of 200 mg tryptophan/100 g cereals. Cereals were produced and manufactured by ORDESA S.L. Laboratories (Barcelona, Spain).
Measurement of sleep
Activity data were collected by a wrist actimeter (Actiwatch©, Cambridge Neurotechnology Ltd, UK) which participants wore in their nondominant hand during the 3 weeks of the trial. The actimetry data were analyzed with the Sleep Analysis 5© v.5.48 (Cambridge Neurotechnology Ltd, UK) software to obtain the following parameters: time in bed, assumed sleep (difference between sleep onset and the final awakening), actual sleep time (assumed sleep minus awake time), sleep latency (time period measured from going to bed until the onset of sleep), sleep efficiency (sleep percentage while the volunteer is in bed), number of awakenings (number of high activity episodes during sleep), immobile time (minutes when mobility is zero), total activity (total activity pulses during sleep), and fragmentation index (indicator value of quality of rest).
Urine sample collection
First-void (07:00 am) and 21:00 pm urines were collected on days 1 and 7 of the treatment week. Control and posttreatment week samples were collected only on day 7 at the same hours as before. Urine was stored at −20°C in Eppendorf vials until use.
6-Sulfatoxymelatonin, 5-hydroxyindoleacetic acid, and total antioxidant capacity measurements
Measurements of melatonin and serotonin were carried out through their excretion metabolites: 6-sulfatoxymelatonin (aMT6s) and 5-hydroxyindoleacetic acid (5-HIAA), respectively. Both aMT6s and 5-HIAA were measured with commercial enzyme-linked immunosorbent DRG© kits. To adjust for variation in the dilution of urine, aMT6s and 5-HIAA were expressed as urinary aMT6s/urine creatinine ratio; creatinine concentration was determined by means of the Jaffe test, as described elsewhere (Garrido et al. 2010). These analyses were carried out at 10:00 a.m. Total antioxidant capacity was measured by a colorimetric assay kit (Cayman©). This assay relies on the ability of antioxidants in the sample to inhibit the oxidation of 2,2′-azino-di-[3-ethylbenzothiazoline sulfonate] (ABTS) to ABTS radical by metmyoglobin. The capacity of the antioxidants in the sample to prevent ABTS oxidation was compared with that of Trolox, a water-soluble tocopherol analog, and quantified as millimolar Trolox equivalents. All procedures were performed following their respective manufacturer's instructions. Microtiter plates were read in a reader (TECAN infinite M200©) with the software Tecan-i-control©.
Anxiety and depression measurements
Before the study and every week during the study, volunteers filled out State-Trait Anxiety Inventory (STAI) anxiety test and Beck Depression Inventory at 10:00 a.m. These tests have been widely validated and are recognized as appropriate methods for the measurement of anxiety and depression (Steer et al. 2000; Telles-Correia and Barbosa 2009). STAI total scores were related to a Spanish version (Spielberger et al. 2008).
Statistical analysis
Data are expressed as mean ± standard error and represented as fold-increase over control levels (expressed as 1). Statistical analysis was made with GraphPad prism© v5.02 using Kruskal–Wallis test; posttest was performed with Dunn's multiple comparison test.
Results
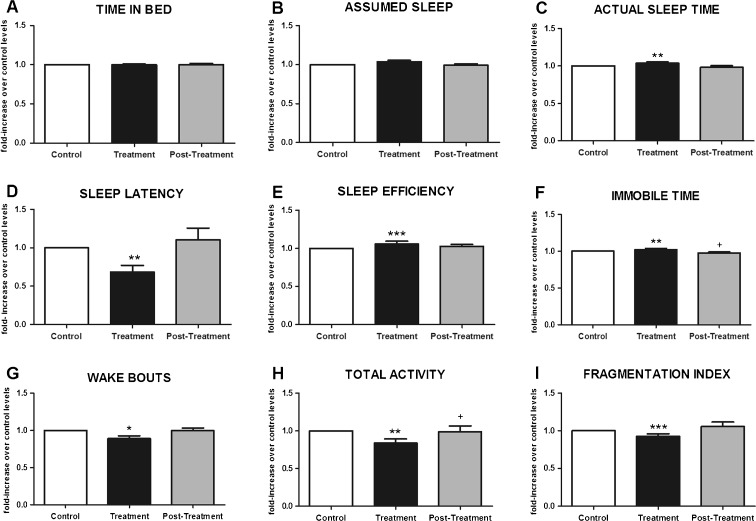
Figure 1 shows that after 1 week of tryptophan-enriched cereal ingestion (60 mg of tryptophan both in breakfast and dinner), most sleep parameters in elderly people improved as compared to both the control week (diet with standard cereals and 22.5 mg of tryptophan per dose) and the third week, in which volunteers had their habitual diet. In particular, an increase in the actual sleep time (Fig. 1c; p < 0.01), sleep efficiency (Fig. 1e; p < 0.001), and immobile time (Fig. 1f; p < 0.01) were obtained. In addition, sleep latency (Fig. 1d; p < 0.01), wake bouts (Fig. 1g; p < 0.05), total activity (Fig. 1h; p < 0.01), and fragmentation index (Fig. 1i; p < 0.001) were lower than control values. Values observed in the third week were similar to the control week values.
Fig. 1.
Effect of tryptophan-enriched cereal intake on time in bed (a), assumed sleep (b), actual sleep time (c), sleep latency (d), sleep efficiency (e), immobile time (f), number of wake bouts (g), total activity score (h), and fragmentation index (i). Results are expressed as fold-increase over control levels (normalized and expressed as 1). Each value represents the mean ± SEM. Control: first week (22.5 mg of tryptophan was consumed in breakfast and dinner); treatment: second week (60 mg of tryptophan was ingested in breakfast and dinner); posttreatment week: habitual diet. *p<0.05 control vs. treatment; **p < 0.01 control vs. treatment; ***p < 0.001 control vs. treatment; +p < 0.05 treatment vs. posttreatment; Kruskal–Wallis test and Dunn's multiple comparison test (posttest) were performed. N = 35
The results of the quantification of serotonin and melatonin through their respective urinary metabolites (5-HIAA and aMT6s) in elderly participants are shown in Table 2. At the end of the treatment week with tryptophan-enriched cereals, an increase in aMT6s (p < 0.05) and 5-HIAA (p < 0.05) was observed with respect to the control week and the third week, where volunteers consumed their habitual diet. With regard to antioxidant capacity, which was quantified in urine by means of Trolox equivalents, an increase was observed (p < 0.05) in its levels.
Table 2.
Effect of tryptophan-enriched cereal intake on 6-sulfatoxymelatonin (aMT6s), 5-hidroxyindoleacetic acid (5-HIAA), and total antioxidant capacity (TAC)
| Control | Treatment | Posttreatment | ||
|---|---|---|---|---|
| 1st day of treatment | 7th day of treatment | |||
| 6-MTS | 1 | 1.04 ± 0.11 | 1.22 ± 0.06* | 0.81 ± 0.10 |
| 5-HIAA | 1 | 1.46 ± 0.27 | 1.90 ± 0.31* | 1.26 ± 0.23 |
| TAC | 1 | 1.04 ± 0.02 | 1.06 ± 0.02* | 0.98 ± 0.02 |
Results are expressed as fold-increase over control levels (normalized and expressed as 1). Each value represents mean ± SEM. Control: first week (22.5 mg of tryptophan was consumed in breakfast and dinner); treatment: second week (60 mg of tryptophan was ingested in breakfast and dinner); posttreatment week: habitual diet
*p < 0.05 control vs. 7th day of treatment; Kruskal–Wallis test and Dunn's multiple comparison test (posttest) were performed; N = 35
After the participants ingested tryptophan-enriched cereals, a decrease was observed (p < 0.05) with respect to the control week in the state anxiety. However, there was no variation in trait anxiety (Table 3). In relation to depression, after 1 week of ingesting tryptophan-enriched cereals, there was a decrease (p < 0.05) at the Beck's depression test with respect the first week of the study (Table 3).
Table 3.
Influence of tryptophan-enriched cereal consumption on Beck Depression Inventory and STAI anxiety test
| Control | Treatment | Posttreatment | |
|---|---|---|---|
| Beck's test | 28.88 ± 1.40 | 24.24 ± 0.85* | 26.19 ± 1.62 |
| State anxiety | 30.13 ± 3.3 | 14.25 ± 2.8* | 26.35 ± 5.4 |
| Trait anxiety | 34.07 ± 5.2 | 29.39 ± 5.8 | 29.39 ± 5.8 |
Volunteers filled out tests on the last day of every experimental week at 10.00 a.m. Results are expressed related to a Spanish typification. Each value represents the mean ± SEM. Control: first week (22.5 mg of tryptophan was ingested in breakfast and dinner); treatment: second week: (60 mg of tryptophan was ingested in breakfast and dinner); posttreatment week: habitual diet
*p < 0.05 control vs. treatment; Kruskal–Wallis test and Dunn's multiple comparison test (posttest) were performed; N = 35
Discussion
It is well-known that quality and quantity of sleep in elderly people decrease (Gilliam 2009; Monjan 2010), due to a reduced amplitude of circadian rhythms (van Someren 2000). In many cases, such problems have been treated with melatonin (Sanchez-Barceló et al. 2010). In previous studies, we showed that tryptophan, the precursor of both serotonin and melatonin, was successful in improving sleep problems in both animals and humans (Cubero et al. 2007; Sánchez et al. 2008a, b; Paredes et al. 2009b). In particular, tryptophan-enriched diets increased the quantity and quality of sleep in newborns (aged 0–6 months) who suffered from sleep problems (more than three nocturnal awakenings in a given night). Our research group found that this tryptophan concentration was successful to improve sleep problems in other ages following a similar schedule (Cubero et al. 2009). For this reason, in the present work, it was decided to administer tryptophan-enriched cereals at breakfast and dinner to elderly people who suffered from sleep onset and sleep consolidation problems.
Actimetry is a noninvasive method in which trials were performed with a high sample with these kinds of assays and gives enough information to evaluate if a diet improves activity/inactivity circadian rhythm (Adamec et al. 2010; Ancoli-Israel et al. 2003). Wrist actimetry is a well-validated technique to study nocturnal sleep, as referenced elsewhere (Martin and Hakim 2011). This method has been used to determine disturbances in actual sleep time, sleep efficiency, sleep latency, wake bouts, and sleep fragmentation index in elderly people (Huang et al. 2002).
In the present work, it was demonstrated that the intake of tryptophan-enriched cereals produced beneficial effects on sleep/wake cycle. Most nocturnal sleep parameters analyzed improved in elderly people who suffered from sleep problems. Tryptophan has been shown to have direct effects on sleep regulation by increasing the availability of brain serotonin, which is involved in sleep latency (Sarris and Byrne 2011; Arnulf et al. 2002; Duncan and Congleton 2010). Melatonin, which is produced from serotonin, is more involved in sleep quality than in sleep onset (Castro-Silva et al. 2010; Bourne et al. 2008). Thus, the aforementioned improvement in sleep may be a consequence of increasing serotonin or melatonin levels since both amines are formed from tryptophan in the brain. Indirect evidence of this is shown by the increase in the urinary metabolites of both molecules obtained in the present work after consuming tryptophan-enriched cereals (Table 2). Other workers have reported similar results (González-Flores et al. 2012; Delgado et al. 2012). In fact, increasing melatonin or serotonin plasma levels through tryptophan administration is a well-documented phenomenon with positive effects against sleep disturbance (Sánchez et al. 2008a; Cubero et al. 2005). Additionally, a significant increase in urinary total antioxidant capacity was observed after the intake of tryptophan-enriched cereals. This could be due to the antioxidant role of the amino acid tryptophan, the neurotransmitter serotonin, and the indole melatonin as has been observed when diets containing foodstuffs with high content in the aforementioned molecules are consumed (Garrido et al. 2010).
Tryptophan availability and consequently serotonin synthesis participate in mood regulation (Mitchell et al. 2011). Many studies by means of acute tryptophan depletion have reported that deficiencies in tryptophan availability produce depressive symptoms (Tocker et al. 2010). Often these symptoms are also associated to circadian rhythm disturbances and anxiety (Soria and Urretavizcaya 2009). In our study, the STAI anxiety test and Beck's depression test showed that tryptophan-enriched cereals improved these aspects associated to sleep problems.
In summary, the present work indicates that the consumption of tryptophan-enriched cereals may be considered as a facilitating tool to improve sleep in the elderly. The findings also suggest that these kinds of diets affect positively mood in elderly populations.
Acknowledgments
This work has been supported by ORDESA S.L. Laboratories. S.D. Paredes was a beneficiary of a grant by Junta de Extremadura—Fondo Social Europeo (REI09009).
References
- Adamec O, Domingues A, Paiva T, Sanches JM. Statistical characterization of actigraphy data during sleep and wakefulness states. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2342–2345. doi: 10.1109/IEMBS.2010.5627774. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Poceta JS, Stepnowsky C, Martín J, Gehrman P. Identifiaction and treatment of sleep problems in the elderly. Sleep Med. 1997;1(1):3–17. doi: 10.1016/S1087-0792(97)90002-2. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Aparicio S, Garau C, Nicolau MC, Rivero M, Rial RV. Chrononutrition: use of dissociated day/night formulas to improve the development of the wake-sleep rhythms. Effects of tryptophan. Nutr Neurosci. 2007;10(3–4):137–143. doi: 10.1080/10284150701455916. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Quintin P, Alvarez J, Vigil L, Touitou Y, Lebre A, Bellenger A, Varoquaux O, Derenne J, Allilaire J, Benkelfat C, Leboyer M (2002) Mid-morning tryptophan depletiond elays REM sleep onset in healthy subjects. Neuropsychopharmacol 27(5):843–851 [DOI] [PubMed]
- Asplund R (2000) Sleep, health and visual impairment in the elderly. Arch Gerontol Geriatr 30(1):7–15 [DOI] [PubMed]
- Barnard AR, Nolan PM. When clocks go bad: neurobehavioural consequences of disrupted circadian timing. PloS Genet. 2008;4(5):e1000040. doi: 10.1371/journal.pgen.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomized trial. Crit Care. 2008;12(2):R52. doi: 10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik GA, Konturek SJ. Melatonin and aging: prospects for human treatment. J Physiol Pharmacol. 2011;62(1):13–19. [PubMed] [Google Scholar]
- Canizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L (2011) Oxidative stress, inflamm-aging and immunosenescence. J Proteome. doi:10.1016/j.jprot.2011.06.005 [DOI] [PubMed]
- Castro-Silva C, Bruin VM, Cunha GM, Nunes DM, Medeiros CA, Bruin PF. Melatonin improves sleep and reduces nitrite in the exhaled breath condensate in cystic fibrosis—a randomized, double-blind placebo-controlled study. J Pineal Res. 2010;48(1):65–71. doi: 10.1111/j.1600-079X.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- Cubero J, Valero V, Sánchez J, Rivero M, Parvez H, Rodríguez AB, Barriga C. The circadian rhythm of tryptophan in breast milk affects the rhythms of 6-sulfatoximelatonin and sleep in newborn. Neuroendocrinol Lett. 2005;26(6):657–661. [PubMed] [Google Scholar]
- Cubero J, Narciso D, Terrón MP, Rial R, Esteban S, Rivero M, Parvez H, Rodríguez AB, Barriga C. Chrononutrition applied to formula milks to consolidate infants’ sleep/wake cycle. Neuroendocrinol Lett. 2007;28(4):360–366. [PubMed] [Google Scholar]
- Cubero J, Chanclón B, Sánchez S, Rivero M, Rodríguez AB, Barriga C (2009) Improving the quality of infant sleep through the conclusion at supper of cereals enriched with tryptophan, adenosine-5′-phosphate, and uridine-5′-phosphate. Nutr Neurosci. doi:10.1179/147683009X423490 [DOI] [PubMed]
- Cubero J, Otalora BB, Bravo R, Sánchez CL, Franco L, Uguz AC, Rodríguez AB, Barriga C. Distribution of 5-HT receptors in the mammalian brain. Trends Cell Mol Biol. 2011;6:41–46. [Google Scholar]
- Delgado J, Terrón MP, Garrido M, Pariente JA, Barriga C, Rodríguez AB, Paredes SD (2012) A cherry nutraceutical modulates melatonin, serotonin, corticosterone, and total antioxidant capacity levels: effect on ageing and chronotype. J Appl Biomed. doi:10.2478/v10136-011-0016-1
- Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homesotasis to age-related changes in human sleep. Chronobiol Int. 2000;17(3):285–311. doi: 10.1081/CBI-100101049. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Congleton MR. Neural mechanisms mediating circadian phase resetting by activation of 5-HT7 receptors in the dorsal raphe: roles of GABAergic and glutamatergic neurotransmission. Brain Res. 2010;1366:110–119. doi: 10.1016/j.brainres.2010.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraut B, Boudjetia KZ, Vanhame L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2011;16(2):137–149. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Magri F. Role of neuroendocrine pathways in cognitive decline during aging. Ageing Res Rev. 2008;7:225–233. doi: 10.1016/j.arr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Franco L, Sánchez CL, Bravo R, Rodríguez AB, Barriga C, Cubero J (2012) The sedative effects of hops (Humulus lupulus), a component of beer, on the activity/rest rhythm. Acta Physiol Hung, in press [DOI] [PubMed]
- Garau C, Aparicio S, Rial RV, Nicolau MC, Esteban S. Age related changes in the activity-rest circadian rhythms and c-fos expression of ringdoves with aging. Effects of tryptophan intake. Exp Gerontol. 2006;41(4):430–438. doi: 10.1016/j.exger.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Garrido M, Espino J, González-Gómez D, Lozano M, Cubero J, Toribio-Delgado AF, Maynar-Mariño JI, Terrón MP, Muñoz JL, Pariente JA, Barriga C, Paredes SD, Rodríguez AB. A nutraceutical product base don Jerte Valley cherries improves sleep and augments the antioxidant status in humans. E-SPEN J. 2009;4:e321–e323. doi: 10.1016/j.eclnm.2009.09.003. [DOI] [Google Scholar]
- Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, Muñoz JL, Reiter RJ, Barriga C, Rodríguez AB. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J Gerontol A Biol Sci Med Sci. 2010;65(9):909–914. doi: 10.1093/gerona/glq099. [DOI] [PubMed] [Google Scholar]
- Gilliam T. Understanding primary insomnia in older people. Nurs Older People. 2009;21(3):30–33. doi: 10.7748/nop2009.04.21.3.30.c7014. [DOI] [PubMed] [Google Scholar]
- González-Flores D, Gamero E, Garrido M, Ramírez R, Moreno D, Delgado J, Valdés E, Barriga C, Rodríguez AB, Paredes SD. Urinary 6-sulfatoxymelatonin and total antioxidant capacity increase after the intake of a grape juice cv. Tempranillo stabilized with HHP. Food Funct. 2012;3(1):34–39. doi: 10.1039/c1fo10146c. [DOI] [PubMed] [Google Scholar]
- Hajak G, Huether G, Blanke J, Freyer B, Poeggeler P, Reimer A, Rodenbeck A, Schulz-Varszegi A, Ruether M. The influence of intravenous l-tryptophan on plasma melatonin and sleep in men. Pharmacopsychiatry. 1991;24(1):17–20. doi: 10.1055/s-2007-1014427. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu R, Wang Q, Van Someren EWJ, Xu H, Zhou J. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76:597–603. doi: 10.1016/S0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- Hussain AM, Mitra AK. Effect of reactive oxygen species on the metabolism of tryptophan in rat brain: influence of age. Mol Cell Biochem. 2004;258(1–2):145–153. doi: 10.1023/B:MCBI.0000012849.16750.00. [DOI] [PubMed] [Google Scholar]
- Hutt HJ, Bennerscheidt P, Thiel B, Arand M (2010) Immunosenescence and vaccinations in the elderly. Med Klin (MUnich) 105(11):802–7 [DOI] [PubMed]
- Ironside S, Davidson F, Corkum P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. J Sleep Res. 2010;19(4):546–541. doi: 10.1111/j.1365-2869.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- Jung-Hynes B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythm: building a bridge between aging and cancer. J Pineal Res. 2010;48(1):9–19. doi: 10.1111/j.1600-079X.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachi Y, Ohwaki K, Yano E. Association of sleep duration with untreated diabetes in Japanese men. Sleep Med. 2012;13(3):307–309. doi: 10.1016/j.sleep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Kotronoulas G, Stamatakis A, Stylianopoulou F. Hormones, hormonal agents, and neuropeptides involved in the neuroendocrine regulation of sleep in humans. Hormones. 2009;8(4):232–248. doi: 10.14310/horm.2002.1239. [DOI] [PubMed] [Google Scholar]
- Lam RW. Adressing circadian rhythm disturbances in depressed patients. J Psychopharmacol. 2008;22(7):13–18. doi: 10.1177/0269881108092591. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131:536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Matarazzo L, Foret A, Mascetti L, Bourdiec AS, Muto V. Contribution of sleep to learning and memory. Biol Aujourdhui. 2010;204(2):139–143. doi: 10.1051/jbio/2010008. [DOI] [PubMed] [Google Scholar]
- Markus CR, Jonkman LM, Lammers JHCM, Deut NEP, Messer MH, Nienke R. Evening intake of α-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am J Clin Nutr. 2005;81:1026–1033. doi: 10.1093/ajcn/81.5.1026. [DOI] [PubMed] [Google Scholar]
- Martin JL, Hakim AD (2011) Wrist Actigraphy. Chest 139(6):1514–1527 [DOI] [PMC free article] [PubMed]
- Meier J, Sturm A. The intestinal epithelial barrier: does it become impaired with age? Dig Dis. 2009;27(3):240–245. doi: 10.1159/000228556. [DOI] [PubMed] [Google Scholar]
- Mendelsohn D, Riedel WJ, Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev. 2009;33:926–952. doi: 10.1016/j.neubiorev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Meydani M (2001) Nutrition interventions in aging and age associated disease. Ann N Y Acad Sci 928:226–235 [DOI] [PubMed]
- Mitchell ES, Slettenaar M, Quadt F, Giesbrecht T, Kloek J, Gerhardt C, Bot A, Eliander A, Wiseman S. Effect of hydrolysed egg protein on brain tryptophan availability. Br J Nutr. 2011;105(4):611–617. doi: 10.1017/S0007114510004150. [DOI] [PubMed] [Google Scholar]
- Mondragón-Rezola E, Arratíbel-Echarren I, Ruiz-Martínez J, Martí-Massó JF. Sleep disorders in Parkinson’s disease: insomnia and sleep fragmentation, daytime hypersomnia, alterations to the circadian rhythm and sleep apnea syndrome. Rev Neurol. 2010;8(50):21–26. [PubMed] [Google Scholar]
- Monjan AA. Perspective on sleep and aging. Front Neurol. 2010;2010(1):124. doi: 10.3389/fneur.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most EIS, Scheltens P, Van Someren EJW. Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light—a randomized clinical trial. Trials. 2010;11:19. doi: 10.1186/1745-6215-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega E, García JJ, de la Fuente M. Ageing modulates some aspects of the non-specific immune response of murine macrophages and lymphocytes. Exp Physiol. 2000;85(5):519–525. doi: 10.1017/S0958067000020509. [DOI] [PubMed] [Google Scholar]
- Paredes SD, Terrón MP, Valero V, Cubero J, Barriga C, Reiter RJ, Rodríguez AB. Tryptophan increases nocturnal rest and affects melatonin and serotonin serum levels in old ringdove. Physiol Behav. 2007;90:576–582. doi: 10.1016/j.physbeh.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Paredes SD, Bejarano I, Terrón MP, Barriga C, Reiter RJ, Rodríguez AB. Melatonin and tryptophan counteract lipid peroxidation and modúlate superoxide dismutase activity in ringdove heterophils in vivo. Effect of antigen-induced activation and age. Age. 2009;31:179–188. doi: 10.1007/s11357-009-9107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes SD, Marchena AM, Bejarano I, Espino E, Barriga C, Vial RV, Reiter RJ, Rodríguez AB. Melatonin and tryptophan affect the activity-rest circadian rhythm, core and peripheral temperatures, and interleukin levels in the ringdove: changes with age. J Gerontol A Biol Sci Med Sci. 2009;3:340–350. doi: 10.1093/gerona/gln054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Mulder RT, Joyce PR, Luty SE. Trytophan and tyrosine availability and response to antidepressant in major depression. J Affect Disord. 2005;86:129–134. doi: 10.1016/j.jad.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Qureshi GA, Parvez SH. Oxidative stress and neurodegenerative disorders. Oxford: Elsevier; 2007. [Google Scholar]
- Reiter RJ, Manchester LC, Tan DX. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition. 2005;21(9):920–924. doi: 10.1016/j.nut.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Terrón MP, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor independent actions. Adv Med Sci. 2007;52:11–28. [PubMed] [Google Scholar]
- Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010;185:91–103. doi: 10.1016/B978-0-444-53702-7.00006-3. [DOI] [PubMed] [Google Scholar]
- Rizwan M, Rodríguez-Blanco I, Harbottle A, Birch-Machin MA, Watson RE, Rhodes LE. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164(1):154–162. doi: 10.1111/j.1365-2133.2010.10057.x. [DOI] [PubMed] [Google Scholar]
- Russell T, Duntley S. Sleep disordered breathing in the elderly. Am J Med. 2011;124(12):1123–1126. doi: 10.1016/j.amjmed.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Sánchez S, Sánchez CL, Paredes SD, Barriga C, Rodríguez AB. Circadian levels of serotonin in plasma and brain after oral administration of tryptophan in rats. Basic Clin Pharmacol. 2008;104:52–59. doi: 10.1111/j.1742-7843.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- Sánchez S, Sánchez CL, Paredes SD, Rodríguez AB, Barriga C. The effect of tryptophan administration on the circadian rhytms of melatonin in plasma and the pineal gland of rats. J Appl Biomed. 2008;6:177–186. [Google Scholar]
- Sánchez S, Sánchez C, Paredes SD, Cubero J, Rodríguez AB, Barriga C. Circadian variations of serotonin in plasma and different brain regions of rats. Mol Cell Biochem. 2008;317(1–2):105–111. doi: 10.1007/s11010-008-9836-z. [DOI] [PubMed] [Google Scholar]
- Sánchez CL, Franco L, Bravo R, Rubio C, Rodríguez AB, Barriga C, Cubero J. Cerveza y salud, beneficios en el sueño. Nutrición Comunitaria. 2010;16(3):160–163. doi: 10.1016/S1135-3074(10)70034-X. [DOI] [Google Scholar]
- Sanchez-Barceló EJ, Mediavilla MD, Tan DX, Reiter RJ. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17(19):2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- Sarris J, Byrne GJ. A systematic review of insomnia and complementary medicine. Sleep Med Rev. 2011;15:99–106. doi: 10.1016/j.smrv.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Shankar SK. Biology of aging brain. J Pathol MIcrobiol. 2010;53:595–604. doi: 10.4103/0377-4929.71995. [DOI] [PubMed] [Google Scholar]
- Silber BY, Schmitt JAJ (2009) Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev. doi:10.1016/j.neubiorev.2009.08.005 [DOI] [PubMed]
- Soria V, Urretavizcaya M. Circadian rhythms and depression. Actas Esp Psiquiatr. 2009;37(4):222–232. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI. Cuestionario de Ansiedad Estado/Rasgo. Madrid: TEA ediciones; 2008. [Google Scholar]
- Steer RA, Rissmiller DJ, Beck AT. Behav Res Ther. 2000;38:311–318. doi: 10.1016/S0005-7967(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Tang JP, Melethil S. Effect of aging on the kinetics of blood–brain barrier uptake of tryptophan in rats. Pharm Res. 1995;12(7):1085–1091. doi: 10.1023/A:1016283003747. [DOI] [PubMed] [Google Scholar]
- Telles-Correia D, Barbosa A. Anxiety and depression in medicine: models and measurement. Acta Med Port. 2009;22(1):89–98. [PubMed] [Google Scholar]
- Tocker L, Amar S, Bersudsky Y, Benjamin J, Klein E, Agam G. The biology of tryptophan depletion and mood disorders. Isr J Psychiatry Relat Sci. 2010;47(1):46–55. [PubMed] [Google Scholar]
- Tsou M. Association between sleep duration and health outcome in elderly Taiwanese. Int J Gerontol. 2011;5:200–205. doi: 10.1016/j.ijge.2011.09.020. [DOI] [Google Scholar]
- Van Someren EJW. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35:1229–1237. doi: 10.1016/S0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- Velayos JL. Medicina del Sueño. Enfoque disciplinario. Madrid: Médica Panamericana; 2009. [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiram M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- Woudstra T, Thomson ABR. Nutrient absorption and intestinal adaptation with ageing. Best Pract Res Clin Gastroenterol. 2002;16(1):1–15. doi: 10.1053/bega.2001.0262. [DOI] [PubMed] [Google Scholar]
- Yao K, Fang J, Yin YL, Feng ZM, Tang ZR, Wu G. Tryptophan metabolism in animals: important roles in nutrition and health. Front Biosci (Schol Ed) 2011;1(3):386–397. doi: 10.2741/s152. [DOI] [PubMed] [Google Scholar]
- Yiengprugsawan V, Banwell C, Seubsman SA, Sleigh AC. Short sleep and obesity in a large national cohort of Thai adults. BMJ Open. 2012;2(1):e000561. doi: 10.1136/bmjopen-2011-000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. 2001;86(10):4727–4730. doi: 10.1210/jc.86.10.4727. [DOI] [PubMed] [Google Scholar]