Abstract
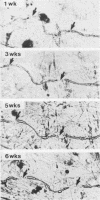
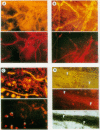
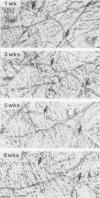
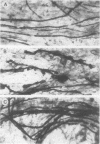
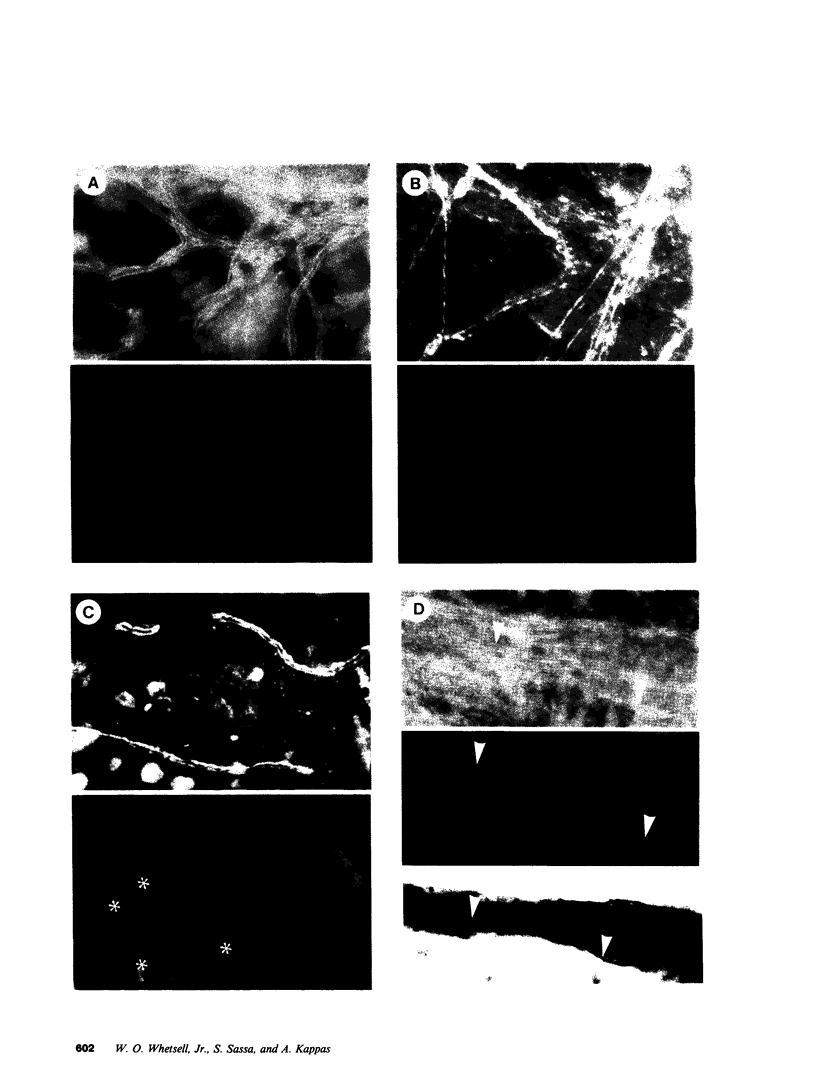
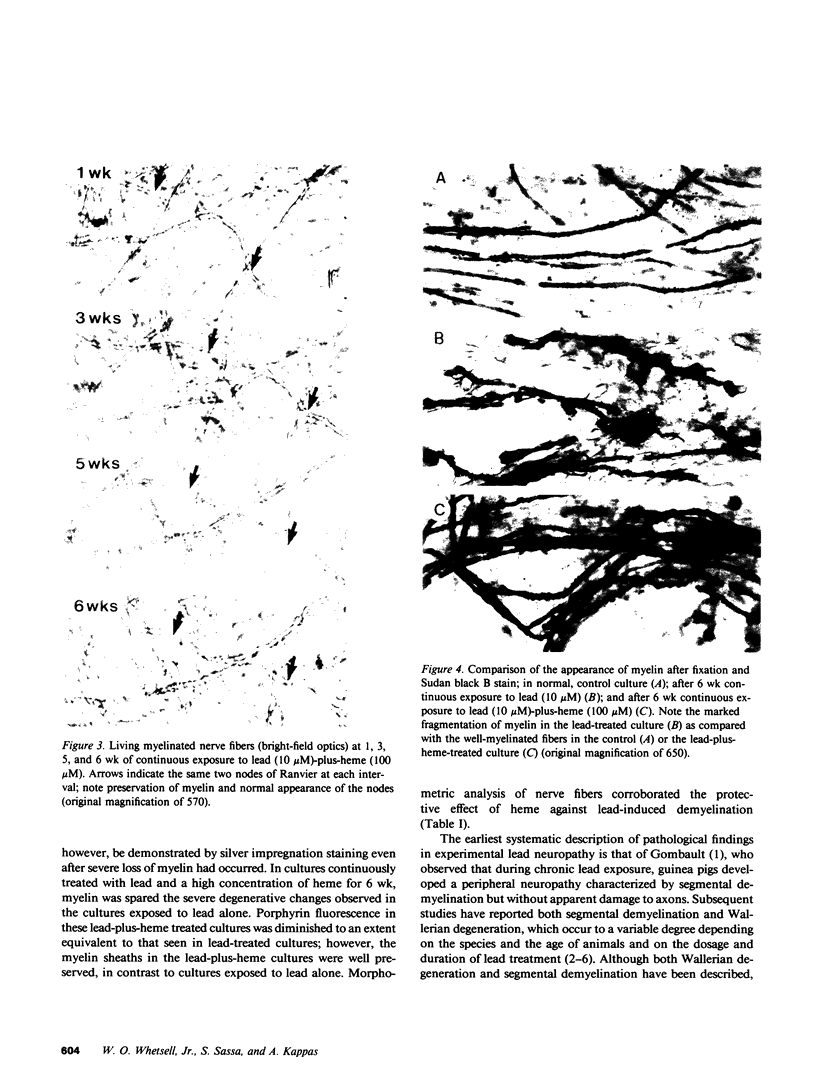
Well-myelinated cultures of mouse dorsal root ganglia incubated for 48 h with sigma-aminolevulinic acid (ALA) showed intense porphyrin fluorescence localized in myelin sheaths but not in axons or neuronal somata. When the cultures were continuously incubated with a high concentration of lead, focal swelling and segmental degeneration of myelin began to develop within 2 wk. Incubation of cultures with ALA after 3 wk of lead treatment revealed markedly decreased porphyrin fluorescence in myelin sheaths compared with untreated controls. After 6 wk of lead treatment, myelin showed severe segmental degeneration. Porphyrin fluorescence from ALA at this time was barely detectable in these cultures. No fluorescence was visible in the demyelinated axons; however, silver-impregnation staining after fixation demonstrated continuity of the axon despite the severe loss of myelin. When cultures were continuously incubated with lead and heme together for 6 wk, the segmental demyelination seen in cultures treated with lead alone did not occur. These findings suggest that the lead-induced segmental demyelination in cultured mouse dorsal root ganglia may be due to toxic effects of the metal on the heme biosynthetic pathway in myelinating cells and that exogenous heme may counteract this toxic effect of lead.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. J., Jr, Murnane M. J., Tonkonow B. L., Berman B. W., Mazur E. M., Cavallesco C., Jenko T., Snyder E. L., Forget B. G., Hoffman R. Embryonic-fetal erythroid characteristics of a human leukemic cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3509–3513. doi: 10.1073/pnas.77.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchet J. P., Roels H., Hubermont G., Lauwerys R. Effect of lead on some parameters of the heme biosynthetic pathway in rat tissues in vivo. Toxicology. 1976 Jun;6(1):21–34. doi: 10.1016/0300-483x(76)90004-4. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Peterson E. R., Murray M. R. A light and electron microscope study of long-term organized cultures of rat dorsal root ganglia. J Cell Biol. 1967 Feb;32(2):439–466. doi: 10.1083/jcb.32.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., London I. M. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981 Oct;26(1 Pt 1):117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- Chisolm J. J., Jr, Barrett M. B., Harrison H. V. Indicators of internal dose of lead in relation to derangement in heme synthesis. Johns Hopkins Med J. 1975 Jul;137(1):6–12. [PubMed] [Google Scholar]
- Ecob-Johnston M. S., Schwartz J., Elizan T. S., Whetsell W. O., Jr Herpes simplex virus types 1 and 2 in organotypic cultures of mouse central and peripheral nervous system. I. Light microscopic observations of myelin degeneration. J Neuropathol Exp Neurol. 1978 Sep;37(5):518–530. doi: 10.1097/00005072-197809000-00007. [DOI] [PubMed] [Google Scholar]
- Fullerton P. M. Chronic peripheral neuropathy produced by lead poisoning in guinea-pigs. J Neuropathol Exp Neurol. 1966 Apr;25(2):214–236. doi: 10.1097/00005072-196604000-00003. [DOI] [PubMed] [Google Scholar]
- Gibson S. L., Goldberg A. Defects in haem synthesis in mammalian tissues in experimental lead poisoning and experimental porphyria. Clin Sci. 1970 Jan;38(1):63–72. doi: 10.1042/cs0380063. [DOI] [PubMed] [Google Scholar]
- Granick J. L., Sassa S., Granick S., Levere R. D., Kappas A. Studies in lead poisoning. II. Correlation between the ratio of activated to inactivated delta-aminolevulinic acid dehydratase of whole blood and the blood lead level. Biochem Med. 1973 Aug;8(1):149–159. doi: 10.1016/0006-2944(73)90018-5. [DOI] [PubMed] [Google Scholar]
- Granick J. L., Sassa S. Hemin control of heme biosynthesis in mouse Friend virus-transformed erythroleukemia cells in culture. J Biol Chem. 1978 Aug 10;253(15):5402–5406. [PubMed] [Google Scholar]
- Ishii D. N., Maniatis G. M. Haemin promotes rapid neurite outgrowth in cultured mouse neuroblastoma cells. Nature. 1978 Jul 27;274(5669):372–374. doi: 10.1038/274372a0. [DOI] [PubMed] [Google Scholar]
- Klüver H. ON NATURALLY OCCURRING PORPHYRINS IN THE CENTRAL NERVOUS SYSTEM. Science. 1944 Jun 16;99(2581):482–484. doi: 10.1126/science.99.2581.482. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Schochet S. S., Jr Demyelination and remyelination in lead neuropathy. Electron microscopic studies. J Neuropathol Exp Neurol. 1968 Oct;27(4):527–545. [PubMed] [Google Scholar]
- Müller W. E., Snyder S. H. delta-Aminolevulinic acid: influences on synaptic GABA receptor binding may explain CNS symptoms of porphyria. Ann Neurol. 1977 Oct;2(4):340–342. doi: 10.1002/ana.410020415. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Bloom F. E. Heavy metals and adenosine cyclic 3',5'-monophosphate metabolism: possible relevance to heavy metal toxicity. Mol Pharmacol. 1976 May;12(3):390–398. [PubMed] [Google Scholar]
- Ohnishi A., Schilling K., Brimijoin W. S., Lambert E. H., Fairbanks V. F., Dyck P. J. Lead neuropathy. 1) Morphometry, nerve conduction, and choline acetyltransferase transport: new finding of endoneurial edema associated with segmental demyelination. J Neuropathol Exp Neurol. 1977 May;36(3):499–518. [PubMed] [Google Scholar]
- Porter P. N., Meints R. H., Mesner K. Enhancement of erythroid colony growth in culture by hemin. Exp Hematol. 1979 Jan;7(1):11–16. [PubMed] [Google Scholar]
- Sassa S., Granick J. L., Granick S., Kappas A., Levere R. D. Studies in lead poisoning. I. Microanalysis of erythrocyte protoporphyrin levels by spectrophotometry in the detection of chronic lead intoxication in the subclinical range. Biochem Med. 1973 Aug;8(1):135–148. doi: 10.1016/0006-2944(73)90017-3. [DOI] [PubMed] [Google Scholar]
- Sassa S., Whetsell W. O., Jr, Kappas A. Studies on porphyrin-heme biosynthesis in organotypic cultures of chick dorsal root ganglia. II. The effect of lead. Environ Res. 1979 Aug;19(2):415–426. doi: 10.1016/0013-9351(79)90066-5. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W. Experimental lead neuropathy: a disease of the supporting cells in the peripheral nervous system. J Neuropathol Exp Neurol. 1969 Jul;28(3):401–418. [PubMed] [Google Scholar]
- Silbergeld E. K., Adler H. S. Subcellular mechanisms of lead neurotoxicity. Brain Res. 1978 Jun 16;148(2):451–467. doi: 10.1016/0006-8993(78)90732-1. [DOI] [PubMed] [Google Scholar]
- Silbergeld E. K., Lamon J. M. Effects of altered porphyrin synthesis on brain neurochemistry. Neurobehav Toxicol Teratol. 1982 Nov-Dec;4(6):635–642. [PubMed] [Google Scholar]
- Verger C. Proliferation and morphology of chick embryo cells cultured in the presence of horse serum and hemoglobin. In Vitro. 1979 Aug;15(8):587–592. doi: 10.1007/BF02623394. [DOI] [PubMed] [Google Scholar]
- Verger C., Sassa S., Kappas A. Growth-promoting effects of iron- and cobalt- protoporphyrins on cultured embryonic cells. J Cell Physiol. 1983 Aug;116(2):135–141. doi: 10.1002/jcp.1041160203. [DOI] [PubMed] [Google Scholar]
- Whetsell W. O., Jr, Sassa S., Bickers D., Kappas A. Studies on porphyrin-heme biosynthesis in organotypic cultures of chick dorsal root ganglion. I. Observations on neuronal and non-neuronal elements. J Neuropathol Exp Neurol. 1978 Sep;37(5):497–507. doi: 10.1097/00005072-197809000-00005. [DOI] [PubMed] [Google Scholar]