Abstract
Throughout life, there is an aging of the immune system that causes impairment of its defense capability. Prevention or delay of this deterioration is considered crucial to maintain general health and increase longevity. We evaluated whether dietary supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 could enhance the immune response in the elderly. This multi-center, double-blind, and placebo controlled study enrolled 61 elderly volunteers who were randomly assigned to receive either placebo or probiotics. Each capsule of probiotics contained at least 3 × 107 L. delbrueckii subsp. bulgaricus 8481. Individuals in the study were administered three capsules per day for 6 months. Blood samples were obtained at baseline (time 0), end of month 3, and month 6. We characterized cell subpopulations, measured cytokines by flow cytometry, quantified T cell receptor excision circle (TREC) by real-time PCR (RT-PCR), and determined human β-defensin-2 (hBD-2) concentrations and human cytomegalovirus (CMV) titers by enzyme-linked immunosorbent assay (ELISA). Elderly responded to the intake of probiotic with an increase in the percentage of NK cells, an improvement in the parameters defining the immune risk profile (IRP), and an increase in the T cell subsets that are less differentiated. The probiotic group also showed decreased concentrations of the pro-inflammatory cytokine IL-8 but increased antimicrobial peptide hBD-2. These effects disappeared within 6 months of stopping the probiotic intake. Immunomodulation induced by L. delbrueckii subsp. bulgaricus 8481 could favor the maintenance of an adequate immune response, mainly by slowing the aging of the T cell subpopulations and increasing the number of immature T cells which are potential responders to new antigens.
Keywords: Immunosenescence, Immunomodulation, T lymphocytes, Differentiation, Lactobacillus delbrueckii
Introduction
Numerous organisms meet the criteria established by the World Health Organization (WHO) for a probiotic: “a live organism, which provides a benefit to the host when provided in adequate quantities.” Probiotics impact metabolism, endocrinology, proper gut development, and regulation of the immune system (Shida and Nanno 2008; Cani and Delzenne 2009). By modifying the microbial community within the gut, we may be able to prevent or treat gut disorders, such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) as well as systemic disorders like eczema, allergies, asthma, and diabetes (Lorea Baroja et al. 2007; Strowski and Wiedenmann 2009; Boyle et al. 2010). Focusing on effects on the immune system, probiotics act on a wide variety of cells in the intestine to modulate immune cells towards a pro- or anti-inflammatory action, depending on the bacterial strain, setting, measured immunological parameters, and types of modulated immune cells (Diaz-Ropero et al. 2007; O'Flaherty et al. 2010).
Lactobillus delbrueckii subsp. bulgaricus has been used to ferment milk for many centuries. Many researchers have shown various beneficial effects for this bacterial species (Kano et al. 2002; Medici et al. 2005). The immunopotentiating activity of lactobacilli varies considerably between strains but little information on the immunopotentiating activity for L. delbrueckii subsp. bulgaricus is available. It was isolated from a region of Bulgaria where its population shows unusual longevity. Since L. delbrueckii subsp. bulgaricus is one of the most common bacteria used in the production of fermented milk in the world, knowledge of its immunopotentiating activity would be useful for the development of dairy products with a more beneficial effect on human health. However, several authors who evaluated the ability of the yogurt bacteria L. delbrueckii subsp. bulgaricus to survive and proliferate in the human intestine found contradictory results (del Campo et al. 2005; Garcia-Albiach et al. 2008). Although live probiotics clearly modulate gut immune and barrier function, studies have shown immunomodulatory effects of probiotic DNA, suggesting that isolated probiotic bacteria DNA is as efficacious in attenuating intestinal inflammation as is treatment with live bacteria (Jijon et al. 2004).
Beyond midlife, the immune system begins to age and cause impairment of its defensive capability, which is known as immunosenescence. Immunosenescence involves multiple changes in both the innate and adaptive responses. Innate immunity seems to be better preserved globally (Le Garff-Tavernier et al. 2010), while the adaptive immune response exhibits profound age-dependent modifications (Haynes and Maue 2009). Elderly donors display a decline in numbers of naïve T cells in peripheral blood and lymphoid tissues (Fagnoni et al. 2000; Sauce et al. 2009); in contrast, they have a marked increase in the proportion of highly differentiated effector and memory T cells like the CD28null T cells (Goronzy et al. 2007; Czesnikiewicz-Guzik et al. 2008). An IRP was defined using a cluster analysis approach (Ferguson et al. 1995). In agreement with a higher IRP, a higher 2-year mortality occurred in a population of very old Swedish individuals who had an inverted CD4/CD8 ratio, an accumulation of CD8+CD28null T cells, and CMV infection (Hadrup et al. 2006). In summary, considerable evidence suggests a clear association between immune function and longevity of individuals (Moro-Garcia et al. 2011), and immunosenescence correlates higher morbidity and mortality. In this sense, prevention or delay of the deterioration of the immune system is considered crucial for maintaining a better overall health and increasing longevity.
L. delbrueckii subsp. bulgaricus 8481 was isolated from a region of Bulgaria known for the longevity of its population (Dixon 2002). The ability of the lactobacilli to resist adverse environmental conditions (Mater et al. 2005; Elli et al. 2006), together with its association with human longevity, led us to postulate the possible beneficial effects of this bacterial strain on the immune system. Thus, the purpose of this double-blind, placebo-controlled, and randomized study was to determine the potential beneficial properties of the selected strain of L. delbrueckii subsp. bulgaricus 8481 on the innate and acquired immune responses of elderly individuals.
Materials and methods
Study population and study design
Elderly volunteers were invited to enroll in this study via interview with their primary care physician and through the publication of a diptych and newspaper advertisement, from October 2008 to April 2011. Inclusion criteria were age greater than 65 years and treatment in the Health Centers of Barredos, Blimea, Laviana, and Sotrondio (Asturias, Spain). Exclusion criteria were conditions with possible influence on the immune system, such as recent or current infection, inflammation, autoimmune or malignant disease, malnutrition, abnormal laboratory data (hemoglobin <12 mg/dL, leukopenia <3,500 cells/μL, neutropenia <1,500 cells/μL, leukocytosis >15,000 cells/μL, platelets <105 cells/μL, and PCR >5 mg/dL), and pharmacological interference (steroids, non-steroidal anti-inflammatory agents, and immunosuppressive drugs). Informed consent was obtained from the elders prior to participation in the study. The study was approved by the Hospital Central de Asturias (Oviedo, Spain) ethics committee.
Patients who met the inclusion criteria were randomly assigned to one of two treatment groups: (1) placebo and (2) probiotics. Each capsule of probiotics contained at least 3 × 107 L. delbrueckii subsp. bulgaricus registered in the National Bank for Industrial Microorganisms and Cell Cultures (NBIMCC) under No. 8481 combined with a strain of Streptococcus thermophillus NBIMCC No. 8357 (European patent No. 2076139). A sample was taken for culture to ensure no pathogenic bacteria, yeasts, or fungus remained in the capsules. All volunteers assigned to the placebo group received capsules with cornstarch; the treatments were identical in appearance. The probiotic capsules were provided by the pharmacy Xalabarder following the necessary requirements for safe human consumption.
This was a multi-center, randomized, double-blind, and placebo-controlled study. Individuals in the study were administered three capsules per day (probiotic or placebo) for a period of 6 months. Peripheral blood samples were obtained from subjects by venipuncture at three time points: time 0 (baseline, immediately before the study began), at the end of month 3, and at the end of month 6. Blood samples were obtained at 6 months after stopping the capsules from nine volunteers who had taken the probiotic.
Throughout the study, the subjects' general health was assessed at each immune measurement time point via direct interview conducted by the health care provider. Volunteers were asked to confirm their compliance with, or deviation from, the dietary regimens.
Bacterial strains
This study included L. delbrueckii subsp. bulgaricus 8481 dairy strain isolated from the district of Stara Planina (Bulgaria) from a fresh cheese made of cow milk, fermented 5 days, with a subsequent selection. The bacterial culture was produced first in laboratory as follows: 1 g of the product was suspended in a 9-mL sterile physiological solution. Repeated dilutions were made, and a 107 dilution was sown onto a Man, Rogosa and Sharpe broth (MRS broth, LAB MTM, IDG Ltd., Lancashire, UK). Cultivation was carried out at 44–45°C for 48 h, and the strain was selected from the final single colonies. The selected strain was sown again in 10-mL sterile skimmed milk till coagulation at pH 4.8 for 6 h. From this culture, named “primary culture,” a series of subcultures were made with the aim to increase the number of cells of the pure strain and to prepare “mother cultures,” which were stored for later use as inoculants during the industrial preparation of bacterial culture of the strain L. delbrueckii subsp. bulgaricus 8481. For preparation of the starter culture, symbiotic culture of strain L. delbrueckii subsp. bulgaricus 8481 and strain Streptococcus thermophilus 8357 were repeatedly sown during 2.6 months until the symbiosis was stabilized. For preparation of dry symbiotic product, the coagulated milk with pH 4.9–5.0 was submitted to two steps of cooling: step one, coagulated milk at 25°C for 20–25 min and step two at 6–7°C for 15–20 min. Within 24 h, it was dried at −38°C to −40°C until the product reached liquor contents of 4–6 % by using the lyophilization method. The lyophilized encapsulated strains were kindly provided from LB Lactis (Scientific-Applied Laboratory for Starter Cultures and Probiotic Products, Plovidv, Bulgaria) culture collection.
Blood sampling and immunological phenotyping
The hematological parameters were determined by using a Sysmex XT-2000i (Syxmex, Hamburg-Norderstedt, Germany) and the biochemistry values in a Cobas 6000 analyser series (Roche Diagnostics, Indianapolis, USA). Cytometric studies were acquired and analyzed in the FACSCalibur Cytometer using CellQuest software (BD Biosciences, San José, CA, USA). CaliBRITE Beads (BD Biosciences) were used to adjust instrument settings, set fluorescence compensation, and check instrument sensitivity. Surface staining of EDTA peripheral blood was performed with Multiset CD3-FITC/CD16 + 56-PE/CD45-PerCP/CD19-APC Reagent, anti-CD4 (APC), anti-CD8 (PE), anti-CD8 (PerCP), anti-CD31 (PE) (Immunostep, Salamanca, Spain), anti-CD4 (PerCP), or anti-NKG2D (PE) (eBioscience, San Diego, CA, USA). One hundred μL of whole-blood from elderly were stained with different combinations of labeled monoclonal antibodies for 20 min at room temperature. Red blood cells in samples were lysed with FACS Lysing Solution (BD Biosciences), and samples were washed in PBS and analyzed with CellQuest software in the FACSCalibur Cytometer. Appropriate isotype control monoclonal antibodies (mAbs) were used for marker settings.
Isolation of CD4+ and CD8+ T cells
To analyze the differentiation status of CD4+ and CD8+ T cells, peripheral blood mononuclear cells (PBMC) were isolated by centrifugation on Ficoll-Hypaque gradients (Lymphoprep; Nycomed, Oslo, Norway). To achieve cell separation, the blood sample mixed with an equal part of phosphate buffered saline (Oxoid Limited, Hampshire, UK) was deposited on a fluid with a density of 1.077 g/mL (Lymphoprep) and separated by centrifugation (1,800 rpm, 30 min). CD4+ and CD8+ T-cells were isolated (Myltenyi Biotec GmbH, Bergisch Gladbach, Germany). Briefly, CD4+ or CD8+ T cells were magnetically labeled with CD4 or CD8 MicroBeads, and the cell suspension was loaded on a column which was placed in the magnetic field. The magnetically labeled cells were retained on the column. The unlabeled cells were not retained, and this cell fraction was depleted of CD4+ or CD8+ cells, respectively. After removal of the column from the magnetic field, the magnetically retained CD4+ or CD8+ cells were eluted as the positively selected cell fractions. Subsequently, cells were stained with anti-CD45RA (FITC) (Immunostep), anti-CCR7 (Alexa Fluor 647) (BD Bioscience), anti-CD27 (PE) (Immunostep), and anti-CD28 (PerCP) (BD Bioscience).
CMV serology
Immunoglobulin G (IgG) titers of CMV-specific antibodies were determined by enzyme-linked immunosorbent assay, Vir-ELISA Anti-CMV-IgG (Viro-Immun Labor-Diagnostika GmbH, Oberursel, Germany), according to the manufacturer's specifications. Patient samples were quantified and interpreted by calculating a ratio (Cutoff Index = OD value of sample/Cut-off value), whereby a ratio of 1.0 is equivalent to the cut-off value. Cutoff indexes >1.1 were considered positive, and the result of this ratio is a semi-quantitative titer.
TREC quantification
DNA from PBMC was extracted by using a QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. Quantification of signal-joint (sj) TREC was performed by using SYBR Green RT-PCR and an iCycler thermocycler (Bio-Rad; Life Science Research Group, Hercules, CA, USA). The primer sequences for TREC were the following: forward primer 5'-CCATGCTGACACCTCTGGTT-3', reverse primer 5'-TCGTGAGAACG GTGAATGAAG-3'. The Cα constant region, which was the internal control for quantity of input DNA because it remains present on TCR genes despite the rearrangement processes, was amplified in every sample tested (forward primer 5'-CCTGATCCTCTTGTCCCACAG-3', reverse primer 5'-GGATTT AGAGTCTCTCAGCTGGTACA-3'). Thermal cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Experimental samples were run in duplicate, and the replicate average value was presented as the sample result.
Immunoglobulin, complement, and cytokine quantification
IgG, IgA, IgM, and complement C3 and C4 were measured in the IMMAGE Immunochemistry System (Beckman Coulter, Minnesota, USA) at baseline (time = 0) and 6 months after the beginning of the probiotic/placebo intake. This fully automated random access immunochemistry system featured high-performance detection technologies which included near-infrared particle immunoassay system (NIPIA) that increased the analytical sensitivity nearly a thousand-fold.
Quantification of cytokines in serum was assessed by Human Th1/Th2 11plex Ready-to-Use Kit FlowCytomix (eBioscience), a fluorescent bead immunoassay. Each type of bead was coated with antibodies specifically reacting with one of the cytokines to be detected in the multiplex system. The beads can be differentiated by their sizes and by their distinct spectral profiles. Coated beads for each relevant cytokine were mixed, and the bead mixtures were incubated with the samples or mixture of standard cytokine concentrations. A biotin-conjugated second antibody mixture was added, and the specific antibodies bound to their respective cytokines captured by the first antibody. After addition of streptavidin–phycoerythrin molecules, they bound to the biotin conjugates and emitted fluorescent signals. This bead-based Analyte Detection System was used to quantitate human IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, TNF-α, and TNF-β by flow cytometry. Quantification of cytokines was performed at time 0 and 6 months.
hBD-2 ELISA
Blood was collected by venipuncture and allowed to clot. Serum was harvested by centrifugation at room temperature, and aliquots were stored at −80°C until use. The secretion of hBD-2 was monitored in serum of the elderly at baseline, 3 months, and 6 months. hBD-2 concentrations were determined by ELISA for Human Beta Defensin 2 (Alpha Diagnostic, San Antonio, TX, USA) according to the manufacturer's specifications. The results were calculated using an immunoassay software package with a four-parameter curve-fit.
Statistical analysis
Results are expressed as median and range, mean and standard deviation (SD), or in some graphs, mean and standard error of the mean (SEM), as indicated. Comparisons between groups were performed with the Wilcoxon non-parametric method when data were not normally distributed, or with Student's t test for paired samples. Analyses were performed using the SPSS 15.0 statistical software package program (SPSS Inc. Chicago, IL), and p-values of 0.05 or less were considered significant.
Results
Demographic and hematologic characteristics of the study population
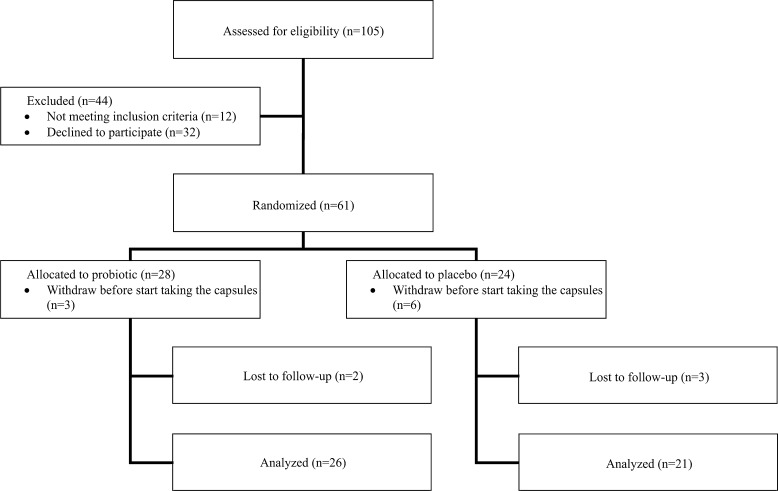
Recruitment took place in four centers in Spain from April 2008 to April 2011. Sixty one participants were in the randomized arm (24 allocated to placebo and 28 to probiotic) (Fig. 1). Of the 61 subjects initially enrolled in the study, 6 from the placebo group and 3 from the probiotic group decided to leave the study before beginning the intervention. Moreover, three elderly from the placebo group and two from the probiotic group were lost to follow up in the study, due to illness not associated with the study. The characteristics of the 47 individuals enrolled in the study are listed in Table 1. The female/male ratio was 4.3:1 in the placebo group and 7.7:1 in the probiotic group. No significant differences in age, sex, hematology values, and biochemistry values at baseline were observed between the groups (Table 1).
Fig. 1.
Flowchart of participants through trial
Table 1.
Characteristics of the study subjects at baseline
| Placebo group (n = 21) | Probiotic group (n = 26) | p value between groupsa | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | |||
| Mean ± SE | 69.5 ± 9.19 | 71.6 ± 5.46 | ns |
| Range | (65–90) | (65–82) | |
| No. of subjects investigated | |||
| Women | 17 | 23 | |
| Men | 4 | 3 | |
| Hematology values (mean ± SD) | |||
| RBCs (106/μL) | 4.63 ± 0.18 | 4.59 ± 0.3 | ns |
| Hemoglobin (g/dL) | 13.84 ± 1.39 | 13.76 ± 0.97 | ns |
| Hematocrit (%) | 41.67 ± 3.34 | 41.06 ± 2.67 | ns |
| MCV (fL) | 90.01 ± 6.44 | 89.6 ± 4.7 | ns |
| Platelets (103/μL) | 246.28 ± 37.39 | 212.56 ± 52.94 | ns |
| WBCs (103/μL) | 6.16 ± 1.73 | 6.2 ± 1.6 | ns |
| Neutrophils (103/μL) | 3.31 ± 1.08 | 3.15 ± 0.92 | ns |
| Monocytes (103/μL) | 0.44 ± 0.14 | 0.49 ± 0.19 | ns |
| Lymphocytes (103/μL) | 2.2 ± 0.97 | 2.31 ± 0.76 | ns |
| Biochemistry values (mean ± SD) | |||
| Glucose (mg/dL) | 106.43 ± 14.87 | 98.92 ± 14.59 | ns |
| Creatinine (mg/dL) | 0.76 ± 0.15 | 0.75 ± 0.13 | ns |
| AST (U/L) | 19.43 ± 6.78 | 20.62 ± 5.82 | ns |
| ALT (U/L) | 16.14 ± 6.44 | 18.29 ± 4.93 | ns |
| AP (U/L) | 81.14 ± 29.69 | 78.21 ± 18.6 | ns |
| Albumin (g/L) | 46.14 ± 3.53 | 43.58 ± 2.46 | ns |
| Immunological values (mean ± SD) | |||
| CD3+/lymphocytes | 25.08 ± 3.8 | 27.44 ± 5.95 | ns |
| CD4+/CD3+ | 38.93 ± 7.03 | 41.42 ± 7.98 | ns |
| CD8+/CD3+ | 30.72 ± 3.4 | 33.13 ± 9.17 | ns |
| CD 16 + 56/CD45+ | 10.74 ± 3.83 | 9.44 ± 3.52 | ns |
| CD 19+/CD45+ | 3.87 ± 1.75 | 3.79 ± 1.79 | ns |
RBCs red blood cells, MCV mean corpuscular volume, AST aspartate aminotransferase, ALT alanine aminotransferase, AP alkaline phosphatase
aCalculated using the Student t test
Blood cell counts and an immune phenotype of majority populations were performed in all individuals included in the study. Between-group analyses indicated no significant overall differences in immune variables between the placebo and probiotic groups at baseline (Table 2). The placebo group showed no difference in the distribution of the measured immune populations at 3 or 6 months. In contrast, the probiotic group had significantly lower percentage of CD8+ T cells at 3 months (Paired t-test, p = 0.008) and significantly higher percentile of NK cells (CD16 + 56+) at 3 and 6 months (Paired t-test, p = 0.012 and p = 0.001, respectively). The double positive CD4 + CD8+ and CD4 + NKG2D + are two other subsets of T cells that have been related to aging (Alonso-Arias et al. 2009; Alonso-Arias et al. 2011). Percentages of these two subpopulations in the probiotic group did not differ significantly between groups at different times of analysis; however, these subpopulations showed a tendency to decrease at 3 and at 6 months (Table 2). In summary, these results demonstrated that consumption of probiotic modulates percentages of different immune populations.
Table 2.
Immunological parameters (mean ± SD) measured at baseline, 3 months, and 6 months
| Placebo group | Probiotic group | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| CD3+/lymphocytes | 25.08 ± 3.8 | 25.62 ± 5.43 | 27.0 ± 6.6 | 27.44 ± 5.95 | 27.44 ± 5.95 | 26.81 ± 5.52 |
| CD4+/CD3+ | 38.93 ± 7.03 | 40.62 ± 8.42 | 41.87 ± 8.75 | 41.42 ± 7.98 | 41.73 ± 8.75 | 41.08 ± 7.27 |
| CD8+/CD3+ | 30.72 ± 3.4 | 29.18 ± 7.14 | 28.39 ± 7.01 | 33.13 ± 9.17 | 30.45 ± 8.0* | 30.67 ± 7.4 |
| CD4+CD8+/CD4+ | 2.79 ± 2.48 | 2.9 ± 2.31 | 2.47 ± 1.66 | 2.86 ± 1.3 | 2.74 ± 1.59 | 2.57 ± 1.45 |
| CD4+NKG2D+/CD4+ | 5.12 ± 2.34 | 5.33 ± 2.14 | 5.35 ± 2.33 | 5.3 ± 3.56 | 4.81 ± 3.01 | 4.74 ± 3.04 |
| CD16+56/CD45+ | 10.74 ± 3.83 | 10.35 ± 4.18 | 11.98 ± 4.85 | 9.44 ± 3.52 | 11.18 ± 4.81* | 11.55 ± 4.09* |
| CD 19+/CD45+ | 3.87 ± 1.75 | 4.25 ± 2.04 | 4.42 ± 2.3 | 3.79 ± 1.79 | 3.85 ± 1.97 | 3.71 ± 1.69 |
*Statistically significant difference (paired Student’s t test, p < 0.05) respect to time 0
IRP parameters and probiotic consumption
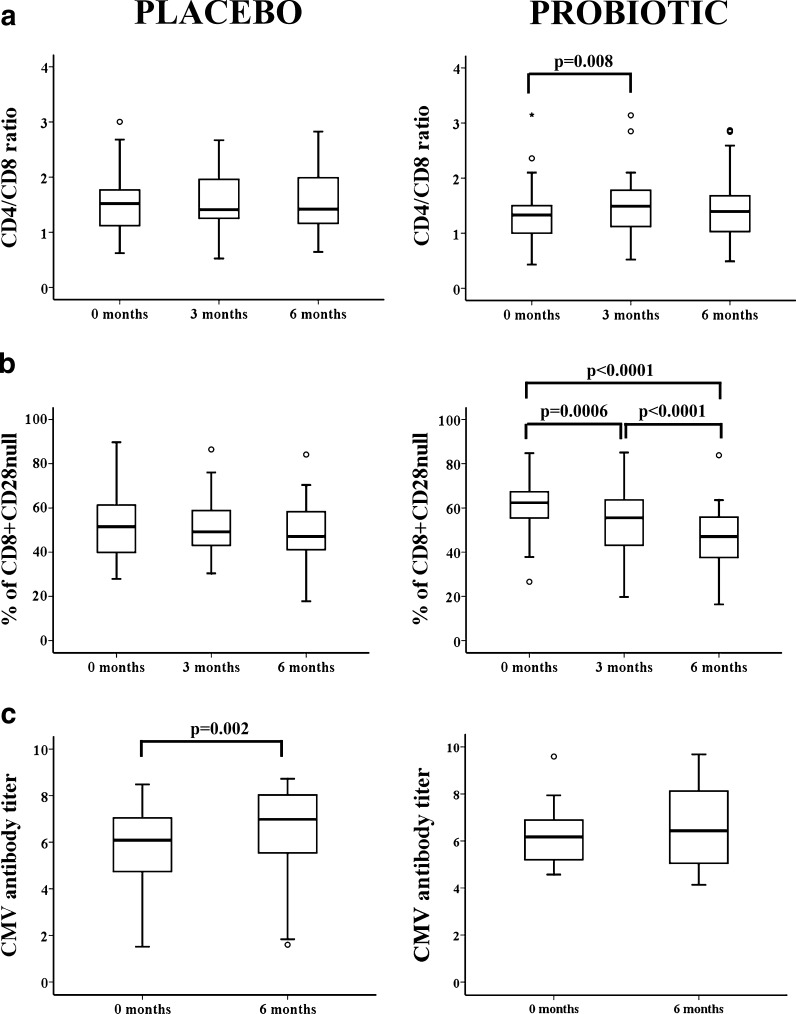
Parameters that define the IRP are the inverted ratio CD4/CD8 (ratio < 1.0), increase of CD8 + CD28null T cells, and CMV infection (Hadrup et al. 2006). As aforementioned, percentages of CD4+ T cells showed no significant differences throughout the study; however, the percentage of CD8+ T cells significantly decreased within 3 months of consumption of the probiotic. Accordingly, the CD4/CD8 ratio was significantly different between 0 and 3 months (Wilcoxon test, p = 0.008) (Fig. 2a). In aging, there is an accumulation of CD8 + CD28null T cells which are oligoclonal and show characteristics of cellular senescence. In the probiotic group, the percentage of CD8 + CD28null T cells significantly declined at both 3 and 6 months compared to that at baseline (Fig. 2b). As expected, for the placebo group, the CD4/CD8 ratio and the percentage of CD8 + CD28null T cells remained unaltered throughout the study (Fig. 2a, b).
Fig. 2.
CD4/CD8 ratio, percentage of CD8+CD28null, and CMV antibody titer in elderly from the placebo and probiotic groups. a CD4/CD8 ratios were analyzed and compared between the two groups at 0, 3, and 6 months post-initiation of probiotic or placebo consumption. Staining was performed with anti-CD3-FITC, anti-CD4-APC, and anti-CD8-PerCP to gate CD4+ and CD8+ T cells. CD4/CD8 ratio less than 1.0 was used to identify individuals with an IRP. b Percentages of CD8+ T cells lacking CD28 expression in peripheral blood of elderly. Whole blood was stained with anti-CD3-FITC, anti-CD28-PE, and anti-CD8-PerCP. Frequencies of CD28null cells in gated CD3+CD8+ lymphocytes were quantified. c Serum anti-CMV antibody titer was measured by ELISA at baseline and 6 months and was compared. Patient samples are quantified and interpreted by comparing the cut-off index ratio (Cutoff Index = OD value of sample/Cut-off value). A ratio of 1.0 is equivalent to the cut-off value. Cutoff index > 1.1 was considered positive, and the result of this ratio is a semi-quantitative titer. Outlier and extreme values, which were calculated by adding 1.5 and 3 times the IR to the 75th percentile, were represented by circles and stars, respectively. Paired t-test was used to compare values between groups, and p-values are depicted in the panels
The relationship between CMV infection and probiotic consumption was also analyzed by comparing titers of antibodies against CMV at the beginning of the study and at 6 months. The elderly group who took the placebo capsules showed a significant increase in the mean CMV titer at 6 months compared to that at the beginning of the study. In contrast, the probiotic group did not show significant differences in CMV titer between baseline and 6 months possibly because their immune system was able to better control the viral reactivations (Fig. 2c).
Therefore, we concluded that those elders who took the probiotic had an improvement in their IRP, an index that is related to increased 2-year mortality in elderly people.
T cell differentiation subsets
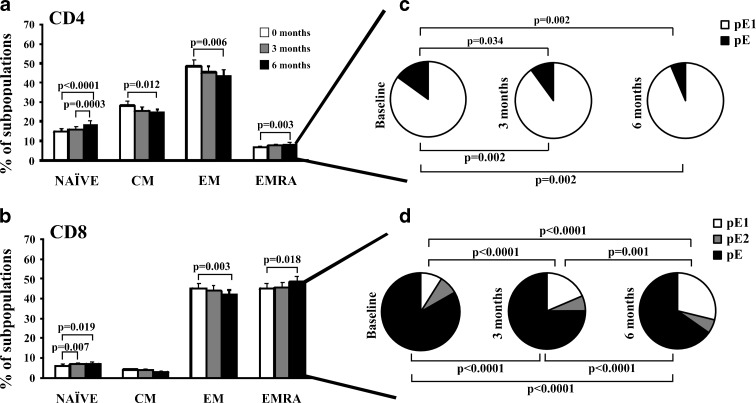
T cells appear to be more sensitive to the aging process than other types of immune cells, and significant changes in both functional and phenotypic profiles occur in elderly humans as a function of time. T cells can be separated into functionally different populations using combinations of cell surface markers such as the tyrosine phosphatase isoform CD45RA and the chemokine receptor CCR7. With these markers, we subdivided the T cells into naïve (NAÏVE; CD45RA + CCR7+), central memory (CM; CD45RA-CCR7+), effector memory (EM; CD45RA-CCR7-), and effector memory RA (EMRA; CD45RA + CCR7-) (Sallusto et al. 1999). The distribution of the distinct T cell subpopulations at 0, 3, and 6 months into the placebo and probiotic groups were compared. First, the elderly in the placebo group showed no significant changes in the percentage of any T cell subpopulations (data not shown). In contrast, probiotic intake in elderly was associated with an increased population of undifferentiated T cell subsets. In fact, the frequency of the NAÏVE CD4+ T cells in elders was significantly increased from baseline and from 3 to 6 months. The percentage of CM and the more differentiated subtype, EM CD4+ T cells declined (Fig. 3a). Similar to CD4+ T lymphocytes, CD8+ T cells showed a significant increase in the younger population, the NAÏVE cells from baseline to 3 and 6 months. CM cells showed no differences, and the CD8+ EM subset, a more differentiated population, exhibited a significant decrease from baseline to 6 months (Fig. 3b).
Fig. 3.
Distribution of T cell subsets in the probiotics group. CD4+ and CD8+ T cells were categorized into NAÏVE, CM, EM, and EMRA cells. Distribution of EMRA in CD4+ and CD8+ T cells into subsets defined by CD28 and CD27 expression. Expression of CD45RA, CCR7, CD27, and CD28 was analyzed by flow cytometry in isolated CD4+ and CD8+ T cells from the two groups of elders at 0, 3, and 6 months. Histograms represent percentage of a CD4+ and b CD8+ T cells in each subset (NAÏVE, CM, EM, and EMRA) in the groups of elderly (time 0: white bars, time 3 months: gray bars, time 6 months: black bars). c, d Individual segments of the pie charts represent the proportions of cells with each combination of CD28 and CD27 in the EMRA CD4+ (c) and CD8+ (d) T cell subsets. EMRA can be divided into pE1 (CD27 + CD28+) and pE2 (CD27 + CD28null, only in CD8 T cells) and E (CD27nullCD28null). Significant differences between subsets related to total CD4+ and CD8+ T cells are indicated (Paired t-test). Each bar in the histograms represented the mean ± SEM
Although it is assumed that EMRA cells are considered the more differentiated cells, the frequency of EMRA cells significantly increased from baseline to 6 months both in CD4+ as in CD8+ T cells. However, EMRA is a heterogeneous population, and the staining with two additional markers, CD27 and CD28, can distinguish between less differentiated (CD27+ and/or CD28+) or more differentiated (CD27nullCD28null) cells (Koch et al. 2008). EMRA subset can be further subdivided into very poorly differentiated pE1 (CD27 + CD28+), pE2 (CD27 + CD28null, only in CD8+ T cells), and most differentiated T-cell subset, E (CD27nullCD28null). We compared the frequencies of these subsets of CD4+ and CD8+ T cells at 0, 3, and 6 months in the placebo and probiotic groups. The less differentiated subset (pE1) in the probiotic group was increased at 3 and 6 months from baseline. Conversely, the most differentiated subset (pE) decreased both in CD4+ T cells as in CD8+ T cells from baseline to 6 months (Fig. 3c, d). Thus, the increase observed in the EMRA population at 6 months in the CD4+ and CD8+ T cells is due to an increase in the less differentiated subset pE1. In contrast, the oldest population of T lymphocytes (pE) declined in the probiotic group. Taken together, these results indicated that the probiotic intake was related to an increase in the less differentiated subsets of both CD4+ and CD8+ T cells. These populations are necessary for the maintenance of an adequate immune response, slowing the aging of the T lymphocyte subpopulations and increasing the number of immature naïve T cells.
Proximity to the thymus of lymphocyte populations
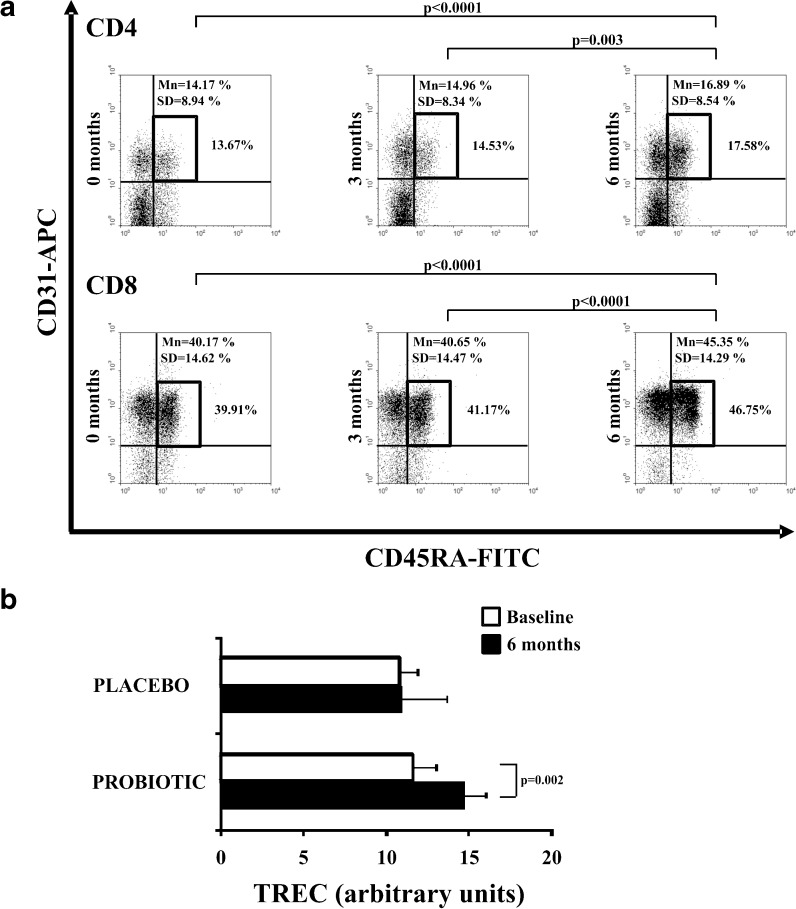
Full thymic activity in young healthy individuals leads to continuous replenishment with naïve T cells with high TREC content. These recent thymic emigrants co-express CD45RA and CD31: This co-expression is high in CD45RA+ T cells from young subjects but decreases continually during ageing (Kimmig et al. 2002). First, we examined whether frequencies of CD45RA+ T cells co-expressing the CD31 molecule changed throughout the study. The CD31 + CD45RA + subset was significantly higher in the elderly probiotic group, both in CD4 and CD8 T cells within 6 months (Fig. 4a). By contrast, no differences were observed in the placebo group (data not shown).
Fig. 4.
Proximity to the thymus of the T cell subsets and TREC content. Whole blood from the elderly of the probiotic group was stained with anti-CD45RA-FITC, anti-CD31-PE, and these CD4+ and CD8+ T cell subsets were evaluated by flow cytometry. a Representative dot-plots showing the frequency of CD45RA+CD31+ in CD4+ and CD8+ T cells from elderly. Percentage of positive cells in each subpopulation in this representative experiment is expressed in the right side, and summarized results from all donors (mean and SD) were also expressed in dot-plots. b The TREC content was measured in PBLs cells from elders belonging to placebo/probiotic group. TREC copy number was determined by real-time PCR. Experiments were conducted in duplicate, and bars represented results from the grouped elders (mean ± SEM)
To further examine the differences in the differentiated status of CD4+ and CD8+ T subsets, we assessed the replicative history of these cells by quantifying the TREC content. TREC is a traceable molecular marker produced in newly naïve T cells; the content of TREC in peripheral T cells is a direct indicator of the number of divisions that the cell has undergone (Douek et al. 1998). TREC content was measured at baseline and 6 months in both groups. No significant differences were detected in the placebo group. In contrast, the TREC content significantly increased between baseline and 6 months in the probiotic group (Paired t-test, p = 0.002) (Fig. 4b).
In summary, these results suggest that the strain of L. delbrueckii subsp. bulgaricus 8481 produces an increase in the generation of immature T cells and their output to peripheral blood needed to mount an adequate immune response.
Changes in humoral immunity
After demonstrating that the consumption of our probiotic produced significant modifications in the cells of the elderly immune system, we decided to investigate if these modifications were reflected in humoral changes in serum. First, we assessed concentrations of immunoglobulin (IgG, IgM, and IgA) and complement molecules C3 and C4 (Table 3). No significant differences were detected between values at baseline and 6 months for either group.
Table 3.
Immunoglobulin and complement quantification (g/L) (mean ± SD) at baseline, 3 months, and 6 months
| Placebo group | Probiotic group | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | p value between groupsa | Baseline | 3 months | 6 months | p value between groupsa | |
| IgG | 9.86 ± 1.47a | 9.44 ± 2.67 | 9.67 ± 1.97 | ns | 10.89 ± 1.9 | 10.56 ± 1.89 | 9.89 ± 1.77 | ns |
| IgM | 0.99 ± 0.59 | 0.95 ± 0.72 | 1.06 ± 0.61 | ns | 1.75 ± 0.95 | 1.65 ± 0.86 | 1.48 ± 0.7 | ns |
| IgA | 2.22 ± 0.84 | 2.12 ± 0.87 | 2.06 ± 0.94 | ns | 3.39 ± 1.38 | 3.3 ± 1.3 | 3.18 ± 1.64 | ns |
| C3 | 1.95 ± 0.49 | 1.95 ± 0.51 | 1.74 ± 0.38 | ns | 1.83 ± 0.12 | 1.65 ± 0.47 | 1.66 ± 0.2 | ns |
| C4 | 0.44 ± 0.1 | 0.45 ± 0.09 | 0.43 ± 0.1 | ns | 0.48 ± 0.08 | 0.45 ± 0.08 | 0.46 ± 0.06 | ns |
aCalculated using the paired Student’s t test
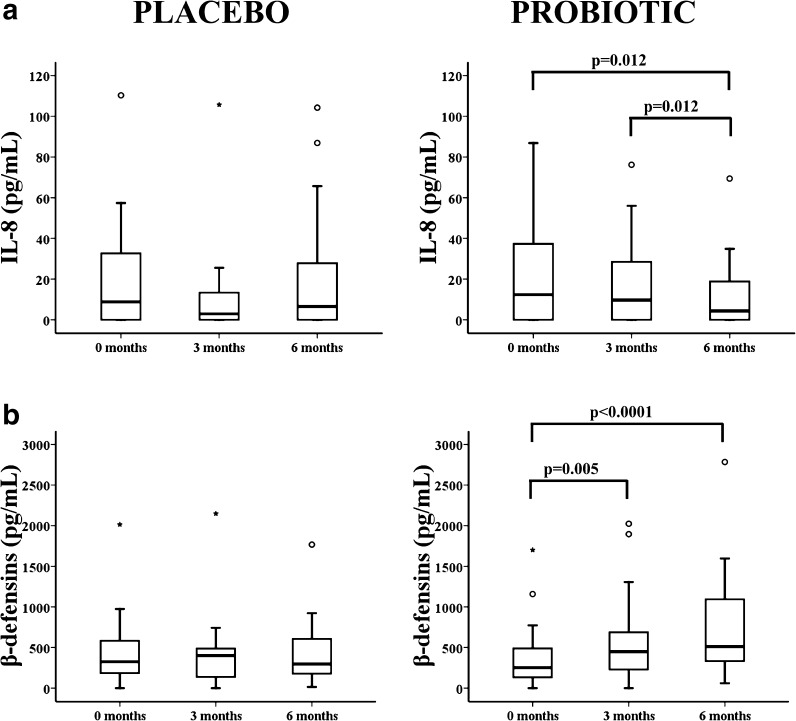
We also assessed the serum concentrations of major cytokines with pro- and anti-inflammatory activities for changes at the three time points by flow cytometry. Concentrations of IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, TNF-α, and TNF-β did not significantly change during the study (data not shown). The concentration of IL-8, one of the major mediators of the inflammatory response, significantly decreased in the probiotic group, but not the placebo group, by 6 months (Fig. 5a).
Fig. 5.
Quantification of pro-inflammatory cytokine IL-8 and hBD-2 antimicrobial peptide in elderly in the placebo/probiotic groups at 0, 3, and 6 months. a Serum IL-8 concentrations into the placebo and probiotic groups were measured by flow cytometry. b Quantification of the hBD-2 peptide in serum from the elderly in the two groups by ELISA. Outlier values were represented by circles and extreme values by stars, calculated by adding 1.5 and 3 times the IR to the 75th percentile, respectively. The Wilcoxon non-parametric method was used to compare frequencies in the groups. P-values are depicted in the panels
It is known that specific probiotic strains including lactobacilli up-regulate expression of the hBD-2 (Wehkamp et al. 2004). As expected, the placebo group had no change in the concentration of hBD-2 during the study. In contrast, the hBD-2 concentration significantly increased in the serum of the elderly of the probiotics group at 3 (Wilcoxon test, p = 0.005) and 6 months (Wilcoxon test, p < 0.0001) (Fig. 5b).
In summary, we can affirm that the changes that we had observed at a cellular level are reflected at a humoral level with changes in some cytokine concentration and antimicrobial molecules.
Cessation of probiotic intake
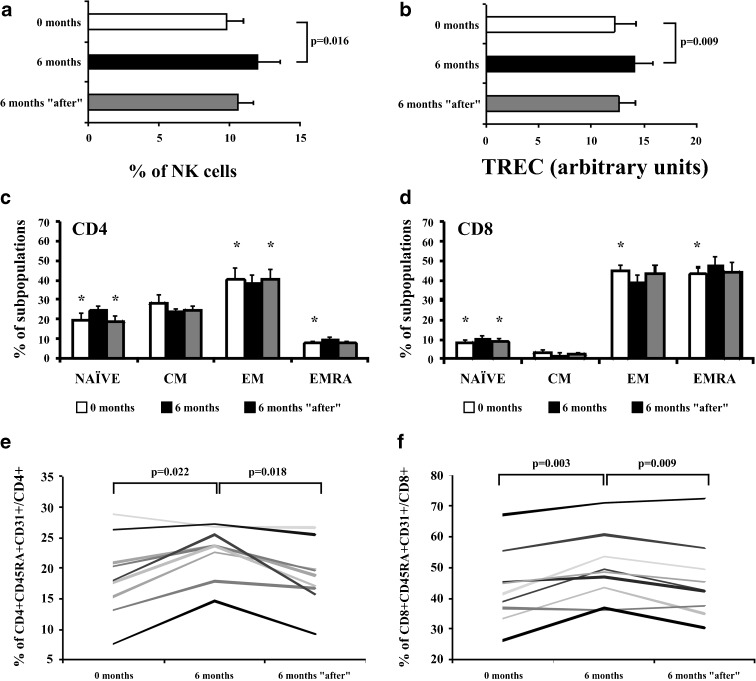
After 6 months of study, we wanted to assess the stability of the changes in immunological variables by the 6-month probiotic intake. Thus, 6 months after stopping the probiotic intake, we managed to obtain blood samples from nine elders who had taken the probiotic capsules. The higher percentage of NK cells, the modulated T cell differentiation subsets, the higher percentage of very poorly differentiated cells CD45+CD31+, and the higher TREC content were not maintained for 6 months without probiotics. These parameters returned to levels equal to or very similar to the baseline before probiotic intake (Fig. 6). Therefore, we concluded that maintenance of the beneficial effects produced by the probiotic requires ongoing probiotic intake.
Fig. 6.
Changes in percentage in NK cells, TREC content, T cell subsets distribution, and percentage of CD45RA+CD31+ into CD4+ and CD8+ T cells 6 months after stopping the probiotic intake. Measurements were made in nine elderly people at baseline, 6 months from the beginning of the study, and 6 months after stopping the probiotic intake. a Percentages of CD16+56+ cells with respect to the total CD45+ cells were compared throughout the study. Staining was performed with “Multiset CD3-FITC/CD16+56-PE/CD45-PerCP/CD19-APC,” and frequencies of CD16+56 cells in gated CD45+ subsets were analyzed. b TREC content in elderly T cells. The TREC content was measured in T cells from elders, and TREC copy number was determined by real-time PCR. Experiments were conducted in duplicate. c, d Distribution of CD4+ and CD8+ T cells into NAÏVE, CM, EM, and EMRA. Expression of CD45RA and CCR7 was analyzed by flow cytometry in isolated CD4+ and CD8+ T cells. e, f Frequency of CD45RA+CD31+ in CD4+ and CD8+ T cells from elderly. Whole blood from the elderly of the probiotic group was stained with anti-CD45RA-FITC, anti-CD31-PE, and anti-CCR7-APC; and CD4+ and CD8+ T cells were evaluated by flow cytometry. Bars in the histograms represented the mean ± SEM. The Paired t-test (when data were normally distributed) and Wilcoxon non-parametric method (when data were not normally distributed) were used to compare frequencies between groups. *Significant differences with values obtained 6 months after beginning the probiotic intake (p < 0.05)
Discussion
The present study used a double-blind, placebo-controlled, and randomized design to determine the effects of L. delbrueckii subsp. bulgaricus 8481 on the elderly immune system. In this study, we have demonstrated a clear association between L. delbrueckii subsp. bulgaricus 8481 consumption and a great benefit on the immune system of elderly people. We can assert that consumption of the probiotic L. delbrueckii subsp. bulgaricus 8481 could promote the maintenance of an adequate immune response, slowing the aging of the subpopulations of T lymphocytes and increasing the number of immature cells potentially responders.
Older people suffer from age-associated changes in the immune system, including decreased immune function, increased incidence and severity of infections, development of autoimmune phenomena, and cancer (DelaRosa et al. 2006; Prelog 2006). NK cells play a critical role in immune surveillance against tumor development and viral infections, and intestinal microflora can modulate NK activity (Takeda and Okumura 2007). NK activity are depressed in the elderly in comparison to younger subjects (Hodes 1997). Therefore, our results strongly suggest that consumption of capsules containing L. delbrueckii subsp. bulgaricus 8481 significantly increased the NK cell percentage in elderly people, which may result in the maintenance of a healthy life and prevention of diseases.
Factors involved in IRP include the inverted CD4/CD8 ratio, an increase in the CD8 + CD28null T cell subset, and CMV seropositivity (Hadrup et al. 2006). Elders of the probiotic group had a significant decrease in the CD8+ T cell subset and a resulting increase in the CD4/CD8 ratio compared to the placebo group. Moreover, we observed a marked decline in the late differentiated memory and effector CD8 + CD28null T cell population throughout the study. Accumulation of CD8 + CD28null T cells is associated with a reduced overall immune response to pathogens and vaccines in the elderly (Riha and Rudd 2010). The cause of loss of CD28 expression in T cells with age has been attributed to repeated antigenic stimulation such as CMV or other persistent viral infections (Vallejo 2005). It is now accepted that CD28null T cells have experienced past episodes of activation and cell cycling. Regards CMV infection, a high CMV antibody titer is associated with a reduced survival time (Caruso et al. 2009). In our study, elderly who took the placebo capsules had a higher CMV titer at 6 months than at baseline, while elderly who took the probiotic maintained the same CMV titer at 6 months and baseline. These results suggest that taking probiotic could prevent CMV replication by controlling their spread and avoiding the occurrence of further viral reactivation via unclear mechanisms.
As people age, differentiated T cells (EM and EMRA subsets) of the immune system accumulate, and the less differentiated immune cells (Naïve and CM) decline in frequency (Appay et al. 2010). The differentiated cells, EM and EMRA, have a memory phenotype, display reduced diversity of the T-cell receptor (TCR), have reduced division potential (Vallejo et al. 2000; Appay et al. 2002), and are less able to respond to pathogens. Consumption of L. delbrueckii subsp. bulgaricus 8481 enhanced the proportions of less differentiated cells and decreased the more differentiated cells in the peripheral circulation. As an alternative mechanism, probiotic consumption stimulated production of naïve T cells and NK cells, which diluted the more differentiated cells and subsequently reduced the memory T cell compartment in the peripheral circulation via mechanisms of T cell homeostasis. These changes in T lymphocytes may be associated with health improvements in the elderly such as an enhanced antibody response to vaccination and markedly reduced duration of infection-related morbidity in elderly subjects.
To further investigate the composition of the T cell repertoire in our elderly, we examined the expression of CD31 and the TREC content (Kimmig et al. 2002; Kohler and Thiel 2009). These two cell markers indicate the time elapsed since these cells left the thymus; less differentiated naïve cells are essential to respond and develop immune responses against new antigens. The thymus gland is greatly reduced in elderly individuals and usually plays a very small role in their immune responses. The CD31+ marker in CD4+ and CD8+ T cells of CD45RA+ phenotype significantly increased in those individuals taking the probiotic. These data suggest that probiotic consumption may stimulate the thymus gland. We also observed that T cells in elders of the probiotic group had a higher mean TREC content, which corroborates the increase in poorly differentiated naïve cells after consumption of the probiotic.
One of the main factors that are postulated as triggers of the process of immunosenescence is a chronic inflammatory state (Ferrucci et al. 2005). Probiotics may also inhibit the production of pro-inflammatory cytokines (Lopez et al. 2008). Elderly who consumed the capsules with L. delbrueckii subsp. bulgaricus 8481 showed a decline over 6 months in the serum concentration of the chemokine IL-8. This chemokine is one of the major mediators of the inflammatory response, so its decline could be very beneficial for the elderly people.
The mucosal immune system consists of an integrated network of tissues, lymphoid and mucous membrane-associated cells, innate effector proteins (mucins and defensins), and previously induced antibody molecules. The gastrointestinal tract in the elderly is particularly susceptible to infectious diseases, in part due to changes in their intestinal microbiota but probably also because of dramatic changes to mucosal immunity itself (Schmucker et al. 1996; Fujihashi and Kiyono 2009). We wanted to determine whether L. delbrueckii subsp. bulgaricus 8481 was able to stimulate the production of hBD-2 and improve the immune innate response in the elderly, with a defective mucosal immune system. Consumption of the probiotic was associated with a very marked increase in hBD-2 antimicrobial molecule in serum, suggesting an enhancement in the innate immunity through defensin induction. Recently, 2 of 46 strains of Lactobacillus fermentum induced hBD-2 production from human intestinal Caco-2 cell line (Ghadimi et al. 2011). Pathogenic strains of Salmonella ssp. and Helicobacter pylori have also been reported to induce hBD-2 expression (Ogushi et al. 2001; Wehkamp et al. 2003); in contrast, lactobacilli and other probiotics appear to induce the intestinal barrier defense system without provoking inflammatory events (Santos Rocha et al. 2011). The increase in hBD-2 production and the increase in the number of NK cells suggest an improvement in innate immunity in individuals consuming L. delbrueckii subsp. bulgaricus. The role played by NK cells in innate immunity is analogous to that of cytotoxic T cells in the adaptive immune response. NK cells provide rapid responses to virally infected cells and respond to tumor formation. Human β-defensin-2 (hBD-2) code for genes which improve the function of the innate immune system (Hellgren and Sheldon 2011), and its production is enhanced by some strains of Lactobacillus (Schlee et al. 2008). These genes are responsible for production of antimicrobial polypeptides found in white blood cells such as macrophages, granulocytes and NK-cells, and antimicrobial β-defensins which are also found in epithelial cells.
In our study, we found that the beneficial effects attributed to probiotic disappeared after 6 months of stopping their consumption. Therefore, it is necessary to ingest these bacteria on a regular and ongoing basis to obtain significant and lasting results. We speculate that the bacteria only pass through the intestinal tract, without establishing residence in the intestinal tract. Whether colonization of part of the intestinal tract with L. delbrueckii subsp. bulgaricus 8481 could be enhanced by altering the diet is unknown. Accordingly, once we stopped using the product, its effect disappeared.
The effects of probiotics seem promising. It is important to note that all parameters studied were evaluated systemically. Most previous immunological studies on the effects of probiotics have shown only local effects in the intestinal mucosa but did not examine their effects in the peripheral blood. Immunomodulation induced by our probiotic could favor the maintenance of an adequate immune response, slowing the aging of the subpopulations of T lymphocytes and increasing the number of immature cells which are potential responders to new antigens. The L. delbrueckii subsp. bulgaricus 8481 probiotic also has a powerful effect on the innate immune system. Therefore, taking the probiotic might help produce a better response to vaccination and greater resistance to contracting various infectious diseases. These results must be confirmed in a larger number of individuals subjected to a longer duration of probiotic consumption. Further studies will help us to determine whether L. delbrueckii subsp. bulgaricus 8481 can promote increased resistance to infection and disease in the elderly and other age groups.
Acknowledgments
We would like to thank Isabel Cuevas Pérez for the excellent technical assistance. The authors are grateful to Mr. Miguel de las Heras, patent holder on the bacteria, and to Dr. Celina Quirós Díaz for their support of the research.
This work was supported by Red de Investigación Renal (REDinREN), Spanish grant FIS PI080566 from Instituto “Carlos III” (European FEDER founds), PEST 08-05 and CNCT-Leche from FICYT.
References
- Alonso-Arias R, Lopez-Vazquez A, Diaz-Pena R, Sampere A, Tricas L, Asensi V, Rodrigo L, Lopez-Larrea C. CD8dim and NKG2D expression defines related subsets of CD4+ T cells in HIV-infected patients with worse prognostic factors. J Acquir Immune Defic Syndr. 2009;51:390–398. doi: 10.1097/FTD.0b013e3181679015. [DOI] [PubMed] [Google Scholar]
- Alonso-Arias R, Moro-Garcia MA, Lopez-Vazquez A, Rodrigo L, Baltar J, Garcia FM, Jaurrieta JJ , Lopez-Larrea C (2011). NKG2D expression in CD4+ T lymphocytes as a marker of senescence in the aged immune system. Age (Dordr). [DOI] [PMC free article] [PubMed]
- Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- Appay V, Sauce D, Prelog M. The role of the thymus in immunosenescence: lessons from the study of thymectomized individuals. Aging (Albany NY) 2010;2:78–81. doi: 10.18632/aging.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle RJ, Ismail IH, Kivivuori S, Licciardi PV, Robins-Browne RM, Mah LJ, Axelrad C, Moore S, Donath S, Carlin JB, Lahtinen SJ , Tang ML (2010). Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy. [DOI] [PubMed]
- Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- Caruso C, Buffa S, Candore G, Colonna-Romano G, Dunn-Walters D, Kipling D, Pawelec G. Mechanisms of immunosenescence. Immun Ageing. 2009;6:10. doi: 10.1186/1742-4933-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo R, Bravo D, Canton R, Ruiz-Garbajosa P, Garcia-Albiach R, Montesi-Libois A, Yuste FJ, Abraira V, Baquero F. Scarce evidence of yogurt lactic acid bacteria in human feces after daily yogurt consumption by healthy volunteers. Appl Environ Microbiol. 2005;71:547–549. doi: 10.1128/AEM.71.1.547-549.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelaRosa O, Pawelec G, Peralbo E, Wikby A, Mariani E, Mocchegiani E, Tarazona R, Solana R. Immunological biomarkers of ageing in man: changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology. 2006;7:471–481. doi: 10.1007/s10522-006-9062-6. [DOI] [PubMed] [Google Scholar]
- Diaz-Ropero MP, Martin R, Sierra S, Lara-Villoslada F, Rodriguez JM, Xaus J, Olivares M. Two Lactobacillus strains, isolated from breast milk, differently modulate the immune response. J Appl Microbiol. 2007;102:337–343. doi: 10.1111/j.1365-2672.2006.03102.x. [DOI] [PubMed] [Google Scholar]
- Dixon B. Secrets of the Bulgarian bacillus. Lancet Infect Dis. 2002;2:260. doi: 10.1016/S1473-3099(02)00247-5. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Elli M, Callegari ML, Ferrari S, Bessi E, Cattivelli D, Soldi S, Morelli L, Goupil Feuillerat N, Antoine JM. Survival of yogurt bacteria in the human gut. Appl Environ Microbiol. 2006;72:5113–5117. doi: 10.1128/AEM.02950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol A Biol Sci Med Sci. 1995;50:B378–382. doi: 10.1093/gerona/50A.6.B378. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K, Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 2009;30:334–343. doi: 10.1016/j.it.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Albiach R, Pozuelo de Felipe MJ, Angulo S, Morosini MI, Bravo D, Baquero F, del Campo R. Molecular analysis of yogurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in human intestinal microbiota. Am J Clin Nutr. 2008;87:91–96. doi: 10.1093/ajcn/87.1.91. [DOI] [PubMed] [Google Scholar]
- Ghadimi D, Hassan M, Njeru PN, de Vrese M, Geis A, Shalabi SI, Abdel-Razek ST, Abdel-Khair AE, Heller KJ , Schrezenmeir J (2011). Suppression subtractive hybridization identifies bacterial genomic regions that are possibly involved in hBD-2 regulation by enterocytes. Mol Nutr Food Res. [DOI] [PubMed]
- Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren O, Sheldon BC. Locus-specific protocol for nine different innate immune genes (antimicrobial peptides: beta-defensins) across passerine bird species reveals within-species coding variation and a case of trans-species polymorphisms. Mol Ecol Resour. 2011;11:686–692. doi: 10.1111/j.1755-0998.2011.02995.x. [DOI] [PubMed] [Google Scholar]
- Hodes RJ. Aging and the immune system. Immunol Rev. 1997;160:5–8. doi: 10.1111/j.1600-065X.1997.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Kano H, Kaneko T, Kaminogawa S. Oral intake of Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 prevents collagen-induced arthritis in mice. J Food Prot. 2002;65:153–160. doi: 10.4315/0362-028x-65.1.153. [DOI] [PubMed] [Google Scholar]
- Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, Thiel A. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- Le Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debre P, Merle-Beral H , Vieillard V (2010). Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. [DOI] [PubMed]
- Lopez M, Li N, Kataria J, Russell M, Neu J. Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J Nutr. 2008;138:2264–2268. doi: 10.3945/jn.108.093658. [DOI] [PubMed] [Google Scholar]
- Lorea Baroja M, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol. 2007;149:470–479. doi: 10.1111/j.1365-2249.2007.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mater DD, Bretigny L, Firmesse O, Flores MJ, Mogenet A, Bresson JL, Corthier G. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol Lett. 2005;250:185–187. doi: 10.1016/j.femsle.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Medici M, Vinderola CG, Weill R, Perdigon G. Effect of fermented milk containing probiotic bacteria in the prevention of an enteroinvasive Escherichia coli infection in mice. J Dairy Res. 2005;72:243–249. doi: 10.1017/S0022029905000750. [DOI] [PubMed] [Google Scholar]
- Moro-Garcia MA, Alonso-Arias R, Lopez-Vazquez A, Suarez-Garcia FM, Solano-Jaurrieta JJ, Baltar J , Lopez-Larrea C (2011). Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age (Dordr). [DOI] [PMC free article] [PubMed]
- O'Flaherty S, Saulnier DM, Pot B, Versalovic J. How can probiotics and prebiotics impact mucosal immunity? Gut Microbes. 2010;1:293–300. doi: 10.4161/gmic.1.5.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogushi K, Wada A, Niidome T, Mori N, Oishi K, Nagatake T, Takahashi A, Asakura H, Makino S, Hojo H, Nakahara Y, Ohsaki M, Hatakeyama T, Aoyagi H, Kurazono H, Moss J, Hirayama T. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J Biol Chem. 2001;276:30521–30526. doi: 10.1074/jbc.M011618200. [DOI] [PubMed] [Google Scholar]
- Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Riha P, Rudd CE. CD28 co-signaling in the adaptive immune response. Self Nonself. 2010;1:231–240. doi: 10.4161/self.1.3.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Santos Rocha C, Lakhdari O, Blottiere HM, Blugeon S, Sokol H, Bermu'dez-Humara'n LG, Azevedo V, Miyoshi A, Dore J, Langella P, Maguin E , van de Guchte M (2011). Anti-inflammatory properties of dairy lactobacilli. Inflamm Bowel Dis. [DOI] [PubMed]
- Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, Ferrand C, Debre P, Sidi D, Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M, Harder J, Koten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol. 2008;151:528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker DL, Heyworth MF, Owen RL, Daniels CK. Impact of aging on gastrointestinal mucosal immunity. Dig Dis Sci. 1996;41:1183–1193. doi: 10.1007/BF02088236. [DOI] [PubMed] [Google Scholar]
- Shida K, Nanno M. Probiotics and immunology: separating the wheat from the chaff. Trends Immunol. 2008;29:565–573. doi: 10.1016/j.it.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Strowski MZ, Wiedenmann B. Probiotic carbohydrates reduce intestinal permeability and inflammation in metabolic diseases. Gut. 2009;58:1044–1045. doi: 10.1136/gut.2009.179325. [DOI] [PubMed] [Google Scholar]
- Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J Nutr. 2007;137:791S–793S. doi: 10.1093/jn/137.3.791S. [DOI] [PubMed] [Google Scholar]
- Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Schirmer M, Weyand CM, Goronzy JJ. Clonality and longevity of CD4 + CD28null T cells are associated with defects in apoptotic pathways. J Immunol. 2000;165:6301–6307. doi: 10.4049/jimmunol.165.11.6301. [DOI] [PubMed] [Google Scholar]
- Wehkamp J, Schmidt K, Herrlinger KR, Baxmann S, Behling S, Wohlschlager C, Feller AC, Stange EF, Fellermann K. Defensin pattern in chronic gastritis: HBD-2 is differentially expressed with respect to Helicobacter pylori status. J Clin Pathol. 2003;56:352–357. doi: 10.1136/jcp.56.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K, Schroder JM, Stange EF. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]